Abstract
We aimed to determine the chemical profile and unveil Anadenanthera colubrina (Vell.) Brenan standardized extract effects on inflammatory cytokines expression and key proteins from immunoregulating signaling pathways on LPS-induced THP-1 monocyte. Using the RT-PCR and Luminex Assays, we planned to show the gene expression and the levels of IL-8, IL-1β, and IL-10 inflammatory cytokines. Key proteins of NF-κB and MAPK transduction signaling pathways (NF-κB, p-38, p-NF-κB, and p-p38) were detected by Simple Western. Using HPLC-ESI-MSn (High-Performance Liquid-Chromatography) and HPLC-HRESIMS, we showed the profile of the extract that includes an opus of flavonoids, including the catechins, quercetin, kaempferol, and the proanthocyanidins. Cell viability was unaffected up to 250 µg/mL of the extract (LD50 = 978.7 µg/mL). Thereafter, the extract's impact on the cytokine became clear. Upon LPS stimuli, in the presence of the extract, gene expression of IL-1β and IL-10 were downregulated and the cytokines expression of IL-1β and IL-10 were down an upregulated respectively. The extract is involved in TLR-4-related NF-κB/MAPK pathways; it ignited phosphorylation of p38 and NF-κB, orchestrating a reduced signal intensity. Therefore, Anadenanthera colubrina's showed low cytotoxicity and profound influence as a protector against the inflammation, modulating IL-1β and IL-10 inflammatory cytokines gene expression and secretion by regulating intracellular NF-κB and p38-MAPK signaling pathways.
Similar content being viewed by others
Introduction
Pathogen-Associated Molecular Patterns (PAMPs) are molecular structures that are commonly found on various pathogens such as bacteria, viruses, fungi, and parasites. These patterns are recognized by the innate immune system as potentially harmful, triggering a series of immune responses aimed at eliminating the invading pathogens1. In the oral cavity, various PAMPs are found in the oral microbiome, including fungal/bacterial cell wall components such as β-Glucans, Mannans and Chitin found in fungi like Candida albicans2,3. Another critical PAMPs in the oral microbiome are the bacterial cell wall components such as Peptidoglycans, Lipoteichoic Acids (LTA) and Lipopolysaccharides (LPS)1,4. The PAMPs recognition can activate strong immune responses, as well as trigger an associated inflammatory pattern of different oral conditions, such as periodontal diseases1,4,5.
Recognition of these PAMPs by pattern recognition receptors (PRRs) expressed on cells of the innate immune system, such as macrophages, dendritic cells, and epithelial cells, triggers signaling pathways that lead to the activation of immune responses. Recognition of oral microbiome PAMPs ligands occurs via some of the Toll-like receptors (TLRs), NOD receptors, RIG receptors, Dectin receptor and C-lectin receptors (CLRs) whereas a signaling cascade is initiated that culminates in proinflamatory cytokine and chemokine production 1,2,6. Thus, an associated inflammatory pattern of the condition is stablished 5,7,8,9.
The signaling sequence through NF‐κB and MAPK pathways may also be activated once the TLRs recognize other microorganisms’ PAMPs such as the LPS produced by Porphyromonas gingivalis, which is a major glycolipid within the outer membrane of gram-negative bacteria. P. gingivalis is highly associated with periodontitis pathogenesis, whereas LPS presence serves as a critical virulence factor triggering an onset inflammation pattern driven by the cytokines and inflammatory mediators release that exacerbate the immune response and local tissues damage5,10,11,12. Although the host immune response is an important factor against infections, when these inflammatory axes become dysregulated, they may also be associated with collateral damage to the host, exacerbation of infection effects, and it plays an important role in carcinogenesis13.
The development of effective anti-inflammatory drugs becomes essential in order to mitigate the risks associated with imbalances in immune response and inflammation14,15. Considering the pharmacological significance of plant-derived substances, significant attention has been drawn to them, especially to discovering potential therapeutic agents for treating a wide range of disorders16,17,18.
In this context, Anadenanthera colubrina (Vell.) Brenan, popularly known as “angico”, is widely applied in Brazilian folk medicine to treat local inflammation19,20. In our prior study, the A. colubrina standardized extract exhibited a significant reduction in C. albicans infection and effectively modulated the Candida-induced inflammatory response in human gingival fibroblasts21. However, it remains unknown whether the therapeutic effect is attributed to a specific active compound or a complex mixture of active substances within the extract—the phytocomplex. Despite the anti-inflammatory properties of A. colubrina, there is limited data on its immunomodulatory effects on transcriptome and proteome levels and molecular signaling transductions. Therefore, in the present study we aimed to investigate the phytochemical profile, the in vitro immunomodulatory effect of the standardized extract of A. colubrina bark, and the underlying cell signaling transduction pathways in LPS-stimulated monocytes.
Results
Cytotoxicity
Using THP-1 cells, we first asked if the A. colubrina extract could treat the cells without killing them. So, we evaluated its cytotoxicity, and the experiments presented LD50 of 978.7 µg/mL and a non-toxic profile on THP-1 cells culture, with no significant effect on cell viability in concentrations up to 250 µg/mL when compared to the vehicle and the cell control (Fig. 1).
Modulatory effect on inflammatory cytokines gene expression
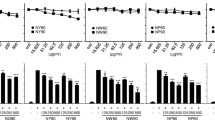
Following prior studies showing the extract presents anti-inflammatory effects, we decided to perform experiments for gene expression where the results showed the A. colubrina extract at 250 µg/mL presented modulatory effects on the gene expression of inflammatory cytokines. The extract itself up-regulated the gene expression of IL-1β cytokine. Upon LPS stimuli, gene expression of IL-1β and IL-10 were downregulated by A. colubrina, while it was ineffective in modulating IL-8 gene expression (Fig. 2a,b,c).
Real-time quantitative information about to relative gene expression of a) IL-8, b) IL-1β, and c) IL-10 of THP-1 cells after 6 h of treatment with A. colubrina extract (250 µg/mL) and stimulation by LPS (100 ng/mL). Values are shown as the fold-change relative to the negative control group (6 h). Values shown with an asterisk (*) are statistically significant when compared to the negative control (p < 0.05).
After that, to better understand the potential of the standardized extract, we also choose to measure the pro and anti-inflammatory cytokines secretion followed by signaling pathways regulation.
Pro and anti-inflammatory cytokines secretion and signaling pathways regulation
The expression of pro-inflammatory (IL-8 and IL-1β) and anti-inflammatory (IL-10) cytokines after treatments with A. colubrina extract (250 µg/mL) were assessed using the THP-1 culture supernatants. The extract itself did not induce any inflammatory response. Also, it did not reduce IL-8 cytokine secretion from the LPS-induced THP-1 cells group (p < 0.05). However, in the LPS-stimulated group treated with A. colubrina, IL-1β, and IL-10 cytokines levels expression were significantly reduced and increased, respectively (p < 0.05), when compared to the LPS-induced only cells group (Fig. 3a, b, c).
Therefore, the Simple Western assay data for the regulatory signaling pathways demonstrated that the phosphorylated forms of NF-κB and p38 were detected in all groups tested, with visual differences in bands’ signal intensity. In addition, the LPS-induced cells group triggered NF-κB and p-38 phosphorylated forms. A. colubrina also activated the MAPK pathway by itself and in conjunction with LPS through phosphorylation of p38. The same groups also activated the NF-κB (Figs. 4, 5).
Phytochemical profile
After the above-cited exciting results, we then performed the phytochemical profile of A. colubrina (standardized hydroethanolic extract); it was assessed by HPLC-ESI-MSn (High-Performance Liquid-Chromatography) and HRESIMS (High Resolution Electrospray Ionization Mass Spectrometry), characterized by the presence of flavonoids, predominantly heterosides of catechins, quercetin, kaempferol; and proanthocyanidins (Table 1). The base peak chromatogram is shown in Fig. 6.
Discussion
The Anadenanthera colubrina is a plant that can positively affect human health. Therefore, we studied the standardized hydroethanolic extract and found the A. colubrina, in its phytocomplex, potentially interacts with TLRs. Nonetheless, it is yet unknown if its therapeutic effect can be delivered by one specific active compound or by a complex mixture of active substances contained in the extract (the phytocomplex). For this reason, identifying bioactive compounds that could act both in modulating the virulence factors of oral microrganism and on the inflammatory response of the host against the pathogen holds promise for improved therapeutic approaches. A. colubrina capacity to reduce C. albicans infection has been shown in our previous study. However, the approach described was employed for the first time to investigate the immunomodulatory effect of A. colubrina extract and its regulation on underlying cell signaling transduction pathways in LPS-induced THP-1 monocytes.
The extract did not affect cell viability at concentrations up to 250 µg/mL, so that it can be a therapeutic application source. Our results are in accordance with several reports that also confirmed the low toxicity of A. colubrina on macrophages22,23,24, keratinocytes, and tumoral cell strains25.
Several studies have investigated the immunomodulatory effects of medicinal plant extracts using THP-1 cells26,27,28 supporting its application as an in vitro model to study human inflammatory diseases. So, in this study, THP-1 cells were set as the in vitro cell model to evaluate the immunomodulation effects of A. colubrina, since these cells possess regulatory proteins which initiate inflammation upon stimulation by LPS26. on the regulation of pro-inflammatory cytokine expression during LPS stimulation, through gene expression (transcriptional level) and secretion (proteomic level) of these inflammation mediators.
Considering that TLRs exert pro-inflammatory effects when activated, and anti-inflammatory effects when downregulated or suppressed, they are thought to play a central role in both mediating and modulating inflammatory response29. In this regard, we chose a panel of pro-inflammatory markers, since the interaction between TRL4 receptor and LPS induces the activation of NF-κB/MAPK signaling pathways and the release of TNF-α, IL-1β, IL-6 and IL-8 cytokines12,30,31. Finally, we evaluated the secretion levels of IL-10 cytokine, as an anti-inflammatory marker.
A. colubrina was not effective in reducing the gene expression of IL-8 pro-inflammatory cytokines in the LPS-induced group. However, upon LPS stimuli, A. colubrina affected the expression of inflammatory cytokines by down-regulating IL-1β and IL-10 genes expression, which suggests the anti-inflammatory effect of the extract on the transcriptional level of regulation. In addition, our results showed that LPS stimulus effectively up-regulates the gene expression of IL-8 and IL-1β pro-inflammatory cytokines, which proves its effect on inducing transcription of genes related to inflammation responses upon interaction with TRL4 receptor32.
Also, we showed that A. colubrina itself did not induce any pro-inflammatory and anti-inflammatory response, which is positive considering that the extract did not significantly affect THP-1 cell viability and function. However, upon A. colubrina treatment, secretion levels of IL-8 cytokines were not affected in LPS-induced groups, which means that the extract was ineffective in decreasing this pro-inflammatory cytokine in response to the inflammatory stimuli produced by LPS. On the other hand, it is known that IL-1β is vital for the inflammatory host response against pathogens since it is involved in the recruitment of immune cells to the site of infection33. Therefore, the study showed a significant decrease in IL-1β secretion by A. colubrina, which may prevent additional inflammatory responses induced by the recruitment of immune cells. In addition, IL-10, a representative anti-inflammatory cytokine that plays a critical role in controlling immune responses and is reported to be involved in the inhibition of IL-1β production34,35, was significantly enhanced in the present study Therefore, these findings suggest that A. colubrina may regulate the immune response by modulating IL-1β and IL-10 cytokines level secretion.
The signaling pathway assay (Simple Western Immunoassay) was used to examine A. colubrina-mediated-signal transduction and determine which pathways were affected on the regulation of pro-inflammatory cytokine gene expression, accessing the expression of key proteins involved in NF-κB/MAPK signaling pathways through the detection of phosphorylated forms of NF-κB and p38. MAPK (JNK, ERK, and p38) and NF-κB are crucial intracellular pathways leading to the inflammatory response36. These biological responses are mediated by their transcription factors, such as activator protein-(AP1) and NF-κB subunit Iκβα, which are phosphorylated and translocated from the cytoplasm to the nucleus, resulting in an inflammatory action through the expression of target genes, including pro-inflammatory cytokines IL-1β, IL-6, and TNF-α as well as iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) proteins37,38.
In this study, we evidenced the boosted activity of phosphorylated NF-κB (NF-κB + p) and p38 (p38 + p) forms upon LPS stimulation alone in THP-1 cells, which proves the activation from NF-κB/MAPK pathways by LPS, resulting in over-production of inflammatory mediators. In addition, we observed phosphorylation from NF-κB and p38, with an attenuated signal intensity band regarding A. colubrina itself and in conjunction with LPS stimuli, especially on the NF-κB transduction factor. These findings suggest that the extract may exert molecular mechanisms involving the modulation of these signaling pathways, particularly on NF-κB protein activation.
Indeed, IL-1β is commonly induced by activating the inflammatory transcription factor NF-κB, which regulates inflammatory responses such as cell proliferation, migration, adhesion, and lymphocyte development39. Thus, the decrease in the release of IL-1β cytokine in Luminex analysis in our study is consistent with the downregulation of the NF-κB signaling pathway. Furthermore, considering that the extract combined with LPS was ineffective in decreasing IL-8 levels but significantly decreased IL-1β secretion. Therefore, we hypothesized that phosphorylation of p38, in conjunction with an attenuated activation of NF-κB, was responsible for the interaction between these pathways, determining this final biological response after LPS mediator stimulation40.
This approach may also help identify potential therapeutic targets in pathophysiological contexts of exacerbated or chronic inflammation15,41. Thus, we reinforce that further analysis to measure the phosphorylation levels of NF-κB and p38 proteins is necessary to verify quantitatively how the extract can regulate the expression of these key proteins related to signaling pathways that trigger the expression and production of pro-inflammatory cytokines.
Besides regulating IL-1β, IL-6, and IL-8, NF-κB/MAPK signaling cascades also monitor the expression of pro-inflammatory cytokine TNF-α27. Our study did not evaluate the TNF-α expression. However, recent studies that evaluated the anti-inflammatory effects of A. colubrina leaves extract23 and protease inhibitors extracted from the same species24 showed a significant reduction in TNF-α and nitric oxide (NO) production levels in LPS-induced macrophages. Along with these findings, our results can contribute to understanding the molecular mechanisms underlying the anti-inflammatory effects of A. colubrina.
Previously, we evaluated the phytochemical composition of the A. colubrina bark extract25. It was found a high total polyphenol content (53.18% gallic acid equivalents); tannins (8.77% catechin equivalents) and flavonoids (0.28% quercetin equivalents: mainly heterosides of catechin, quercetin, and kaempferol), and proanthocyanidins. In this current study, we also assessed the chemical profile of the extract by HPLC coupled to Ion Trap and TOF Mass Spectrometry (HPLC-IT-MS/HPLC-TOF-MS). Flavonoids are polyphenolic compounds commonly present in most plants with anti-inflammatory properties42,43,44, supporting the A. colubrina immunomodulatory property here evaluated. Additionally, flavonoids can modulate the immune response through the inhibition of molecules that play an essential role in the modulation of mediators related to inflammation response, such as regulatory enzymes and transcription factors, such as NF-κB10,44,45,46.
Several studies have reported that extracts containing flavonoids, such as catechin and glycosylated derivatives of quercetin, can modulate several inflammatory and oxidative stress mediators through the negative regulation of pro-inflammatory cytokines and chemokines (TNF-α, IL-6, IL-1β, IL-8), nitric oxide (NO) and COX-2 in LPS-activated macrophages42,43,47. Thus, consistent with these studies, the immunomodulatory effects of A. colubrina extract observed in the present study could be related to the presence of flavonoids.
In conclusion, the present in vitro study is the first to show that A. colubrina extract has anti-inflammatory properties in LPS-induced THP-1 cells. Furthermore, these effects can be related to modulations in the secretion of IL-1β and IL-10 cytokines through the regulation of intracellular NF-κB and p38-MAPK signaling pathways. Further understanding of additional signaling pathways and activation effects implicated in extract properties might provide novel insights into the immunomodulation and new opportunities for A. colubrina rational application. Future analyses are warranted to identify and characterize each substance or group of the substance responsible for the potential anti-inflammatory activity highlighted in the current study; nevertheless, our experimental approach, employing the standardized hydroethanolic extract, is worthy of consideration.
Methods
Preparation of the standardized extract
Barks of Anadenanthera colubrina (Vell.) Brenan were collected during September in the semi-arid region of Paraíba state, Brazil (7º 22′ 25′′ S, 35º 59′ 32′′ W). It should be noted that all methods used in this study involving plants followed institutional, national, and international guidelines and legislation. Additionally, all necessary permissions and licenses needed for the collection and processing were obtained. Botanical specimens were deposited in the Manuel de Arruda Câmara Herbarium (ACAM) at the State University of Paraíba (UEPB), Campus I, Campina Grande, Paraíba, Brazil, under nº 1936/ACAM. Also, this research was conducted under authorization of Genetic Heritage Management Council, attached to the Brazilian ministry of the environment, SisGen authorization number A289DF4.
The plant material was dried, macerated, and immersed in 80% ethanol for 48 h (10 mg: 25 mL) to obtain a hydroethanolic extract48. In addition, the material was filtered three times, vacuum concentrated (Tecnal TE-211, Piracicaba, SP, Brazil), and lyophilized (Martin Christ 1–2 LDplus, Germany), with an extraction yield of 31.7%.
Cell viability assay
The effect on cell viability of A. colubrina extract was evaluated on THP-1 monocyte cells (THP-1 ATCC® TIB-202) and assessed by a resazurin fluorometric method (Cell Titer Blue Viability Assay, Promega Corp, Madison, WI). THP-1 cells were cultured in Roswell Park Memorial Institute medium (RPMI-1640, VWR Life Science, Radnor, PA) supplemented with 10% Fetal Bovine Serum (FBS, Gibco, Invitrogen, Waltham, MA), Penicillin (10,000 U/mL), Streptomycin (10,000 µg/mL), and 2-Mercaptoethanol 50 nM (VWR Life Science, Radnor, PA), at 37 °C in 5% CO2. THP-1 cells (2.5 × 105 cells/mL) were seeded in a 24-well plate (Greiner Bio-One North America, Inc Monroe, NC, USA) in RPMI with 10% FBS. The A. colubrina extract was diluted in Dimethyl Sulfoxide 1% (DMSO, BDH Solvents, Dawsonville GA), with a final concentration inside the wells of 0.1%, and then added to the cultured cells wells (2500–0.25 µg/mL). After 24 h of incubation at 37 °C in 5% CO2, the resazurin (30 µL) was added, and the plates were incubated for 3 h. The fluorescence of the supernatant was read in a microplate reader (SpectraMax M3, Molecular Devices, Sunnyvale, CA), with excitation of 555 nm, emission of 585 nm, and 570 nm cut-off49. (n = 3 for each group, analyzed on three independent experiments).
Cell treatment and LPS-induced inflammation assay
As mentioned above, THP-1 cells were seeded in 24-well plates at the same density and culture conditions from the cell viability assay protocol, as mentioned above. Then, the cells were exposed to A. colubrina extract and LPS from Porphyromonas gingivalis (InvivoGen, San Diego, CA), according to the following groups: stimulation with LPS (100 ng/mL), treatment with A. colubrina (250 µg/mL), simultaneous exposure to LPS (100 ng/mL) and A. colubrina (250 µg/mL), and a control group with no treatment. The plates were incubated for 6 h at 37 °C in 5% CO250. Cell supernatants were collected by centrifugation at 1500 rpm for 5 min for quantitative analysis of cytokines. The cells kept on the bottom of the wells were processed for RNA extraction and whole-cell lysate collection for gene expression by RT-PCR and Wes Simple Western assays, respectively.
Real-time quantitative PCR
RNA was isolated from LPS-induced THP-1 cells and treated with A. colubrina using RNeasy® Mini Kit (Qiagen, Hilden, Germany) per the supplier’s specification. In this study were chosen the same host inflammatory cytokine genes that were described in our first report using A.colubrina50: IL-8 (Qiagen Gene ID#: 3576; Qiagen, Hilden, Germany); IL-10 (Qiagen Gene ID#: 3587; Qiagen, Hilden, Germany); IL-1β (Qiagen Gene ID#: 3553; Qiagen, Hilden, Germany) and GAPDH (housekeeping) (Qiagen Gene ID#: 2597; Qiagen, Hilden, Germany). In addition, the gene expressions were normalized to GAPDH. Reactions of qPCR were performed using g QuantiFast® SYBR® Green RT-PCR One Step Kit (Qiagen, Hilden, Germany) in a thermocycler (QuantStudio 3 Real-Time PCR System, Thermo Fischer Scientific, Rockford, IL). The qPCR protocol was as follows: 50 °C for 10 min, 95 °C for 5 min, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. Analysis of relative gene expression was accomplished according to the ΔΔCt method51. (n = 3 for each group, analyzed on three independent experiments).
Luminex for quantitative analysis of inflammatory cytokines
Supernatants from TPH-1 cells treated with A. colubrina and exposed to LPS were collected and assayed for analysis expression of pro-inflammatory cytokines IL-8, IL1β, and anti-inflammatory IL-10 using Human Magnetic Premixed Multi-Analyte Luminex Assay Kit (R&D Systems, Minneapolis, MN). Culture supernatants and cytokine capture bead cocktails were incubated overnight. Samples were incubated for 1 h with a biotin-labeled antibody and 30 min with streptavidin-PE. Using Luminex 200 Milliplex System, the data were collected and analyzed using Milliplex Analyst software52. (n = 3 for each group, analyzed on three independent experiments).
WES simple western immunoassay of NF-κB and p38-MAPK
THP-1 cells were lysed with 200 µL of ice-cold Pierce RIPA Lysis Buffer (Thermo Fischer Scientific, Rockford, IL) for 5 min at room temperature to obtain the whole-cell lysate. According to the manufacturer’s instructions, total protein concentration was determined using Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) to normalize the lysates’ protein content. Wes Simple Western system (ProteinSimple, San Jose, CA) was used to detect the protein expression of NF-κB, p38, phosphorylated NF-κB + p, and phosphorylated p38 + p. According to the manufacturer’s protocol, the Separation Module of 12–230 kDa, Anti-Rabbit Detection Module, and capillary cartridges (ProteinSimple, SM-W002-1, SanJose, CA) were applied. Lysate samples were reduced to 0.4 M dithiothreitol (DTT), mixed with Fluorescent Master Mix, and denatured at 95 °C for 5 min. A biotinylated ladder (12–230 kDa) was used for molecular weight determination. Primary antibodies (1:1000) were used for protein detection of NF-κB and NF-κB -p (MW: 120 kDa), p38 (MW: 40 kDa), and p38 -p (MW:43 kDa) (Cell Signaling Technology, Danvers, MA). The samples, the blocking reagent, the primary antibodies, the HRP-conjugated secondary antibodies, and the chemiluminescent substrate were added to the plate. The WES machine and software (Compass™) provided data as virtual blots (electropherograms) in which the molecular weight and signal intensity are presented53,54. (n = 3 for each group, analyzed on three independent experiments).
Phytochemical profile analysis by LC-ESI-MSn and LC-HRESIMS
The phytochemical profiles of A. colubrina extract were analyzed using an HPLC (Shimadzu, Kyoto, Japan) equipped with a C18 column (Kromasil—250 mm × 4.6 mm × 5 μm) coupled to Ion-Trap (Amazon X, Bruker, Berlin, Germany) or microTOF mass spectrometers (Bruker, Berlin, Germany) with an Electrospray Ionization (ESI).First the extract was diluted in methanol (1 mg/mL) and then filtered in a 0.45 µM PVDF (Polyvinylidene Difluoride) membrane. For the chromatography method, methanol (solvent B) and ultrapure milli-Q water were used; the solution was acidified with formic acid (0.1% v/v), following gradient elution of concentration (5 to 100% of solvent B in 60 min). The injection volume was 10 µL, and the flow was set to 0.6 mL/min. Ion Trap and TOF acquisition parameters were as follows: negative ionization mode; spray voltage of 4.4 kV; offset of 500 V; sheath gas at 35 pi; drying gas N2; the flow of 8 mL/min and heater temperature of 300 °C. The analysis of the compounds was based on MS/MS data available in the literature.
Statistical analysis
Data were expressed as the mean ± SEM using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests to the vehicle using GraphPad Prism software (version 8.02). (p ≤ 0.05 was set as the threshold of significance).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Li, D. & Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. https://doi.org/10.1038/s41392-021-00687-0 (2021).
D’Enfert, C. et al. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. https://doi.org/10.1093/femsre/fuaa060 (2021).
Carpino, N., Naseem, S., Frank, D. M. & Konopka, J. B. Modulating host signaling pathways to promote resistance to infection by Candida albicans. Front. Cell. Infect. Microbiol. https://doi.org/10.3389/fcimb.2017.00481 (2017).
Naclerio, G. A. & Sintim, H. O. Multiple ways to kill bacteria via inhibiting novel cell wall or membrane targets. Future Med. Chem. 12, 1253–1279 (2020).
Xu, W., Zhou, W., Wang, H. & Liang, S. Roles of porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 120, 45–84 (2020).
Lopes, J. P. & Lionakis, M. S. Pathogenesis and virulence of Candida albicans. Virulence 13, 89–121 (2022).
Nikou, S. A. et al. The Candida albicans toxin candidalysin mediates distinct epithelial inflammatory responses through p38 and EGFR-ERK pathways. Sci. Signal. https://doi.org/10.1126/scisignal.abj6915 (2022).
Ho, J. et al. Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology 162, 11–16 (2021).
Zhou, Y., Cheng, L., Lei, Y. L., Ren, B. & Zhou, X. The Interactions Between Candida albicans and Mucosal Immunity. Front. Microbiol. https://doi.org/10.3389/fmicb.2021.652725 (2021).
Li, S. et al. Glutamine protects against LPS-induced inflammation via adjusted NODs signaling and enhanced immunoglobulins secretion in rainbow trout leukocytes-ScienceDirect. Dev. Comp. Immunol. 98, 148–156 (2019).
Yin, P. et al. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κβ and MAPK pathway. Res. Vet. Sci. 126, 164–169 (2019).
Zhu, Z. et al. Effect of berberine on LPS-induced expression of NF-κB/MAPK signalling pathway and related inflammatory cytokines in porcine intestinal epithelial cells. Innate Immun. 26, 627–634 (2020).
Murata, M. Inflammation and cancer. Environ Health Prev Med https://doi.org/10.1186/s12199-018-0740-1 (2018).
Sugimoto, M. A., Sousa, L. P., Pinho, V., Perretti, M. & Teixeira, M. M. Resolution of inflammation: What controls its onset?. Front. Immunol. https://doi.org/10.3389/fimmu.2016.00160 (2016).
Kim, H. et al. Src/NF-κB-targeted anti-inflammatory effects of potentilla glabra var. Mandshurica (maxim.) hand.-mazz. ethanol extract. Biomolecules 10(4), 648 (2020).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803 (2020).
Adegbaju, O. D., Otunola, G. A. & Afolayan, A. J. Anti-inflammatory and cytotoxic evaluation of extracts from the flowering stage of celosia argentea. BMC Complement Med. Ther. https://doi.org/10.1186/s12906-020-02941-4 (2020).
Sadeghi, A., Bastin, A. R., Ghahremani, H. & Doustimotlagh, A. H. The effects of rosmarinic acid on oxidative stress parameters and inflammatory cytokines in lipopolysaccharide-induced peripheral blood mononuclear cells. Mol. Biol. Rep. 47, 3557–3566 (2020).
Delices, M., de Muller, J. A. I., Arunachalam, K. & Martins, D. T. Anadenanthera colubrina (Vell) Brenan: Ethnobotanical, phytochemical, pharmacological and toxicological aspects. J. Ethnopharmacol. https://doi.org/10.1016/j.jep.2022.115745 (2023).
Weber, C. R. et al. Anadenanthera colubrina: A therapeutic potential study. Rev. Bras. Farm 92, 235–244 (2011).
de Maia, C. M. A., Pasetto, S., Nonaka, C. F. W., de Costa, E. M. M. B. & Murata, R. M. Yeast-host interactions: Anadenanthera colubrina modulates virulence factors of C. albicans and inflammatory response in vitro. Front. Pharmacol. 12, 1–13 (2021).
Silva, D. R. et al. Anadenanthera Colubrina vell Brenan: Anti-Candida and antibiofilm activities, toxicity and therapeutical action. Braz Oral Res 33, (2019).
Junior, O. C. et al. In vitro and in vivo evaluation of anti-inflammatory activity and free radical scavenging potential of leaves extract from Anadenanthera colubrina. Nat Prod Res 35, 4819–4823 (2021).
Guarneire, G. J. et al. Effect of Anadenanthera colubrina protease inhibitors as an antiinflamatory mediator. Nat Prod Res 35, 1690–1695 (2021).
Lima, R. D. F. et al. Antimicrobial and antiproliferative potential of Anadenanthera colubrina (Vell.) brenan. Evidence-based Complement. Alternat. Med. 2014, 1–7 (2014).
Sullivan, A. C., Pangloli, P. & Dia, V. P. Kafirin from Sorghum bicolor inhibition of inflammation in THP-1 human macrophages is associated with reduction of intracellular reactive oxygen species. Food Chem. Toxicol. 111, 503–510 (2018).
Albrahim, T. et al. Potential anti-inflammatory and anti-apoptotic effect of Coccinia grandis plant extract in LPS stimulated-THP-1 cells. Environ. Sci. Pollut. Res. 27, 21892–21904 (2020).
Zhang, G., Gao, X., Zeng, H., Li, Y. & Guo, X. Virosecurinine induces apoptosis in human leukemia THP-1 cells and other underlying molecular mechanisms. Oncol Lett 15, 849–854 (2018).
Calabrese, E. J., Agathokleous, E., Kapoor, R., Kozumbo, W. J. & Rattan, S. I. S. Chemico-biological interactions. Chem Biol Interact 314, 108844 (2019).
Park, H. S. et al. Cis- and Trans-gnetin H from Paeonia suffruticosa suppress inhibitor kappa B kinase phosphorylation in LPS-stimulated human THP-1 cells. J Ethnopharmacol 189, 202–209 (2016).
Lee, J. O. et al. Compound K, a ginsenoside metabolite, plays an antiinflammatory role in macrophages by targeting the AKT1-mediated signaling pathway. J Ginseng Res 43, 154–160 (2019).
Elmarakby, A. A. et al. A dual role of 12/15-lipoxygenase in LPS-induced acute renal inflammation and injury. Biochim Biophys Acta Mol Cell Biol Lipids 1864, 1669–1680 (2019).
Mantovani, A., Dinarello, C. A., Molgora, M. & Garlanda, C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 50, 778–795 (2019).
Hara, K. et al. Interferon-tau attenuates uptake of nanoparticles and secretion of interleukin-1β in macrophages. PLoS One 9, e113974 (2014).
Yamazaki, T., Ohshio, K., Sugamata, M. & Morita, Y. Lactic acid bacterium, Lactobacillus paracasei KW3110, suppresses inflammatory stress-induced caspase-1 activation by promoting interleukin-10 production in mouse and human immune cells. PLoS One https://doi.org/10.1371/journal.pone.0237754 (2020).
Jang, J. et al. Sorbaria kirilowii ethanol extract exerts anti-inflammatory effects in vitro and in vivo by targeting src/nuclear factor (Nf)-κb. Biomolecules 10(5), 741 (2020).
Cui, L. et al. Progesterone inhibits inflammatory response in E. coli- or LPS-Stimulated bovine endometrial epithelial cells by NF-κB and MAPK pathways. Dev Comp Immunol 105, 103568 (2020).
Li, C. et al. IκBα phosphorylation and associated NF-κB activation are essential events in lymphocyte activation, proliferation, and anti-bacterial adaptive immune response of Nile tilapia. Dev Comp Immunol 103, 103526 (2020).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2, e17023 (2017).
Hsieh, Y.-H., Deng, J.-S., Pan, H.-P., Liao, J.-C. & Huang, S.-S. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int. Immunopharmacol. 44, 16–25 (2017).
Afonina, I. S., Zhong, Z., Karin, M. & Beyaert, R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol 18, 861–869 (2017).
Lee, H. N. et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 41, 888–898 (2018).
Chen, G. L., Fan, M. X., Wu, J. L., Li, N. & Guo, M. Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 277, 706–712 (2019).
Maleki, S. J., Crespo, J. F. & Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chemestry 30, 125124 (2019).
Chen, L. et al. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit. Rev. Food Sci. Nutr. 58, 2908–2924 (2018).
Sharma, A., Sharma, R., Kumar, D. & Padwad, Y. Berberis lycium Royle fruit extract mitigates oxi-inflammatory stress by suppressing NF-κB/MAPK signalling cascade in activated macrophages and Treg proliferation in splenic lymphocytes. Inflammopharmacology 28, 1053–1072 (2020).
Lin, W., Wang, W., Wang, D. & Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 61(9), 1700031 (2017).
Cheeran, V. & Munuswamy-Ramanujam, G. Sesquiterpene lactone Zaluzanin D alters MMP-9 promoter methylation in differentiated THP-1 monocytic cells and down regulates inflammatory cytokines IL-1β and TNF-α. Int. Immunopharmacol. 87, 106803. https://doi.org/10.1016/j.intimp.2020.106803 (2020).
O’Brien, J., Wilson, I., Orton, T. & Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426 (2000).
Wei, W. et al. TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J. Ethnopharmacol. 179, 243–252. https://doi.org/10.1016/j.jep.2015.12.060 (2016).
Chen, E. et al. Fungal-host interaction: Curcumin modulates proteolytic enzyme activity of Candida albicans and inflammatory host response in vitro. Int. J. Dent. 2018, 1–7 (2018).
Allin, N. et al. Inflammatory response influences treatment of localized aggressive periodontitis. J. Dent. Res. 95, 635–641 (2016).
Misiewicz-Krzeminska, I. et al. A novel nano-immunoassay method for quantification of proteins from CD138-purified myeloma cells: Biological and clinical utility. Haematologica 103, 880–889 (2018).
Petővári, G. et al. Inhibition of metabolic shift can decrease therapy resistance in human high-grade glioma cells. Pathol. Oncol. Res. 26, 23–33 (2020).
Acknowledgements
Research reported in this publication was supported by Brazilian Coordination of Improvement of Higher Education Personnel–CAPES (PDSE–Call 47/2017), and by the National Institutes of Health-NIH under award number R03DE031190.
Author information
Authors and Affiliations
Contributions
Conceptualization: RMM, EMMBC, CMAM, SP. Methodology: CMAM, SP Investigation and Data curation: CMAM, SP, WCG, JPRS. Formal analysis: RMM, EMMBC, CMAM, SP, VP, WCG, JPRS, JTF. Validation: RMM, EMMBC, CMAM, SP. Writing – original draft: CMAM. Writing – review and editing: CMAM, PGSV, SP, VP, RMM, EMMBC. Funding Acquisition: EMMBC. Supervision: RMM, EMMBC.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maia, C.M.A., Vasconcelos, P.G.S., Pasetto, S. et al. Anadenanthera colubrina regulated LPS-induced inflammation by suppressing NF-κB and p38-MAPK signaling pathways. Sci Rep 14, 16028 (2024). https://doi.org/10.1038/s41598-024-66590-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66590-0
- Springer Nature Limited