Abstract
The structural, surface, and upconversion (UC) luminescence properties of Y2O3:Ho3+,Yb3+ films grown by pulsed laser deposition, for different numbers of laser pulses, were studied. The crystallinity, surface, and UC luminescence properties of the thin films were found to be highly dependent on the number of laser pulses. The X-ray powder diffraction analysis revealed that Y2O3:Ho3+,Yb3+ films were formed in a cubic structure phase with an Ia \(\overline{3 }\) space group. The thicknesses of the films were estimated by using cross-sectional scanning electron microscopy, depth profiles using X-ray photoelectron spectroscopy (XPS), and the Swanepoel method. The high-resolution XPS was used to determine the chemical composition and oxidation states of the prepared films. The UC emissions were observed at 538, 550, 666, and 756 nm, assigned to the 5F4 → 5I8, 5S2 → 5I8, 5F5 → 5I8, and 5S2 → 5I7 transitions of the Ho3+ ions. The power dependence measurements confirmed the involvement of a two-photon process in the UC process. The color purity estimated from the Commission International de I’Eclairage coordinates confirmed strong green UC emission. The results suggested that the Y2O3:Ho3+,Yb3+ UC transparent films are good candidates for various applications, including solar cell applications.
Similar content being viewed by others
Introduction
Transmission losses, which describe the incident energy lost to unabsorbed low-energy photons, are one of the significant loss processes in solar cells1. Upconversion (UC) can convert the low-energy photons that are unabsorbed by solar cells into high-energy photons. These high-energy photons can, therefore, be absorbed by the solar cell. This process can reduce transmission losses, and in turn, boosts the efficiency of the solar cells2. Thus far, materials reported that display visible UC emissions focused more on ceramics or powders3,4,5,6,7,8,9,10,11, which limits their practical solar cell use due to their poor compatibility with solar cell technology. Therefore, UC transparent films are more suitable for applications due to their good compatibility with solar cell technology. For solar cell applications, UC materials are applied underneath the solar cells as a layer with a controllable thickness, therefore, this film study is more suitable for such applications12.
As a typical host for a variety of luminescent materials, Y2O3 exhibits wide transmittance from 0.2 to 8 μm12, which is wide enough to cover the whole solar spectrum for solar cells. Furthermore, Y2O3 is an important host material for UC due to its excellent chemical durability, thermal stability, wide bandgap of 5.8 eV, low phonon energy of ~ 500 cm−1, and high refractive index of ~ 24,13,14. Doping Y2O3 with Ho3+ ions can offer certain advantages for UC since Ho3+ ions have been studied as excellent UC activators due to their high UC efficiency15. However, to enhance the UC efficiency further, the Yb3+ ion is typically co-doped as an ideal UC sensitizer for the Ho3+ ion due to its large absorption cross-section in the near-infrared (NIR) region around 980 nm16. Y2O3 thin films doped with lanthanide ions have been the subject of numerous studies17,18,19,20,21,22. Qiao et al.17 reported the UC properties of Y2O3:Er films prepared by the sol–gel method and obtained red and green emissions. Dikovsa et al.18 prepared the Er, Yb co-doped Y2O3 thin films by pulsed laser deposition (PLD) and studied the structural and optical properties of the prepared films. The authors obtained a smoother surface and a better crystalline structure for the Er, Yb co-doped Y2O3 films deposited at low oxygen pressure. Lian et al.19 reported on the deposition of the Eu3+ doped Y2O3 ultrathin films deposited on alumina nanoparticles by a solution synthesis method. The authors observed a strong photoluminescence (PL) in the film annealed at high temperatures. Pandey et al.23 investigated the temperature-induced UC behavior in Ho3+,Yb3+ co-doped Y2O3 thin films using PLD and obtained an intense green polycrystalline film of high quality with a smooth surface. They barely addressed the thickness effect on the UC emission of the prepared films, except for the thickness obtained from the Auger results. However, considering UC films as coatings on solar cells, the thickness of the film is vital. Hence, investigating the role of the Y2O3:Ho3+, Yb3+ UC thin film thickness is essential.
In this work, Y2O3:Ho3+, Yb3+ UC thin films were prepared using the pulsed laser deposition (PLD) technique for a different number of laser pulses. The structural, surface, and optical properties of the PLD Y2O3:Ho3+, Yb3+ UC films were investigated using X-ray powder diffraction (XRPD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and atomic force microscopy (AFM). The UC luminescence emissions were produced by exciting the thin films under 980 nm laser excitation. The photons involved in the UC process were confirmed by power dependence measurements.
Experimental
The Y2-x-yO3:Hox=0.005,Yby=0.05 films were prepared by the PLD method using the Nd:YAG laser with a wavelength of 266 nm under oxygen (O2) gas pressure of 50 mTorr and a various number of laser pulses (10,000, 20,000, 30,000, 40,000, 50,000, and 60,000). The PLD target was prepared by mixing 8 g of Y2-x-yO3:Hox=0.005, Yby=0.05 powder with ethanol (99.9%) and compressed at 75 tons for an hour to produce a pellet (target). Ethanol was used as a binder to help compact the powder more evenly. The target was then annealed at 1110 °C for 10 h to ensure that all impurities and ethanol had been eliminated. The preparation procedure of the Y2-x-yO3:Hox,Yby powders was previously reported by Makumbane et al.24. The researchers studied the UC luminescence of Y2-x-yO3:Hox=0.005,Yby (y = 0, 0.002, 0.006, 0.01, 0.05, 0.1, 0.2) phosphors. The UC emission intensity was optimized at a concentration of y = 0.05 Yb3+. Hence, Y2-x-yO3:Hox=0.005,Yby = 0.05 was used to prepare a pellet for this study. Moreover, the difference in the optimal concentrations with the work done by Pandey et al.23 could be due to the differences in the synthesis conditions used. They have used different starting materials, and annealing temperatures to synthesized their phosphor materials.
The deposition was carried out on soda lime glass substrates. The substrates were thoroughly cleaned for 15 min in an ultrasonic bath using acetone, ethanol, and distilled water. Nitrogen gas was then used to dry the cleaned substrates. The prepared target and a single cleaned substrate were then placed inside the PLD chamber system, where the chamber was pumped down to a base pressure of 1.5 × 10−5 Torr and back-filled with O2 gas at a pressure of 50 mTorr. The target-to-substrate distance was kept constant at 4 cm, while the substrate temperature was kept fixed at 350 °C. The laser energy and repetition rate were approximately 39 mJ/pulse and 30 Hz, respectively.
The structural properties of the Y2-x-yO3:Hox=0.005,Yby=0.05 films were characterized by XRPD (Bruker AXS GmbH, Karlsruhe, Germany) using a Bruker D8 Advance diffractometer. The morphology and elemental analysis were measured using a JEOL JSM-7800F field emission scanning electron microscope (FE-SEM) equipped with energy-dispersive X-ray spectroscopy (EDS) (JEOL, Tokyo, Japan). Shimadzu SPM-9600 AFM images taken in contact mode were used to determine the surface roughness. The Shimadzu SPM-9600 AFM software was used to examine the topography scans of the surfaces and estimate the root mean square (RMS) roughness. The transmittance was recorded using a Lambda 950 UV–Vis-NIR spectrophotometer (PerkinElmer Ltd., Beaconsfield, United Kingdom). A PHI Quantes Scanning Dual X-ray Photoelectron Microprobe System was used to carry out high-resolution XPS. The XPS spectra fittings were done using Multipack software version 8.2. The depth profile XPS measurements were acquired by bombarding the films with an Ar+ ion sputtering gun (2 kV, 1 μA). The sputter rastering area was 1 × 1 mm at a sputter rate of about 15 nm/min. The UC emission measurements were acquired using a 980 nm emitting diode laser as the excitation source. The decay curves were recorded using an FLS980 fluorescence spectrometer (Edinburgh Instruments) equipped with a photomultiplier (PMT) detector with a 980 nm emitting diode laser as the excitation source.
Results and discussion
Structural analysis
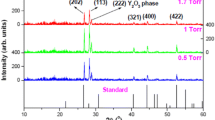
Figure 1a displays the XRPD patterns of Y2-x-yO3:Hox=0.005,Yby=0.05 thin films deposited at different numbers of laser pulses. The patterns of all prepared films are in good agreement with the standard reflection peaks of JCPDS# 71-009925, which indicates that a single-phase cubic structure with an Ia \(\overline{3 }\) space group of the Y2O3 crystal was formed. The films preferred both the (222) and (400) plane orientations, depending on the number of laser pulses. At a low number of laser pulses, the Y2-x-yO3:Hox=0.005,Yby=0.05 films preferred the (222) plane. With increasing the number of laser pulses, the thin films prefer the (400) plane orientation. This behaviour could be attributed to the oxygen gas pressure of 50 mTorr, which can affect the preferential orientation of the films26. Moreover, with increasing the number of laser pulses, some of the defined peaks were observed, which indicates the formation of thicker films and an improvement in the film crystallinity. Structural parameters such as crystallite size (D) and strain (\(\varepsilon )\) were estimated using the Williamson-Hall (W–H) equation27:
where \(\lambda\) is the wavelength of radiation (0.154 nm), k is a constant related to the crystallite shape (taken as 0.9), \(\beta\) is the full width at half-maximum of the diffraction peak, and \(\theta\) is the peak position. The lattice strain and average crystallite sizes were estimated by plotting βcosθ as a function of 4sinθ as shown in Fig. 1b–f. The stress of the prepared films was estimated by Hooke’s law which defines the relation between strain and stress within the elastic region (\(stress=\sigma \times\) \(\varepsilon )\), where the elastic modulus (\(\sigma\)) of Y2O3 thin film is 150 GPa28.
The average crystallite sizes, lattice strain, and stress are listed in Table 1. The average crystallite size varied between 11–18 nm. It is observed that the crystallite size increased with increasing the number of laser pulses. The increase in the crystallite size is ascribed to the increase in material on the films, which in turn improves the crystallinity of the films. However, the strength of the film, cracking, and hardness have been reported as some of the factors that affect the film’s strain29. From Table 1, it can be seen that as the number of laser pulses increased, the strain decreased throughout the lattice, implying that at the lower number of laser pulses, the thin films have a relatively higher lattice mismatch30. Additionally, the reduction in strain with an increasing number of laser pulses also indicates the production of thin films with good crystalline properties and a very low number of lattice defects31. Liu and Kumar et al.32,33 reported that the stress on the thin films can be related to impurities and defects in the crystal (intrinsic stress), the lattice mismatch, growth condition, and mismatch in the thermal expansion coefficient of the thin film, and the substrate (extrinsic stress). As the number of laser pulses increased, the stress gradually decreased, which suggests that increasing the number of laser pulses helps to reduce the tension in the films. It was reported that as the film thickness increases, the lattice disorder decreases because the atoms in the lattice have more time to alter their positions. As a result, the stress tends to be relaxed in thicker thin films34.
Surface and elemental analysis
Surface and cross-sectional SEM images of the Y2-x-yO3:Hox=0.005,Yby=0.05 thin films for different number of laser pulses are displayed in Fig. 2. The surface morphology of the films differs depending on the number of laser pulses. When the number of laser pulses increased, the surface morphology of the films exhibited agglomerated, uniform particles. The particle agglomeration occurred during the PLD deposition process, where bigger particles were created when the produced particles started merging35. Similar results have been reported by Qu et al.26 while studying the highly efficient antireflective downconversion of Y2O3 films grown by PLD. Furthermore, SEM cross-sectional views were used to determine the thicknesses of the films, see Fig. 3. The thicknesses of the films were determined to be 241, 446, 583, 691, 757, and 972 nm for 10,000, 20,000, 30,000, 40,000, 50,000, and 60,000 pulses, respectively. The thickness of the films increased with the number of laser pulses since more material was deposited on the substrate.
The average particle sizes of the prepared films were calculated using the ImageJ software from surface SEM images. Figure 4a–e displays the histograms of the particle size distribution for the films deposited at images 20,000–60,000 laser pulses. The average particle sizes were found to be 70, 79, 91, 97, and 128 nm for 10,000, 20,000, 30,000, 40,000, 50,000, and 60,000 pulses, respectively. As seen, the average particle size increased due to particle agglomeration at high laser pulses.
The elemental composition and maps of the Y2-x-yO3:Hox=0.005,Yby=0.05 films were obtained through the EDS analysis, Fig. 5. All the expected elements (Y, O, Yb, and Ho) in the films were observed. Common impurities such as carbon (C) also appeared in the spectra, which probably could be from the carbon tape used during the measurements. Additionally, the Si, Na, and Ca peaks were detected, which are associated with the chemical composition of the soda-lime glass substrate36. As can be seen, the Si, Na, and Ca peaks decreased at higher laser pulses as expected since more material was being deposited on the surface and thicker films were formed. From the elemental maps (inset), all elements were evenly distributed on the surface of the thin films.
The 3D surface topographic images of the films for different number of laser pulses are presented in Fig. 6. All the AFM images revealed that the films’ surfaces consisted of particles of varying sizes and shapes. The prepared films showed uniformly distributed particles at a higher number of laser pulses. The root mean square (RMS) roughnesses of the thin films grown at laser pulses of 10,000–60,000 were determined to be 3.4, 5.3, 5.7, 6.2, 6.2, and 10.5 nm, respectively. With an increase in laser pulses, the surface roughness of the thin films increased. Peng et al.37 studied the effect of thickness on the structural and optical properties of CuI films, where an increase in surface roughness was observed as the thickness of the films increased. Similar observations were reported by Vankhade et al.38. The authors investigated the role of thickness on the structural and optical properties of PbS films and reported that as the film thickness increases, the average surface roughness gradually increases. The increase in surface roughness in the films would be expected to enhance the UC emission.
To understand the surface chemical composition of the film, XPS analysis was carried out as displayed in Fig. 7. The XPS survey of the Y2-x-yO3:Hox=0.005,Yby=0.05 thin films confirmed the presence of Y, O, Ho, and Yb basic elements in the film. The photoelectron peaks of Y 3d, O 1s, Ho 4d, Yb 4d, and C 1s appear at approximately 157.1, 529.2, 157.1, 192.2, and 284.8 eV, respectively39,40. The Ho 4d peak normally overlaps with Y 3d, whereas the Yb 4d peak falls under the same energy range as the energy loss peak of Y 3d.
The depth profiling analysis of the thin films for an increasing number of laser pulses is displayed in Fig. 8. All the Y and O main elements have been detected alongside C and Si. The C signal disappeared within a few seconds after sputtering was started, which confirmed that the C was due to surface contamination41. From Fig. 8, no segregation of the substrate atoms’ compositions was observed. The soda-lime glass substrates interfaces were reached after 12, 31, 36, 47, 71, and 52 min for the films grown at 10,000, 20,000, 30,000, 40,000, 50,000, and 60,000 laser pulses, which indicates that the films became thicker as the number of laser pulses increased. The observed enhancement in the film thickness is in correlation with the cross-sectional SEM and Swanepoel method results, except for the sample deposited at 50,000 laser pulses, see Table 2. The depth profile for the 50,000 pulses film might have been measured at a position where there were agglomerated particles sitting on the surface of the film, as shown in Fig. 3.
High-resolution XPS spectra for Y 3d and O 1s peaks are displayed in Fig. 9. In the fitting procedures, the C 1s XPS peak at 284.8 eV was used as a reference. Figure 9a Y 3d spectra fitted into four peaks at 156.4, 157.8, 158.5, and 159.9 eV, which are associated with Y 3d5/2 and Y 3d3/2 for two different sites (C2 and S6) of Y3+ ion in the Y2O3 material. The 156.4 and 158.5 eV peaks were the spin–orbit splitting of Y 3d5/2 and Y 3d3/2 in the C2 site, while 157.8 and 159.9 eV peaks were the spin–orbit splitting in the S6 site of cubic Y2O3 crystal, respectively40,42. The binding energy separation between the Y 3d5/2 and Y 3d3/2 peaks was 2.1 eV and the integrated area ratio was 0.7 due to spin-orbital splitting. The Y 3d5/2 and Y 3d3/2 peak values correlated well with previously reported values in the Y2O3 structure43. The Ho 4d peak overlaps with Y 3d and because the Ho3+ concentration in this study was 0.5 mol% there was a low probability of detecting the Ho 4d peak. The high-resolution XPS for the O 1s peak was deconvoluted into three peaks as shown in Fig. 9b. The O 1s peak located at 529.1 eV corresponded to the lattice O in the Y2O3 host. The peak situated at 531 eV is associated with the O defects inside the material. The binding energy peak positioned at 532.1 eV is ascribed to the O–C=O bond, which might be due to the residual carbon from the fuel (CH4N2O) used while preparing the target or due to exposure to air44,45. Jafer and Shivaramu et al.43,44 fitted two peaks of the lattice O corresponding to two different sites in the Y2O3 host. However, the cubic crystal structure of Y2O3 (Fig. 9d), shows that all the O are in an identical environment (situated at 48e Wyckoff positions). Each of the O is bonded to 4Y ions (3Y ions at the C2 site and 1Y ion at the S6 site). The O-Y bond lengths in the C2 site are 2.23, 2.24, and 2.27 Å. In the S6 site, all the O-Y bond lengths are 2.28 Å46. Therefore, the binding energy of the O 1s electron of all these O in the lattice should be the same, thus, a single peak related to the O lattice should be fitted. Moreover, the Yb 4d5/2 peak located at 184.7 eV is displayed in Fig. 9c, correlating well with the reported results47.
High-resolution XPS core-level spectra for the (a) Y 3d, (b) O 1s, (c) Yb 4d in the Y2-x-yO3:Hox=0.005,Yby=0.05 films at 60,000 laser pulses, and (d) Y2O3 crystal structure showing O coordination in equivalent positions (Y ions are not shown, but their positions can be inferred from the green and grey bonds).
Optical analysis
The optical transmittance spectra of the Y2-x-yO3:Hox=0.005,Yby=0.05 films are displayed in Fig. 10, in the 300–1500 nm spectral range. The prepared films exhibited interference fringes which are caused by reflections of the light at the front and back of the film. The interference fringe information implies that the deposited films are uniform and homogeneous48,49. It can be seen that the transmittance of the films decreased as the laser pulses increased due to the film thickness effect50.
Swanepoel method51 was used to estimate the thicknesses of the thin films from the transmittance data. This method relies on the oscillating peak's maximum and minimum transmissions (TM, Tm) as shown in Fig. 11a,b. Transmission spectra have two regions as per absorption in the films in general; region of strong absorption, and weak and medium absorption. According to Swanepoel’s method, the refractive index (n1) value of the thin films in the medium and weak absorption is given by52:
where
And S is the refractive index of the substrate.
Taking the basic equation of interference fringes into account:
where n is the refractive index, d is the thickness of the thin film, m is the interference order (half-integer for minima and integer for maxima) and λ is the wavelength. The thickness of the film \(d\) can be estimated from the refractive indices (n1, n2) at two adjacent maxima or minima at λ1 and λ2 with λ1 > λ2 through51:
The thickness values of the thin films estimated by the Swanepoel method were found to be 200, 267, 442, 682, 952, and 1218 nm for 10,000, 20,000, 30,000, 40,000, 50,000, and 60,000 pulses, respectively, see Table 2. It can be seen that the films became thicker as the laser pulses increased. The discrepancy observed from the Swanepoel method arose due to the interference fringes that were taken into account. Swanepoel’s approach does not always result in a good accuracy of the films’ thickness when few fringes are considered53, but clearly showed the same trend of increasing thickness with an increase in the number of pulses. The difference in thickness, if SEM and XPS are compared, might be due to the uneven features on the surface that can influence the thickness, especially with the XPS depth profiles. Figure 12 shows the comparison of the thicknesses as determined by SEM, XPS, and Swanepoels method. The Swanepoel method can give a rough indication of the thickness without any destructive measurements of the thin films with an underestimation at thinner films and an overestimation at thicker films.
UC analysis
The UC emission spectra of the thin films at different laser pulses under 980 nm excitation are presented in Fig. 13a. The UC emission exhibited intense green emission bands assigned to the 5F4 → 5I8 and 5S2 → 5I8 transitions at 538 and 550 nm as well as weaker red and infrared emissions at 666 and 756 nm corresponding to the 5F5 → 5I8 and 5S2 → 5I7 transitions of the Ho3+ ions, respectively24. It is observed that the UC emission intensity depends on the number of laser pulses. From Fig. 13b, it can be seen that the UC emission intensity of all emission bands increased with an increase in the number of laser pulses. The enhancement in UC emission performance is related to the reduction of internal reflections due to the surface roughness54. Jafer et al.55 observed such luminescence improvement in the Y2O3:Bi3+ films due to the crystallinity and the surface roughness of the prepared films. Therefore, the UC emission intensity enhancement with an increasing number of laser pulses can also be due to the crystallinity improvement of the thin films (Fig. 1a).
The luminescence decay curves of the 5S2 → 5I8 (550 nm) and 5F5 → 5I8 (666 nm) transitions of the Ho3+ ion under 980 nm excitation are displayed in Fig. 14a,b.
All the luminescence decay curves were fitted with a triple exponential function56:
where \(I\) is the intensity at time \(t\), \(A, B,\) and \(C\) are the fitting constants, and \({\tau }_{1}, {\tau }_{2},\) and \({\tau }_{3}\) are the lifetime constants. The average lifetime (\({\tau }_{ave})\) were determined using \({\tau }_{ave}=(A{{\tau }_{1}}^{2}+B{{\tau }_{2}}^{2}+C{{\tau }_{3}}^{2})/(A{\tau }_{1} +B{\tau }_{2}+C{\tau }_{3})\). The multiexponential decay curves' behavior has been reported to commonly arise from doping ions occupation in the host lattice, ion-ion interactions between the donor and the activator, and the probability of non-radiative transition between ion–ion pairs57,58,59. It can be seen that the laser pulses slightly influenced the luminescence lifetime of the films, Table 3. The lifetimes of the 5S2 → 5I8 (550 nm) and 5F5 → 5I8 (666 nm) levels decreased as the number of laser pulses increased.
The UC emission mechanism was further studied at various excitation laser powers to determine the excited number of photons needed for each emitted photon. Figure 15a presents the UC emission spectra of Y2-x-yO3:Hox=0.005,Yby=0.05 film at 60,000 pulses under different pumping power of 980 nm laser. The UC emission spectra display four emission bands, a strong green (~ 538 nm), dominant green (~ 550 nm), weak red (~ 666 nm), and weak infrared (~ 756 nm) assigned to 5F4 → 5I8, 5S2 → 5I8, 5F5 → 5I8, and 5S2 → 5I7 transitions of the Ho3+ ions, respectively60. From Fig. 15b, the increase in excitation power significantly enhanced the UC emission intensity of each emission band. This UC emission enhancement is due to the increase of populations in the excited states of the dopant ions23. The number of excitation photons needed for each UC emission band can be determined from the relation \(I \alpha {P}^{n}\)61, where \(I\) is the emission intensity, \(P\) is the pumping power, and \(n\) is the number of photons. In Fig. 15c, the \(In \left(power\right)\)–\(In (intensity)\) plot has \(n\) as the slope of the straight lines fit, which was found to be 1.83, 1.84, 1.54, and 1.59 for the 538, 550, 666, and 756 nm UC emission peaks. These \(n\) values are close to 2 suggesting that a two-photon UC process is responsible for the observed 5F4 → 5I8, 5S2 → 5I8, 5F5 → 5I8, and 5S2 → 5I7 emissions. Figure 15d shows the Commission International de I’Eclairage (CIE) chromaticity diagram of the film under 980 nm laser excitation at 845 mW power. The calculated CIE coordinates (0.30, 0.71), estimated using UC emission spectra (Fig. 15a), shows that the UC emission has a pure green colour. The UC luminescence of Y2-x-yO3:Hox=0.005,Yby=0.05 occurred following the UC mechanism shown in Fig. 16. In this process, the Yb3+ ion is excited from the 2F7/2 ground state via the 980 nm excitation to the 2F5/2 excited state. Due to the close energy match between 2F5/2 (Yb3+) and 5I6 (Ho3+), the excited Yb3+ ion can easily transfer the absorbed energy to the 5I6 excited level of Ho3+ ion via non-radiative resonance energy transfer. Energy transfer upconversion (ETU) further excites the electron in the 5I6 level to the 5F4, 5S2 levels of the Ho3+, which results in intense green emission through 5F4 → 5I8 and 5S2 → 5I8 transitions at 538 and 550 nm, and weaker infrared emission through the 5S2 → 5I7 transitions at 756 nm. Furthermore, weak red emission at 667 nm through 5F5 → 5I8 transitions was observed after the 5F5 level may have been populated by the non-radiative multiphonon relaxation process from the 5F4, 5S2 levels62.
Conclusions
High-quality Y2-x-yO3:Hox=0.005,Yby=0.05 thin films have been deposited on soda-lime glass substrates at various numbers of laser pulses using the PLD technique. It was discovered that the number of laser pulses in the films has a significant impact on crystallinity, surface properties, and UC luminescence. The XRPD results showed the films were formed as a single-phase cubic structure of Y2O3 with different preferred plane orientations under various laser pulses. High-resolution XPS confirmed the two sites of Y2O3. The thin film thicknesses were determined by cross-sectional SEM measurements, depth profile XPS, and the Swanepoel method. The cross-sectional SEM thicknesses were determined to be 241, 446, 583, 691, 757, and 972 nm for 10,000, 20,000, 30,000, 40,000, 50,000, and 60,000 pulses, respectively. The depth profile XPS thicknesses were determined to be 180, 465, 540, 705, 1065, and 780 nm for 10,000, 20,000, 30,000, 40,000, 50,000, and 60,000 pulses, respectively. The Swanepoel method thicknesses were determined to be 267, 442, 682, 952, and 1218 nm for 20,000, 30,000, 40,000, 50,000, and 60,000 pulses, respectively.
The thicknesses of the films determined by cross-sectional SEM, depth profile XPS, and the Swanepoel methods indicated that the films became thicker as the laser pulses increased. Power dependence and lifetime measurements confirmed the involvement of the two-photon UC process. The UC luminescence showed a dominant green emission under 980 nm excitation, making the Y2-x-yO3:Hox=0.005,Yby=0.05 UC transparent thin films phosphor a good candidate for solar cell applications.
Data availability
Data is not publicly available, but data will be made available on request.
References
Hamadani, B. H. Understanding photovoltaic energy losses under indoor lighting conditions. Appl. Phys. Lett. 117, 043904 (2020).
Sark, W., Wild, J., Rath, J. K., Meijerink, A. & Schropp, R. E. Upconversion in solar cells. Nanoscale Res. Lett. 8, 81 (2013).
Yu, X. et al. Near infrared to visible upconversion emission in Er3+/ Yb3+ co-doped NaGd(WO4)2 nanoparticles obtained by hydrothermal method. J. Lumin. 154, 111 (2014).
Hou, X., Zhou, S., Jia, T., Lin, H. & Teng, H. Investigation of up-conversion luminescence properties of RE/Yb co-doped Y2O3 transparent ceramic (RE = Er, Ho, Pr, and Tm). Physica B 406, 3931 (2011).
Alonso, A. S., Ramos, J. M., Yanes, A. C., del Catillo, J. & Rodriguez, V. D. Up-conversion in sol–gel derived nano-glass–ceramics comprising NaYF4 nano-crystals doped with Yb3+, Ho3+, and Tm3+. Opt. Mater. 32, 903 (2010).
Zhang, L., Yang, J., Yu, H. & Pan, W. Highly efficient up-conversion in Nd:Y2O3 transparent ceramics. Scr. Mater. 178, 281 (2020).
Newport, A. et al. Up-conversion emission phosphors based on doped silica glass ceramics prepared by sol–gel methods: Control of silica glass ceramics containing anatase and rutile crystallites. J. Mater. Chem. 11, 1447 (2001).
Sun, Y., Liu, H., Wang, X., Kong, X. & Zhang, H. Optical spectroscopy and visible upconversion studies of YVO4:Er3+ nanocrystals synthesized by a hydrothermal process. Chem. Mater. 18, 2726 (2006).
Ledemi, Y. et al. Infrared to visible up-conversion emission in Er3+/Yb3+ codoped fluoro-phosphate glass ceramics. J. Am. Ceram. Soc 96, 825 (2013).
Lahoz, F. Ho3+– doped nanophase glass ceramics for efficiency enhancement in silicon solar cells. Opt. Lett. 33, 2982 (2008).
Lahoz, F. et al. Upconversion mechanisms in rare-earth doped glasses to improve the efficiency of silicon solar cells. Sol. Energy Mater. Sol. Cells 95, 1671 (2011).
Guo, M. et al. Study on water absorption characteristics of cubic Y2O3 films. Opt. Mater. 109, 110304 (2020).
Karle, S. et al. Metal-organic CVD of Y2O3 thin films using Yttrium tris-amidinates. Chem. Vap. Deposit. 21, 335 (2015).
Ramana, C. V. et al. Enhanced optical constants of nanocrystalline yttrium oxide thin films. Appl. Phys. Lett. 98, 031905 (2011).
Sangeetha, N. M. & van Veggel, F. C. J. M. Lanthanum silicate and lanthanum zirconate nanoparticles co-doped with Ho3+ and Yb3+: Matrix-dependent red and green upconversion emissions. J. Phys. Chem. C. 113, 14702 (2009).
Tadge, P. & Ray, S. Upconversion and quantum cutting emission in Y2O3 co-doped Ho3+ and Yb3+ oxide phosphor synthesized by solution route: A comparative study. Int. J. Recent Trends Sci. Technol. 2018, 199–202 (2018).
Qiao, Y. & Guo, H. Upconversion properties of Y2O3:Er films prepared by sol-gel method. J. Rare Earth 27, 406 (2009).
Dikovska, A. O. et al. Structural and optical properties of Er, Yb co-doped Y2O3 thin films. Appl. Surf. Sci. 252, 4569 (2006).
Lian, J. et al. Deposition of ultrathin rare-earth doped Y2O3 phosphor films on alumina nanoparticles. Nanotechnology 17, 1351 (2006).
Castro, Y. et al. Preparation, structural and optical characterization of rare earth doped mesoporous Y2O3 thin films by EISA method. Micropor. Mesopor. Mater. 103, 273 (2007).
Hrudey, P. C. et al. Evaporated nanostructured Y2O3:Eu thin films. J. Nanosci. Nanotechnol. 5, 229 (2005).
Cho, K. G., Kumar, D., Holloway, P. H. & Singh, R. K. Luminescence behavior of pulsed laser deposited Eu:Y2O3 thin film phosphors on sapphire substrates. Appl. Phys. Lett. 73, 3058 (1998).
Pandey, A., Kumar, V., Kroon, R. E. & Swart, H. C. Temperature induced upconversion behaviour of Ho3+–Yb3+ codoped yttrium oxide films prepared by pulsed laser deposition. J. Alloys Compd. 672, 190 (2016).
Makumbane, V., Yagoub, M. Y. A., Xia, Z., Kroon, R. E. & Swart, H. C. Up-conversion luminescence and optical temperature sensing behaviour of Y2O3:Ho3+, Yb3+ phosphors. Crystals 13, 1288 (2023).
Khan, S., Park, B. I., Han, J. S., Lee, S. Y. & Cho, S. H. Flame synthesized Y2O3:Tb3+–Yb3+ phosphors as spectral convertors for solar cells. Res. Chem. Intermed. 44, 4619 (2008).
Qu, M., Wang, R., Zhang, Y., Li, K. & Yan, H. High efficient antireflective down-conversion Y2O3:Bi, Yb films with pyramid preferred oriented nano-structure. J. Appl. Phys. 111, 093108 (2012).
Tabaza, W. A. I., Swart, H. C. & Kroon, R. E. Optical properties of Bi and energy transfer from Bi to Tb in MgAl2O4 phosphor. J. Lumin. 148, 192 (2014).
Ivaniĉ, R. et al. Properties of Y2O3 thin films applicable in micro-electrochemical cells. Electr. Eng. 54, 83 (2003).
Kumar, V. et al. The role of growth atmosphere on the structural and optical quality of defect-free ZnO films for strong ultraviolet emission. Laser Phys. 24, 105704 (2014).
Hasabeldaim, E., Ntwaeaborwa, O. M., Kroon, R. E., Coetsee, E. & Swart, H. C. Effect of substrate temperature and post annealing temperature on ZnO:Zn PLD thin film properties. Opt. Mater. 74, 139 (2017).
Sayeed, M. A., Rouf, H. K. & Hassain, K. M. A. Effect of thickness on characteristics of ZnSe thin film synthesized by vacuum thermal evaporation. J. Theor. Appl. Phys. 14, 251 (2020).
Liu, M., Man, B. Y., Lin, X. C. & Li, X. Y. The effect of substrate material on pulsed laser deposition of HgCdTe films. Appl. Surf. Sci. 255, 4848 (2009).
Kumar, V. et al. Role of film thickness on the properties of ZnO thin films grown by sol-gel method. Thin Solid Films 539, 161 (2013).
Thornton, J. A. & Hoffman, D. W. Stress-related effects in thin films. Thin Solid Films 171, 5 (1989).
Murr, L. E. Effects of substrate temperature, pressure, and high evaporation rates on nucleation, epitaxy, and structure of palladium thin films. Thin Solid Films 7, 101 (1971).
Karazi, S. M., Ahad, I. M. & Benyounis, K. Y. Laser Micromachining for Transparent Materials, Reference Module in Materials Science and Materials Engineering (Elsevier, 2017).
Peng, W., Li, L., Yu, S., Zheng, H. & Yang, P. Structure, binding energy and optoelectrical properties of p-type CuI thin films: The effects of thickness. Appl. Surf. Phys. 502, 144424 (2020).
Vankhade, D. & Chaudhuri, T. K. Effect of thickness on structural and optical properties of spin-coated nanocrystalline PbS thin films. Opt. Mater. 98, 109491 (2019).
Song, L. et al. Synthesis and up-conversion properties of Ho3+-Yb3+-F- tri-doped TiO2 nanoparticles and their application in dye-sensitized solar cells. Mater. Res. Bull. 88, 1 (2017).
Shivaramu, N. J., Lakshminarasappa, B. N., Coetsee, E., Kroon, R. E. & Swart, H. C. Structural, thermoluminescence and optical properties of Nd3+ doped Y2O3 nanophosphor for dosimeter and optoelectronics applications. Mater. Res. Bull. 161, 112153 (2023).
Yagoub, M. Y. A., Swart, H. C. & Coetsee, E. Structural, surface and luminescent properties of SrF2:Eu annealed thin films. Vacuum 191, 110362 (2021).
Jones, S. C., Kumar, D. & Singh, R. K. Luminescence of pulsed laser deposited Eu doped yttrium oxide films. Appl. Phys. Lett. 71, 404 (1997).
Jafer, R. M. et al. X-ray photoelectron spectroscopy and luminescent properties of Y2O3:Bi3+ phosphor. Appl. Surf. Sci. 332, 198 (2015).
Shivaramu, N. J., Coetsee, E. & Swart, H. C. Cathodoluminescence degradation of Y2O3:Dy3+ nanophosphor for field emission displays. J. Vac. Sci. Technol. A 37, 061405 (2019).
Moulder, J. F., Stickle, W. F., Sobol, P. W. & Bomben, K. D. Handbook of X-ray Photoelectron Spectroscopy (Perkin-Elmer, 1992).
Putz, H. & Brandenburg, K. Diamond-crystal and molecular structure visualization.
Saeed, N. A. M. Luminescent Properties of YOF Phosphor for Solar Cell Application (University of the Free State, 2019).
Penzkofer, A., Holzer, W., Tillmann, H. & Horhold, H. H. Leaky-mode emission of luminescent thin films on transparent substrates. Opt. Commun. 229, 279 (2004).
Tirca, I., Boerasu, I., Radu, M. S. & Osiac, M. Refractive index of WO3 thin films grown under various temperatures determined by the Swanepoel method. Physics B 620, 413266 (2021).
Xiaotao, H., Jin, M. & Deheng, Z. Thickness dependence of structural, optical and electrical properties of ZnO:Al films prepared on flexible substrates. Appl. Surf. Sci. 183, 137 (2001).
Swanepoel, R. Determination of surface roughness and optical constants of inhomogeneous amorphous silicon films. J. Phys. E Sci. Instrum. 17, 896 (1984).
Shaaban, E. R., Kansal, I., Mohamed, S. H. & Ferreira, J. M. F. Microstructural parameters and optical constants of ZnTe thin films with various thicknesses. Physica B 404, 3571 (2009).
Tirca, I., Boerasu, I., Radu, M. S. & Osiac, M. Refractive index of WO3 thin films grown under various temperatures determined by the Swanepoel method. Phys. B 620, 413266 (2021).
Bae, J. S. et al. Photoluminescence characteristics of pulsed laser-ablated Y2-xGdxO3/Eu3+ thin film phosphors grown on Si (100) substrate. Thin Solid Films Lett. 476, 35 (2005).
Jafer, R. M., Swart, H. C., Yousif, A. & Coetsee, E. The effect of different substrate temperatures on the structure and luminescence properties of Y2O3:Bi3+ thin films. Solid State Sci. 53, 30 (2016).
Ogugua, S. N., Ntwaeaborwa, O. M. & Swart, H. C. Luminescence, structure and insight on the inversion degree from normal to inverse spinel in a ZnAl(2–x)Fex3+O4 system. Boletín de la Soc Iedad Española de Cerámica y Vidrio 60, 147 (2021).
Chen, Q. et al. Influence of the Tb3+ concentration on the luminescent properties of high silica glass. Opt. Mater. 86, 606 (2018).
Ding, J. et al. Influence of Tb3+ concentration on the optical properties and Verdet constant of magneto-optic ABS-PZZ glass. Opt. Mater. 69, 202 (2017).
Stouwdam, J. W. & van Veggel, F. C. J. M. Near-infrared emission of redispersible Er3+, Nd3+, and Ho3+Doped LaF3 nanoparticles. Nano Lett. 2, 733 (2002).
Dwivedi, Y., Bahadur, A. & Rai, S. B. Optical avalanche in Ho:Yb:Gd2O3 nanocrystals. J. Appl. Phys. 110, 043103 (2011).
Li, J. G., Wang, X. J. & Liu, W. G. (La0.97RE0.01Yb0.02)2O2S nanophosphors converted from layered hydroxyl sulfate and investigation of upconversion photoluminescence (RE=Ho, Er). Nanoscale Res. Lett. 12, 508 (2017).
An, L., Zhang, J., Liu, M. & Wang, S. Up-conversion properties of Yb3+, Ho3+:Lu2O3 sintered ceramic. J. Lumin. 122, 125 (2007).
Acknowledgements
This work is based on research supported by the National Research Foundation of South Africa and the Department of Science and Technology's South African Research Chairs Initiative (grand 84415). This acknowledges the financial support for this research provided by the National Research Foundation (NRF), CSIR-HCD IBS, and the University of the Free State.
Author information
Authors and Affiliations
Contributions
V.M.: Methodology, investigation, formal analysis, and writing original draft, R.E.K.: Co-supervision, review and editing, M.Y.A.Y.: Support, review and editing, L.J.B.E.: Swanepoel calculations, E.C.: XPS measurements, review and editing, and H.C.S.: Supervision, conceptualization, funding acquisition, resources, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makumbane, V., Kroon, R.E., Yagoub, M.Y.A. et al. The role of thickness on the structural and luminescence properties of Y2O3:Ho3+, Yb3+ upconversion films. Sci Rep 14, 17758 (2024). https://doi.org/10.1038/s41598-024-68367-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68367-x
- Springer Nature Limited