Abstract
The platelet/high-density lipoprotein ratio (PHR) has been identified as a significant indicator of inflammation and a hypercoagulable state, demonstrating a strong link with the severity of nonalcoholic fatty liver disease (NAFLD) and metabolic syndrome (MetS). However, its correlation with hyperuricemia has not yet been documented. This study utilized a cross-sectional design, analyzing data collected from the National Health and Nutrition Examination Survey (NHANES) between 2007 and 2016 in the United States. The platelet/high-density lipoprotein ratio (PHR) was determined by dividing the number of platelets (PLT) by the level of high-density lipoprotein cholesterol (HDL-C). We employed multivariable logistic regression analyses, generalized additive models, and subgroup analyses to investigate the correlation between PHR and hyperuricemia. The study revealed a hyperuricemia prevalence of 18.56%. Analysis indicated a significant positive correlation between PHR and the risk of hyperuricemia (OR 1.11, 95% CI 1.08, 1.14). This correlation remained consistent across different subgroups including age, ethnicity, gender, and body mass index (BMI). Smooth curve fitting demonstrated a saturation effect between PHR and the risk of hyperuricemia. PHR is positively correlated with hyperuricemia and may serve as a novel biomarker for predicting the onset of this condition. Additionally, targeted interventions to improve PHR might help reduce the incidence of hyperuricemia.
Similar content being viewed by others
Introduction
Serum uric acid (SUA) represents the final product of purine breakdown in humans. Initially, purine is metabolized to hypoxanthine, which is then oxidized by xanthine dehydrogenase (XDH) to xanthine before further oxidation to uric acid (UA)1. Elevated levels of SUA can induce endothelial dysfunction, affecting vascular tone, thrombosis, inflammation, and oxidative stress2. High SUA levels can trigger gout and play a significant role in the development of cardiovascular diseases, including hypertension, atrial fibrillation, heart failure, and coronary artery disease. They are also closely associated with diabetes and metabolic syndrome (MetS)3,4,5,6,7,8. Despite a lack of extensive clinical study evidence, UA-lowering treatments have shown clinical significance in managing chronic kidney disease, blood sugar control, and cardiovascular outcomes9,10,11. In the United States, during 2015–2016, 47.1 million adults met the criteria for hyperuricemia, with a prevalence rate of 20.1%12. This condition and its associated comorbidities significantly increase the public health burden, highlighting the importance of early prediction, identification, and treatment of hyperuricemia.
Hyperuricemia is closely associated with coagulation abnormalities. SUA, through reactive oxygen species (ROS)-mediated oxidative stress, induces an inflammatory state and vascular damage, triggering thrombosis13. Platelets (PLT) serve a crucial function in the coagulation process, and several studies have demonstrated an independent correlation between PLT counts and the width of the PLT distribution representing the activation of PLT and with SUA14,15,16,17. The lipid profile associated with hyperuricemia typically features reduced concentrations of high-density lipoprotein cholesterol (HDL-C) and elevated levels of triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C), where HDL-C is regarded as a protective factor against elevated SUA levels18. Given these factors, a close relationship between the platelet/HDL-C ratio (PHR) and hyperuricemia is hypothesized. As a marker of inflammation and a hypercoagulable state, PHR has been found to be closely related to the severity of nonalcoholic fatty liver disease (NAFLD) and MetS, yet its correlation with hyperuricemia remains unexplored19,20,21,22.
Thus, this research employs data from the National Health and Nutrition Examination Survey (NHANES) to explore the connection between PHR and hyperuricemia.
Methods
Study population
The NHANES is a project aimed at evaluating the health and nutritional condition of the American population. NHANES combines interviews with physical examinations to collect data on health, socioeconomic status, and diet, among other areas. This data helps identify disease prevalence, risk factors, and nutritional status, providing crucial information for public health policies and health measurement standards such as height and weight.
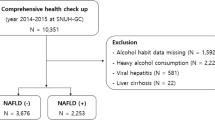
This study analyzed data from the 2007–2016 cycle with 50,588 participants. After excluding participants under the age of 20, those using uric acid-lowering medications, or lacking data on HDL-C, PLT, or UA, a total of 26,202 subjects remained eligible for analysis. Figure 1 outlines the criteria for inclusion and exclusion used in the study.
Study variables
The exposure variable, PHR, is calculated as the ratio of PLT (1000 cells/uL) to HDL-C(mg/dL). Trained researchers collect blood samples from each participant to perform a complete blood cell count using VCS (Volume, Conductivity, and Scatter) technology. From 2007 to 2012, the hematology analyzer used was the Beckman Coulter MAXM; however, from 2013 to 2016, the Beckman Coulter DXH 800 was employed for this purpose. Serum samples were stored at minus 30 degrees Celsius for the direct measurement of HDL-C using immunoassay methods. During the 2007–2012 cycle, HDL-C testing was conducted with the Roche Modular P Chemistry Analyzer, while in the 2013–2016 cycle, both the Roche Modular P and the Roche Cobas 6000 chemistry analyzers were utilized.
Hyperuricemia, the outcome variable, is defined as a SUA level exceeding 420 μmol/L in adult males and 360 μmol/L in adult females.
Traditional risk factors for hyperuricemia were considered as potential confounders and included in the final multivariate logistic regression model. Covariates encompassed demographic, health, and biochemical indicators: gender, age, race, education level, Body Mass Index (BMI), hypertension, diabetes, hyperlipidemia presence, alcohol consumption, smoking status, and levels of creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), Hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR), LDL-C, and hemoglobin (HGB). Hypertension, diabetes and hyperlipidemia were self-reported through questionnaires. Smoking was characterized by the consumption of more than 100 cigarettes in a person's lifetime, while alcohol intake was defined as consuming over 12 alcoholic beverages annually. The eGFR was calculated utilizing the CKD-EPI formula23.
Statistical analysis
Following the guidelines of the Centers for Disease Control and Prevention, sample weights were incorporated to address the complex probability sampling of NHANES, ensuring adequate national representation, particularly in terms of oversampling in certain survey cycles.
Continuous variables are presented as mean ± standard deviation (SD), while categorical variables are depicted as numbers (percentages). To evaluate differences among participants divided by PHR tertiles, a weighted linear regression model and chi-square tests were employed. To explore the link between PHR and hyperuricemia, multivariate logistic regression analyses were conducted across three distinct models: Model 1, which did not include any adjustments; Model 2, which adjusted for factors such as gender, age, and race; and Model 3, which incorporated adjustments for all the covariates mentioned earlier. A sensitivity analysis was also carried out, in which PHR was treated as a categorical variable, and the median value of each category was used as a continuous variable to evaluate linear trends. Subgroup analyses were conducted to assess the robustness of the outcomes and examine interactions, ensuring a thorough investigation of the data. Potential nonlinear relationships were investigated using generalized additive models and smoothing curves, with two-phase linear regression applied to pinpoint significant trend changes at inflection points. The threshold for statistical significance was established at P < 0.05. All analyses and visualizations were conducted using R version 4.2.0 and Empower Stats version 4.0.
Ethics statement
The research involving human participants underwent a thorough review and received approval from the Research Ethics Review Board of the NCHS. All patients or participants gave their written informed consent to be part of this study.
Results
Subject characteristics
Table 1 displays that a total of 26,202 participants were included in the study. The mean age of the cohort was 47.36 ± 16.85 years, with 48.21% males and 51.79% females. The ethnic composition was as follows: 8.64% Mexican American, 5.71% Other Hispanic, 67.31% Non-Hispanic White, 10.69% Non-Hispanic Black, and 7.65% Other races. The average PHR level was 4.99 ± 2.07. There were 4,861cases identified with hyperuricemia, representing 18.56% of the total population. Individuals with higher PHR levels tended to be younger males, more likely to smoke, less likely to drink alcohol, and had lower levels of education. They also had a higher likelihood of being obese, diabetic, and suffering from hyperlipidemia and hypertension. Additionally, these individuals exhibited lower AST levels, and higher levels of HbA1c, LDL-C, SUA, GFR and ALT.
Association between PHR and risk of hyperuricemia
Table 2 presents the results of multivariable logistic regression analyses. In the unadjusted model, PHR was positively correlated with the risk of hyperuricemia (OR 1.12, 95% CI 1.10, 1.13). This positive correlation persisted in both the minimally adjusted model (OR 1.16, 95% CI 1.14, 1.18) and the fully adjusted model (OR 1.11, 95% CI 1.08, 1.14), even after adjusting for confounders. When PHR was divided into tertiles, compared to the reference group, the odds ratios for hyperuricemia for participants in the second and third tertiles were 1.29 (95% CI 1.12, 1.49) and 1.78 (95% CI 1.54, 2.06), respectively, with a significant trend observed across the tertiles of PHR (trend P < 0.05).
Subgroup analyses demonstrated a consistent positive correlation between PHR and the risk of hyperuricemia across stratified participant groups based on gender, age, ethnicity, BMI categories (< 25 kg/m2, 25–30 kg/m2, ≥ 30 kg/m2), smoking status, alcohol consumption, hypertension, diabetes, and hyperlipidemia (Fig. 2).
Subgroup analysis of the correlation between PHR and the risk of hyperuricemia. In addition to stratification variables, each subgroup analysis was adjusted for sex, age, race, education, BMI, hypertension, diabetes mellitus, hyperlipidemia, smoking status, drinking status, Cr, eGFR, AST, ALT, HbA1c, LDL-C.
Figure 3 illustrates the nonlinear relationship between PHR and the prevalence of hyperuricemia via a smoothed curve fit using a generalized additive model. An inflection point was identified at 8.18 using a two-stage linear regression model (Table 3). Below this inflection point, a lower PHR corresponds to a reduced risk of hyperuricemia.
Discussion
This study is the first to assess the relationship between PHR and hyperuricemia. Results show a positive correlation between PHR and the risk of hyperuricemia, which remains significant even after adjusting for a variety of confounding factors such as ethnicity, age, educational level, and BMI. Subgroup analysis and interaction tests indicate that this positive correlation is stable across different demographic environments. Smooth curve fitting reveals a saturation effect between PHR and the risk of hyperuricemia, suggesting that an appropriate range of PHR (less than 8.18) may be beneficial for assessing the risk of hyperuricemia.
A previous study investigated the relationship between the PHR and MetS, revealing that PHR correlates with all five characteristics of MetS: abdominal obesity, hypertriglyceridemia, low HDL-C, hyperglycemia, and hypertension. Moreover, the level of PHR increases with the severity of MetS19. There is a significant bidirectional association between hyperuricemia and MetS. A four-year longitudinal study demonstrated that hyperuricemia is a significant and independent predictor of MetS in subjects, with the risk of MetS escalating alongside the increase in SUA levels24. On the other hand, MetS and its five components are significantly associated with the incidence of new-onset hyperuricemia, with the incidence rate rising with a rise in the count of MetS components25. This mechanism might be due to microvascular damage associated with the elevated blood pressure in MetS enhancing UA production, while the hyperinsulinemia related to MetS increases the reabsorption of UA by the proximal renal tubules26. Since MetS and hyperuricemia share common features like inflammation, insulin resistance, and a hypercoagulable state, it is therefore logical to propose that PHR is closely related to hyperuricemia.
The relationship between PHR and hyperuricemia involves complex pathophysiologic mechanisms that are not yet fully understood. First, there is a negative correlation between HDL-C levels and renal function27; lower HDL-C levels indicate a decrease in eGFR, which may lead to decreased UA excretion.
Second, there is a noteworthy inverse relationship between HDL-C concentration and insulin resistance, which affects SUA levels. Insulin resistance is known to affect UA metabolism, suggesting that changes in HDL-C levels may indirectly affect SUA levels through changes in insulin sensitivity28.
In addition, as a combination of PLT and HDL-C, PHR may represent inflammatory and prothrombotic states, both of which are strongly associated with hyperuricemia20.
Inflammation significantly influences the onset of hyperuricemia. Research findings have revealed that factors such as the dietary inflammatory index, C-reactive protein (CRP) levels, the Systemic Inflammatory Response Index (SIRI), and the ratio of monocytes to HDL-C (MHR) all have a direct positive relationship with the condition of hyperuricemia29,30,31,32. During the inflammatory response, platelets engage with neutrophils and lymphocytes, prompting the adhesion and movement of monocytes, which leads to the release of a range of inflammatory mediators. These inflammatory cytokines could potentially enhance the activity of xanthine oxidase through the upregulation of its gene expression, resulting in an increase in UA production33. HDL-C has been confirmed as a protective factor against the elevation of serum uric acid in multiple studies, primarily due to its anti-inflammatory effects18,34,35. HDL inhibits endothelial inflammation and reduces the expression of key cellular adhesion molecules36. Apolipoprotein A-1, a major component of HDL-C, exerts anti-inflammatory effects by decreasing the activation of CD11b on monocytes37. UA, in turn, induces inflammation, which is the main driving mechanism for the various complications of hyperuricemia38. Even at physiologic concentrations, SUA promotes inflammatory responses and impairs vascular endothelial cell proliferation/migration and nitric oxide (NO) release39. Several in vitro experiments confirm this. In rat vascular endothelial cells studied in vitro, UA triggers inflammatory pathways, leading to an increased expression of COX-2 and the chemokine monocyte chemotactic protein-1 (MCP-1)40. In human liver cancer HepG2 cells, it was discovered that UA enhances the expression of CRP in a dose-dependent manner.41. In addition, because of the free radical-scavenging and antioxidant properties of serum urate, elevated levels of UA may also be a compensatory mechanism designed to counteract the oxidative damage and inflammatory responses associated with atherosclerosis and aging42. UA-induced inflammation triggers activation of the coagulation system. Multiple research efforts have documented a connection between SUA levels and the incidence of thrombotic events. One of the first studies to report an association between SUA levels and venous thromboembolism (VTE) was the Atherosclerosis Risk in Communities (ARIC) study, which analyzed 14,126 participants, including 632 cases of VTE events, and found that SUA levels were positively associated with these cases after adjusting for multiple confounders14. Other studies have also found that elevated SUA levels are associated with VTE severity, thrombus recurrence, and left atrial thrombosis13,39,43,44. Inflammation induces activation of coagulation by damaging the vascular endothelium and upregulating the expression of tissue factor on the surface of circulating monocytes and neutrophils45. Elevated UA levels contribute to the upregulation of let-7c, which activates the (myocyte enhancer factor-2c) MEF2C and nuclear factor-κB (NF-κB) pathways, leading to thrombosis46. Thus, PHR is strongly associated with hyperuricemia, but it is not clear whether it is a pathogenic risk factor or simply a marker for hyperuricemia.
Study strengths and limitations
This study demonstrates two primary strengths that enhance the credibility and efficacy of its outcomes. Firstly, the substantial sample size and the consistency between the initial analysis and sensitivity analysis results underscore the robustness of our findings. Secondly, the use of curve fitting has uncovered a nonlinear relationship between PHR and hyperuricemia, suggesting that PHR within an appropriate range (< 8.8) could be advantageous for predicting hyperuricemia.
However, there are limitations to acknowledge. The cross-sectional nature of the study inhibits any deduction of causality. Moreover, due to the observational design, despite adjustments for selected variables, the potential for unaddressed confounding factors exists. Another limitation of this study is the absence of detailed information on participants with blood or liver diseases, which could affect platelet counts. The participants of the study were solely American adults, which may restrict the applicability of the results to other geographical and ethnic groups. Prospective cohort studies are necessitated to corroborate our findings.
Conclusion
PHR is positively correlated with the risk of hyperuricemia. This discovery underscores the potential of PHR as a biomarker, which could be used to improve the management of hyperuricemia.
Data availability
This data can be found here: https://www.cdc.gov/nchs/nhanes.
References
Kimura, Y., Tsukui, D. & Kono, H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 22(22), 12394 (2021).
Maruhashi, T., Hisatome, I., Kihara, Y. & Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 278, 226–231 (2018).
Verdecchia, P. et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension: The PIUMA study. Hypertension 36(6), 1072–1078 (2000).
Kojima, S. et al. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am. J. Cardiol. 96(4), 489–495 (2005).
Anker, S. D. et al. Uric acid and survival in chronic heart failure: Validation and application in metabolic, functional, and hemodynamic staging. Circulation 107(15), 1991–1997 (2003).
Kawasoe, S. et al. Uric acid level and prevalence of atrial fibrillation in a Japanese general population of 285,882. Circ. J. 80(12), 2453–2459 (2016).
van der Schaft, N., Brahimaj, A., Wen, K.-X., Franco, O. H. & Dehghan, A. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: The Rotterdam Study. PLoS ONE 12(6), e0179482 (2017).
Choi, H. K., Ford, E. S., Li, C. & Curhan, G. Prevalence of the metabolic syndrome in patients with gout: The Third National Health and Nutrition Examination Survey. Arthritis Care Res. 57(1), 109–115 (2007).
Kanbay, M. et al. Effect of uric acid-lowering agents on cardiovascular outcome in patients with heart failure: A systematic review and meta-analysis of clinical studies. Angiology 71(4), 315–323 (2020).
Chen, J. et al. Effects of uric acid-lowering treatment on glycemia: A systematic review and meta-analysis. Front. Endocrinol. 11, 577 (2020).
Tsukamoto, S. et al. Prevention of kidney function decline using uric acid-lowering therapy in chronic kidney disease patients: A systematic review and network meta-analysis. Clin. Rheumatol. 41, 911–919 (2022).
Chen-Xu, M. Contemporary prevalence of Gout and Hyperuricemia in the United States (National Health and Nutrition Examination Survey [NHANES] 2015–2016) and decadal trends (NHANES 2007–2016). 2018 ACR/ARHP Annual Meeting (ACR, 2018).
Țăpoi, L., Șalaru, D. L., Sascău, R. & Stătescu, C. Uric acid: An emergent risk marker for thrombosis?. J. Clin. Med. 10(10), 2062 (2021).
Kubota, Y., McAdams-DeMarco, M. & Folsom, A. R. Serum uric acid, gout, and venous thromboembolism: The atherosclerosis risk in communities study. Thromb. Res. 144, 144–148 (2016).
Bluhm, G. B. & Riddle, J. M. (eds) Platelets and Vascular Disease in Gout. Seminars in Arthritis and Rheumatism (Elsevier, 1973).
Tayefi, M. et al. Relationship between platelet count and platelet width distribution and serum uric acid concentrations in patients with untreated essential hypertension. BioFactors 44(6), 532–538 (2018).
Liu, X. et al. Association between platelet distribution width and serum uric acid in Chinese population. BioFactors 45(3), 326–334 (2019).
Liang, J. et al. The comparison of dyslipidemia and serum uric acid in patients with gout and asymptomatic hyperuricemia: A cross-sectional study. Lipids Health Dis. 19, 1–7 (2020).
Jialal, I., Jialal, G. & Adams-Huet, B. The platelet to high density lipoprotein-cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes/Metab. Res. Rev. 37(6), e3403 (2021).
Lu, C.-f et al. Association between the platelet/high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: Results from NHANES 2017–2020. Lipids Health Dis. 22(1), 130 (2023).
Zhang, H., Xu, Y. & Xu, Y. The association of the platelet/high-density lipoprotein cholesterol ratio with self-reported stroke and cardiovascular mortality: A population-based observational study. Lipids Health Dis. 23(1), 121 (2024).
Ni, J., Lv, L., Wu, P. & Xu, C. Associations between the platelet/high-density lipoprotein cholesterol ratio and likelihood of nephrolithiasis: A cross-sectional analysis in United States adults. Front. Endocrinol. 15, 1289553 (2024).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Nagahama, K. et al. Hyperuricemia predicts future metabolic syndrome: A 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens. Res. 37(3), 232–238 (2014).
Tu, Y.-C., Liu, Y.-H., Chen, S.-C. & Su, H.-M. Metabolic syndrome and its components are associated with new-onset hyperuricemia in a large taiwanese population follow-up study. Nutrients 15(5), 1083 (2023).
Tomiyama, H. et al. Relationships among hyperuricemia, metabolic syndrome, and endothelial function. Am. J. Hypertens. 24(7), 770–774 (2011).
Frutuoso, J. & Bourbon, M. Cholesterol levels and glomerular filtration rate–Is there any association?. Atherosclerosis 252, e150 (2016).
Wasada, T., Katsumori, K., Saeki, A. & Iwatani, M. Hyperuricemia and insulin resistance. Nihon Rinsho Jpn. J. Clin. Med. 54(12), 3293–3296 (1996).
Yang, T. et al. Association between high-sensitivity C-reactive protein and hyperuricemia. Rheumatol. Int. 36, 561–6 (2016).
Chen, M.-Q., Wang, H.-Y., Shi, W.-R. & Sun, Y.-X. Estimate of prevalent hyperuricemia by systemic inflammation response index: Results from a rural Chinese population. Postgrad. Med. 133(2), 242–249 (2021).
Chen, M.-Q., Shi, W.-R., Shi, C.-N., Zhou, Y.-P. & Sun, Y.-X. Impact of monocyte to high-density lipoprotein ratio on prevalent hyperuricemia: Findings from a rural Chinese population. Lipids Health Dis. 19(1), 1–9 (2020).
Wang, L. et al. The correlation between dietary inflammatory index and risk of hyperuricemia in the US population. Medicine 102(20), e33374 (2023).
Xie, F. et al. Association between systemic immune-inflammation index and serum uric acid in US adolescents: A population-based study. Nutr. Metab. Cardiovasc. Dis. 34(1), 206–213 (2024).
Li, Y., Liu, X. & Luo, Y. Monocyte to high-density lipoprotein cholesterol ratio and serum uric acid in Chinese adults: A cross-sectional study. BMC Endocr. Disord. 22(1), 48 (2022).
Murphy, A. J. & Woollard, K. J. High-density lipoprotein: A potent inhibitor of inflammation. Clin. Exp. Pharmacol. Physiol. 37(7), 710–718 (2010).
Murphy, A., Chin-Dusting, J., Sviridov, D. & Woollard, K. J. The anti inflammatory effects of high density lipoproteins. Curr. Med. Chem. 16(6), 667–675 (2009).
Murphy, A. J. et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 28(11), 2071–2077 (2008).
Jung, S. W., Kim, S.-M., Kim, Y. G., Lee, S.-H. & Moon, J.-Y. Uric acid and inflammation in kidney disease. Am. J. Physiol. 318, 1327–1340 (2020).
De Lucchi, L. et al. Serum uric acid levels and the risk of recurrent venous thromboembolism. J. Thromb. Haemostas. 19(1), 194–201 (2021).
Kanellis, J. et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41(6), 1287–1293 (2003).
Spiga, R. et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 37(6), 1241–1249 (2017).
Nieto, F. J., Iribarren, C., Gross, M. D., Comstock, G. W. & Cutler, R. G. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis?. Atherosclerosis 148(1), 131–139 (2000).
Shimizu, Y. et al. Serum uric acid level increases in proportion to the severity of pulmonary thromboembolism. Circ. J. 66(6), 571–575 (2002).
Tang, R.-B. et al. Serum uric acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can. J. Cardiol. 30(11), 1415–1421 (2014).
Yu, H. et al. Hyperuricemia enhances procoagulant activity of vascular endothelial cells through TMEM16F regulated phosphatidylserine exposure and microparticle release. FASEB J. 35(9), e21808 (2021).
Cheng, X. et al. Prothrombotic effects of high uric acid in mice via activation of MEF2C-dependent NF-κB pathway by upregulating let-7c. Aging (Albany NY) 12(18), 17976 (2020).
Author information
Authors and Affiliations
Contributions
SYZ was involved in the study design. XYH, LSY and SSW were involved in data analysis and graph preparation. LSY was responsible for writing and revising the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, L., Hu, X., Wu, S. et al. Association of platelet to high-density lipoprotein cholesterol ratio with hyperuricemia. Sci Rep 14, 15641 (2024). https://doi.org/10.1038/s41598-024-66747-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66747-x
- Springer Nature Limited