Abstract
Identifying novel epigenetic biomarkers is a promising way to improve the clinical management of patients with breast cancer. Our study aimed to determine the methylation pattern of 25 tumor suppressor genes (TSG) and select the best methylation biomarker associated with clinicopathological features in the cohort of Slovak patients diagnosed with invasive ductal carcinoma (IDC). Overall, 166 formalin-fixed, paraffin-embedded (FFPE) tissues obtained from patients with IDC were included in the study. The methylation status of the promoter regions of 25 TSG was analyzed using semiquantitative methylation-specific MLPA (MS-MLPA). We identified CDH13 as the most frequently methylated gene in our cohort of patients. Further analysis by ddPCR confirmed an increased level of methylation in the promoter region of CDH13. A significant difference in CDH13 methylation levels was observed between IDC molecular subtypes LUM A versus HER2 (P = 0.0116) and HER2 versus TNBC (P = 0.0234). In addition, significantly higher methylation was detected in HER2+ versus HER2- tumors (P = 0.0004) and PR− versus PR+ tumors (P = 0.0421). Our results provide evidence that alteration in CDH13 methylation is associated with clinicopathological features in the cohort of Slovak patients with IDC. In addition, using ddPCR as a methylation-sensitive method represents a promising approach characterized by higher precision and technical simplicity to measure the methylation of target CpGs in CDH13 compared to other conventional methods such as MS-MLPA.
Similar content being viewed by others
Introduction
According to the latest official statistics, breast cancer (BC) now represents the most commonly diagnosed malignancy and the leading cause of cancer-related deaths in women worldwide1,2. Although mammography, the most widely used technique for BC screening, is an effective tool for early BC detection and significantly reduces the mortality of BC patients, its sensitivity and specificity remain dissatisfactory3. Therefore, early BC detection based on more sensitive and specific screening methods is still desirable, which could be reflected in the better prognosis and selection of less severe but effective treatment options3,4. Novel molecular biomarkers extensively analyzed by high-throughput omics-based approaches seem to be promising tools not only for improving the diagnosis and prognosis of BC but also for setting up effective targeted personalized therapy in BC patients in the near future.
BC is a very heterogeneous disease at both the molecular and clinical levels, associated with various genetic and epigenetic dysregulations. As epigenetic changes are reversible, they represent an attractive target for BC treatment. DNA methylation is the most extensively studied epigenetic modification that plays a crucial role in the regulation of gene expression. In the human genome, it occurs predominantly on cytosine-phosphate-guanine (CpG) dinucleotides found mostly at the 5´ promoter regions generally called CpG islands (CGIs) of about 70% of genes5,6,7,8. The DNA methylation of CGIs regulates gene expression through gene-specific repression of transcription9. Aberrant promoter methylation of tumor suppressor genes (TSG), which normally inhibits tumor cell progression, invasion, and metastasis, is a frequent and early event in the process of carcinogenesis10,11,12,13. A wide body of evidence from cancer research has demonstrated that hypermethylation of DNA CGIs, together with hypoacetylated and hypermethylated histones, represent three frequent epigenetic events responsible for the repression of TSG14,15,16. The results from preclinical and clinical BC studies indicate that aberrant DNA methylation patterns of multiple TSG correlate with tumor stage and size, positive lymph nodes, and premenopausal age at diagnosis, and therefore, might be used as a biomarker for diagnosis and therapeutic strategies in BC patients17,18,19. However, as BC is a very heterogeneous disease, methylation status and the type of TSG are inconsistent among different BC studies, and the proper frequency and utility of DNA methylation as a biomarker in BC patients has not yet been established. Further studies in this area are certainly necessary.
Aberrantly methylated genes in promoter regions of TSG represent frequent events in BC carcinogenesis20. Recent genome-wide studies revealed abnormal methylation state of key genes participating in the occurrence, development, and maintenance of cancer21. Accelerating the development of approaches based on sequencing or array technologies improved DNA methylation profiling, which allows a deeper understanding of the biological processes behind the methylation landscape22. On the other hand, these methods for DNA methylation profiling require expensive reagents, labor time, and bioinformatics analysis to summarize a large amount of data; thus, it is unlikely that these technologies will be implemented into routine clinical practice in the near future23. Semiquantitative methods such as MS-MLPA can overcome shortcomings associated with genome-wide DNA methylation analysis, mainly in cost and bioinformatics processing. MS-MLPA allows the simultaneous analysis of the DNA methylation state in several genes, but this technique is limited by its dependence on the presence of the HhaI restriction site24. Despite the mentioned limitations, MS-MLPA can be used as a laboratory assay for robust, reliable, and semiquantitative detection of DNA methylation in promoter regions of several TSGs.
The detection of methylation state of DNA in a single gene is a rapidly advancing field of molecular medicine, offering the potential to distinguish between different tumor types and predict their response to chemotherapeutic intervention regarding their specific methylation profile. Currently, several methods are available to determine the methylation status of DNA samples based on bisulfite modification, biological identification, or chemical cutting25. Selecting an appropriate method for determining the methylation profile of specific promoter regions of individual TSG with high specificity, sensitivity, and cost-effectiveness is critical for acquiring significant experimental data and subsequent clinical application26. Droplet digital PCR (ddPCR) is a relatively novel method that enables the precise detection and quantification of target DNA. This method allows distinguished changes in DNA with single-base resolution, and therefore, it represents a suitable approach to detecting differently methylated CpG in the promoter region of TSG27. Moreover, detecting methylated DNA derived from primary tumors is currently a challenge and requires a highly sensitive and specific assay28.
Based on the above-mentioned knowledge, the main aim of our research work was to identify the novel methylation biomarkers in tissue samples of Slovak patients with invasive ductal carcinoma (IDC) that would correlate with their clinicopathological characteristics.
Materials and methods
The study group consisted of 166 women diagnosed with IDC. The clinicopathological characteristics of the patients are summarized in Table 1. Histologically normal tissue adjacent to tumor from 10 patients was used as control samples. Formalin-fixed, paraffin-embedded (FFPE) tissue samples were provided by the Department of Pathological Anatomy, Jessenius Faculty of Medicine in Martin, Comenius University and University Hospital in Martin. The study was designed in line with the ethical principles of the Helsinki Declaration and approved by the Ethics Committee of Jessenius Faculty of Medicine (EK1822/2016). All the patients enrolled in the study signed informed consent.
DNA isolation and bisulfite modification
FFPE tissue samples were deparaffinized with xylene. The DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) was then used for DNA isolation. The obtained DNA concentration was measured with a fluorometer Qubit 3.0 (Thermo Fisher Scientific, Waltham, MA, USA) using a dsDNA BR Assay kit (Thermo Fisher Scientific). For methylation-specific MLPA (MS-MLPA) analysis, the isolated DNA was adjusted to 15 ng/µl and stored at -20 °C. For further methylation analysis utilizing droplet digital PCR (ddPCR), one µg of isolated DNA was modified with an EpiTect Bisulfite kit (Qiagen, Hilden, Germany) following the manufacturer´s instructions.
Methylation-specific MLPA
MS-MLPA analysis was employed to detect methylation levels of 25 tumor suppressor genes in SALSA MLPA ME002 Tumour suppressor mix2 (MRC Holland, Amsterdam, The Netherlands). Restriction endonuclease HhaI (Promega, Southampton, UK) was used to cleave the samples. The probe mix was composed of 41 probes, 27 containing the cleavage sites for methylation detection. Samples were prepared and analyzed according to the kit manufacturer's instructions. The individual reaction steps were carried out on Sure Cycler 8800 thermocycler (Agilent Technologies, Santa Clara, CA, USA). PCR amplifications were followed by fragment analysis on the ABI 3500 genetic analyzer (Thermo Fisher Scientific). The results were analyzed using Coffalyser.Net software (MRC Holland). The individual baseline methylation level was calculated for each methylation-specific probe as suggested by the manufacturer, by taking the mean methylation of control samples per probe and adding two times the standard deviation. The calculated value was considered the cut-off for determining the methylation status of each sample.
Methylation analysis of CDH13 gene
Increased methylation in the CDH13 gene detected by MS-MLPA was further investigated using ddPCR analysis. Primers and probes were designed using the MethPrimer31 and Primer3Plus32 online programs. However, it was unfeasible to achieve precise alignment with the specific CpG site detected by the MS-MLPA probe (chr16:82,627,075 in hg38 assembly). Instead, the designed probes targeted three nearby CpG sites (chr16:82,626,843; chr16:82,626,845; chr16:82,626,859) in the CDH13 promotor region. The similarity of methylation patterns between the CpG site detected by the MS-MLPA probe and the CpG sites targeted by our probes was evaluated using The Cancer Genome Atlas data. The methylation array in the Breast Cancer project covered two of our CpG sites (chr16:82,626,845 and chr16:82,626,859) and a CpG site 10 bp downstream from the MS-MLPA probe (hg38; chr16:82,627,065). The methylation pattern of those CpG sites in 295 IDC samples was very similar. The designed primers and probes sequences are presented in Table 2.
The ddPCR assay was optimized for simultaneous detection of methylated and unmethylated sequences using a probe targeting the methylated DNA (M-Probe, FAM labeled) and a probe targeting the unmethylated DNA (UnM-Probe, HEX labeled) in one reaction mix. Duplex ddPCR mixture contained 10 μL of Supermix for Probes (No dUTP) (Bio-Rad Laboratories, Hercules, CA, USA), 0.45 μL of each primer, 0.45 μL of each probe, 2.5 μL of bisulfite converted DNA adjusted with 6.1 μL of water up to final volume of 20 μL. For control purposes, commercially available fully methylated and unmethylated EpiTect DNA controls (Qiagen, Hilden, Germany) were used, along with ultrapure water, to ascertain the absence of reagents contamination. The entire reaction volume, along with 70 μL of Droplet Generation Oil for Probes, was loaded into a DG8 cartridge and inserted into the QX200 Droplet Generator (Bio-Rad Laboratories) to generate approximately 20,000 droplets per sample. The resulting droplet emulsion (40 μL) was transferred into a 96-well PCR plate, covered with a pierceable foil, and heat-sealed using Bio-Rad's PX1 system. Subsequent PCRs were carried out in the T100 thermal cycler (Bio-Rad Laboratories). Optimized PCR protocol was initialized with denaturation (95 °C for 10 min), followed by 40 cycles of denaturation (94 °C for 30 s) and annealing/extension (57 °C during for 1 min) with ramp rate decreased to 1 °C/s. The enzyme was deactivated at 98 °C for 10 min, and the product was held at 12 °C overnight according to the recommendations by Rowlands et al.33. Following the amplification step, the plate was loaded onto the QX200 Droplet Reader (Bio-Rad Laboratories) for droplet analysis of each individual well.
The QuantaSoft v.1.7 Software (Bio-Rad Laboratories) was utilized to analyze and categorize each droplet based on fluorescence emission detected in HEX or FAM channels. The droplets were sorted into four clusters 1) methylated DNA with FAM signal, 2) unmethylated DNA with HEX signal, 3) DNA with both signals, and 4) empty droplets lacking the target DNA. The threshold amplitudes were determined for FAM and HEX signals at values 2600 and 1745, respectively. The correlation coefficient (R2) was calculated by performing serial dilutions of methylated and unmethylated EpiTect DNA controls in water, with 8000, 4000, 2000, 1000, 500, 250, 125, and 62 copies per reaction (Fig. 1). For each dilution specificity and sensitivity was calculated: specificity as copies of the unmethylated control DNA detected by the UnM-Probe divided by all copies of the unmethylated control DNA (detected by both probes); sensitivity as the methylated control DNA detected with the M-Probe divided by all copies of the methylated control DNA. As no copies of unmethylated control DNA were detected by the M-Probe, the specificity reached 100%. The average sensitivity across all dilutions was 96.5%.
The level of methylation was expressed as a percentage, representing the ratio of methylated sequences to the sum of methylated and unmethylated sequences.
Statistical analysis
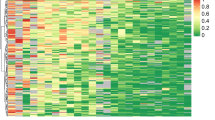
Statistical analysis was performed for 135 cases with available clinicopathological data that underwent analysis by ddPCR. The Shapiro–Wilk test was used to check the normality assumption. The association between CDH13 methylation levels and clinicopathological features, such as ER, PR, and HER2 status, was analyzed using a Mann–Whitney test. The Kruskal–Wallis one-way ANOVA was used to evaluate the association between CDH13 methylation levels and grade, tumor status, nodal status, molecular classification, and age. Associations between clinicopathologic features and the number of samples with detected methylation (cut-off 10%) were analyzed using Fisher´s exact test. A value of P < 0.05 was considered as statistically significant. All statistical analyses were performed in GraphPad Prism 8.1.1 (GraphPad, San Diego, CA, USA). Additionally, a heatmap was constructed to visualize the results obtained from MS-MLPA analysis.
Results
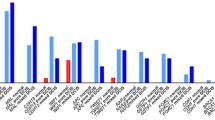
In the first part of our study, we performed MS-MLPA analysis to obtain an overview of changes in promotor methylation of 25 tumor suppressor genes. Using calculated baseline methylation level for each tumor suppressor gene, we observed an increase of methylation in 13 genes (TP73, MSH6, RARB, PAX5, KLLN, MGMT, PAX6, WT1, GSTP1, CADM1, THBS1, CDH13, and GATA5). Significantly increased methylation was most often present in CDH13 (67.5% of analyzed samples), MSH6 (65.1%), KLLN (61.5%), PAX5 (44.0%), THBS1 (43.4%), and WT1 (31.9%). The rest of the examined genes (VHL, ESR1, CDKN2A, CD44, ATM, CHFR, BRCA2, RB1, PYCARD, TP53, BRCA1 and GATA5) did not exhibit any change in methylation or methylation was increased in less than 10% of analyzed samples. The methylation levels of all 25 tumor suppressor genes detected by MS-MLPA are depicted in Fig. 2. We selected CDH13 as the most frequently methylated gene for further analysis and result validation in the next part of our study.
Using ddPCR, we successfully analyzed 135 samples. We were unable to analyze the whole set of 166 samples due to the insufficient amount of some tissue samples or the necessity to exclude the bisulfite-converted DNA of inadequate quality. The sample was considered methylated if methylation level exceeded 10%. We detected methylation in 25.2% of BC samples with an average methylation of level 31.0%. An example of data output from ddPCR analysis is presented in Fig. 3. In the same sample set, MS-MLPA showed methylation in 67.4% of tumor samples with average methylation level 30.9%. The comparison of methylation levels detected in individual samples is depicted in Fig. 4.
Representative plots of ddPCR results. Two-fold dilution series, diluted from 8000 to 62 copies per reaction, of methylated (top left) and unmethylated control DNA (top right). Two-dimensional plot corresponding to methylated sample (bottom). Blue dots represent a number of DNA copies considered methylated; green dots represent unmethylated copies of DNA. The resulting level of sample methylation is calculated as the ratio between methylated copies and all DNA copies detected by both channels. The purple lines define the threshold amplitudes.
In subsequent statistical analysis, we investigated the correlation between clinicopathological characteristics and methylation detected by ddPCR in the promoter region of the CDH13 gene. Observed methylation was evaluated as methylation levels of all individual samples as well as a number of samples with detected methylation using the cut-off of 10%. A significant difference in CDH13 methylation was observed between BC molecular subtypes LUM A vs. HER2 (average methylation level 6.54% vs. 17.3%, P = 0.0116; 17.0% of methylated samples vs. 43.3%, P = 0.0183) and HER2 vs. TNBC (average methylation level 17.3% vs. 7.09%, P = 0.0234). Although, the percentage of samples with detected methylation in the HER2 subgroup was 43.3. % vs. 20.9% in the TNBC subgroup, the difference was not considered statistically significant (P = 0.0683). The statistically significant differences were also revealed when tumors were compared according to their ER, PR, and HER2 status. Significantly higher methylation was detected in HER2-positive vs. HER2-negative tumors (17.0% vs. 6.79%, P = 0.0004; 41.0% vs. 18.8%, P = 0.0092) and PR-negative vs. PR-positive tumors (11.9% vs. 6.29%, P = 0.0421; 31.3% vs. 15.4%, P = 0.0431). Although higher methylation was observed in grade 3 tumors, the difference was not considered statistically significant. A significant difference was also observed between the numbers of methylated samples with pT1 and pT2 tumor status (18.3% vs. 37.5%, P = 0.0322); however, the difference between the average methylation levels did not prove statistically significant (8.07% vs. 12.9%, P = 0.3390). No significant correlation was observed between CDH13 methylation and the rest of the recorded clinicopathological characteristics (, nodal status, age). Differences between CDH13 methylation levels in individual clinicopathological subgroups are presented in Fig. 5. Table 3 summarizes differences according to the number of methylated samples in each group.
Discussion
BC is a heterogeneous disease that develops due to the accumulation of genetic and epigenetic changes in cells34. DNA methylation, as one of the epigenetic mechanisms, is strongly associated with malignant transformation. Biologically, the specific methylation pattern in individual genes can act as an initial event of carcinogenesis or play an essential role in the promotion and progression of BC35. Methylation of the CpG islands of the gene promoter regions is a highly dynamic process linked to gene expression and regulation that potentially fluctuates over time36.
In the current study, we determined the methylation status of the promoter region of 25 TSGs, focusing on CDH13 and its association with clinicopathological characteristics in the cohort of Slovak patients with IDC. To evaluate the hyper-methylation of CDH13, we used semiquantitative MS-MLPA as a prescreening method and quantitative ddPCR to confirm the obtained results. Here, we move toward the possible clinical application of CDH13 promoter methylation by 1) determining the appropriate methods for methylation detection and 2) evaluating clinical association with clinicopathological features of BC to determine the impact of methylation on clinical outcome.
The choice of appropriate high throughput methods for DNA methylation assessment with single-base resolution plays an essential step in identifying methylation patterns associated with clinical outcomes. In the present study, we used two diagnostic approaches to detect differently methylated CpGs in the promoter region of CDH13. The MS-MLPA techniques using methylation-sensitive digestion represent an appropriate molecular approach to identify methylation changes in multiple TSG, providing semiquantitative data without bisulfite conversion required for other detection methods such as methylation-specific PCR or quantitative methylation-specific PCR26. On the contrary, there are some limitations of MS-MLPA. Each probe can recognize only one specific CpG site; thus, the methylation of another or nearby CpG island cannot be included. To improve the accuracy of target-DNA detection and measurement, we used methylation-specific ddPCR. We developed and optimized a unique ddPCR assay for CDH13 to evaluate the methylation status of the gene promoter region. This approach overcomes the limitation of MS-MLPA and enables the absolute quantification of methylated DNA in FFPE samples37.
MS-MLPA analysis has been used for over a decade to detect epigenetic alterations in the genes associated with cancer development38. In the present study, we used an MS-MLPA probe set to evaluate the methylation status of TSG in patients with IDC. We identified an increased methylation frequency in 6 genes, including MSH6, PAX5, WT1, KLLN, THBS6, and CDH13, in the BC group compared to the control samples. It has been shown that methylation of these genes may serve as a potential marker for the diagnosis and prognosis of BC39,40,41. Marzese et al. documented frequent WT1 promoter methylation in a cohort of patients with IDC42. Furthermore, in a retrospective study by Moelans et al., the authors confirmed a higher MSH6, CDH13, WT1, and PAX5 methylation rate in archival samples obtained from BC patients43.
Results acquired from MS-MLPA revealed the most prevalent methylation in the CDH13 gene. CDH13 (also known as T-cadherin or H-cadherin), localized to chromosome 16q24, is a unique transmembrane glycoprotein belonging to the cadherin superfamily, characterized by being devoid of a transmembrane domain40. Cadherins, including CDH13, play an essential role in cell–cell adhesion, and inactivation of this cadherin-mediated cell adhesion due to cadherin downregulation is associated with cancer progression44. Abnormal CDH13 methylation is frequently observed in different types of tumors, including BC45,46,47,48. Re-expression of CDH13 is associated with the suppression of invasiveness, tumor growth, and neovascularization in BC in vitro40. For this reason, effective screening and early, accurate detection of aberrantly methylated CDH13 promoter region could be used as a promising diagnostic and prognostic marker for various malignancies, including breast cancer. To verify the correct performance of MS-MLPA analysis, we developed an in-house methylation-specific ddPCR assay for CDH13. We confirmed increased levels of CpG methylation in the target site of the promoter region in tumor samples. As expected, CDH13 methylation was absent in tumor-adjacent (morphologically normal) tissue, and none of our control samples showed any methylation (cut-off 10%).
Subsequently, we investigated the association between CDH13 methylation levels and molecular classification and clinicopathological characteristics of BC. We detected higher methylation levels in tumors classified as HER2 compared to LUM A (P = 0.0116) and TNBC (P = 0.0234). These data are consistent with a previously published study, stating that CDH13 methylation is significantly higher in HER2 subtypes than in LUM A and TNBC49. Our study showed that higher methylated CDH13 was associated with immunohistochemically verified HER2-positive status (P = 0.0004). These data are in agreement with previous studies, which reported increased methylation of CDH13 in a cohort of HER2-positive BC patients50. Another study by Pang et al. identified CDH13 gene hypermethylation associated with HER2-amplification among patients with ductal carcinoma in situ (DCIS)51. Taken together, our results indicate that CDH13 methylation may play a significant role in different BC phenotypes.
Furthermore, methylation-sensitive ddPCR analysis revealed higher CDH13 methylation levels in patients with PR-negative status than those with PR-positive tumors (P = 0.0421). Consistent with existing literature, our findings corroborated the link between increased CDH13 methylation and PR-positive status51,52. In general, PR-negative status has been shown to have a worse prognosis and survival outcomes compared to PR-positive patients with BC53.
In conclusion, we showed that increased CDH13 methylation is significantly associated with the aggressive phenotype of BC with the potential to serve as a diagnostic and prognostic biomarker. Identifying a specific methylation pattern in the DNA promoter region of targeted genes could enhance the ability to distinguish BC molecular subtypes when combined with routine immunohistochemistry staining, thus enhancing specificity and sensitivity in distinguishing aggressive from indolent BC. CDH13 serves as an important tumor suppressor responsible for cell–cell adhesion, often deactivated by abnormal promoter hypermethylation during carcinogenesis. Therefore, selecting this cell adhesion-related gene to assess breast cancer prognosis is a logical approach for future clinical implementation.
Precise and accurate measurements of breast cancer-associated biomarkers require developing novel diagnostic methods based on analysis of DNA methylation status. Using ddPCR represents a promising approach for confirming data obtained from semiquantitative analysis such as MS-MLPA, and thus, it could be helpful in the management of Slovak patients with BC.
Data availability
All data analyzed during this study are included in this published article. The generated datasets are available from the corresponding author upon reasonable request.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Luo, C. et al. Advances in breast cancer screening modalities and status of global screening programs. Chronic Dis. Transl. Med. 8, 112–123 (2022).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541 (2022).
Kim, M. & Costello, J. DNA methylation: An epigenetic mark of cellular memory. Exp. Mol. Med. 49, e322–e322 (2017).
Babenko, V. N., Chadaeva, I. V. & Orlov, Y. L. Genomic landscape of CpG rich elements in human. BMC Evolut. Biol. 17, 19 (2017).
Lim, W.-J., Kim, K. H., Kim, J.-Y., Jeong, S. & Kim, N. Identification of DNA-methylated CpG islands associated with gene silencing in the adult body tissues of the ogye chicken using RNA-Seq and reduced representation bisulfite sequencing. Front. Genet. 10, 346 (2019).
Lu, Y. et al. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer 19, 79 (2020).
Curradi, M., Izzo, A., Badaracco, G. & Landsberger, N. Molecular mechanisms of gene silencing mediated by DNA methylation. Mol. Cell Biol. 22, 3157–3173 (2002).
Cooper, G. M. The Cell. (Sinauer Associates, ISBN: 978-0-87893-106-4, 2000).
Widschwendter, M. & Jones, P. A. DNA methylation and breast carcinogenesis. Oncogene 21, 5462–5482 (2002).
Fackler, M. J. et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 64, 4442–4452 (2004).
Sherr, C. J. Principles of tumor suppression. Cell 116, 235–246 (2004).
Esteller, M. & Herman, J. G. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J. Pathol. 196, 1–7 (2002).
Cheng, Y. et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Sig. Transduct. Target Ther. 4, 1–39 (2019).
Al Aboud, N. M., Tupper, C. & Jialal, I. Genetics, Epigenetic Mechanism. in StatPearls (StatPearls Publishing, Treasure Island (FL), 2023).
Christensen, B. C. et al. Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genet. 6, e1001043 (2010).
Callahan, C. L. et al. DNA methylation and breast tumor clinicopathological features: The Western New York Exposures and Breast Cancer (WEB) study. Epigenetics 11, 643–652 (2016).
de Almeida, B. P., Apolónio, J. D., Binnie, A. & Castelo-Branco, P. Roadmap of DNA methylation in breast cancer identifies novel prognostic biomarkers. BMC Cancer 19, 219 (2019).
Kristiansen, S., Jørgensen, L. M., Guldberg, P. & Sölétormos, G. Aberrantly methylated DNA as a biomarker in breast cancer. Int. J. Biol. Mark. 28, 141–150 (2013).
Shi, Y., Gong, W., Gong, X., Wang, P. & Zhao, X. Genome-wide DNA methylation analysis of breast cancer MCF-7/taxol cells with MeDIP-Seq. PLoS ONE 15, e0241515 (2020).
Yong, W.-S., Hsu, F.-M. & Chen, P.-Y. Profiling genome-wide DNA methylation. Epigenet. Chromatin 9, 26 (2016).
Foley, J. W., Zhu, S. X. & West, R. B. Cost-effective DNA methylation profiling by FML-seq. bioRxiv 2023.01.13.523849 (2023) https://doi.org/10.1101/2023.01.13.523849.
Christians, A. et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS ONE 7, e33449 (2012).
Khodadadi, E. et al. Current advances in DNA methylation analysis methods. Biomed. Res. Int. 2021, 8827516 (2021).
Šestáková, Š, Šálek, C. & Remešová, H. DNA methylation validation methods: A coherent review with practical comparison. Biol. Proc. Online 21, 19 (2019).
Arroyo, K. et al. Development of a Droplet Digital™ PCR DNA methylation detection and quantification assay of prenatal tobacco exposure. BioTechniques 72, 121–133 (2022).
Venetis, K. et al. Liquid biopsy: Cell-free DNA based analysis in breast cancer. J. Liq. Biopsy 1, 100002 (2023).
Palacín-Aliana, I. et al. Clinical utility of liquid biopsy-based actionable mutations detected via ddPCR. Biomedicines 9, 906 (2021).
Izquierdo, E. et al. Droplet digital PCR-based detection of circulating tumor DNA from pediatric high grade and diffuse midline glioma patients. Neurooncol. Adv. 3, vdab013 (2021).
Li, L.-C. & Dahiya, R. MethPrimer: Designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 (2002).
Untergasser, A. et al. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 35, W71-74 (2007).
Rowlands, V. et al. Optimisation of robust singleplex and multiplex droplet digital PCR assays for high confidence mutation detection in circulating tumour DNA. Sci. Rep. 9, 12620 (2019).
Takeshima, H. & Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. npj Precis. Onc. 3, 1–8 (2019).
Pouliot, M.-C., Labrie, Y., Diorio, C. & Durocher, F. The role of methylation in breast cancer susceptibility and treatment. Anticancer Res 35, 4569–4574 (2015).
Ciccarone, F., Tagliatesta, S., Caiafa, P. & Zampieri, M. DNA methylation dynamics in aging: How far are we from understanding the mechanisms?. Mech. Ageing Dev. 174, 3–17 (2018).
Yu, M., Willbanks, A. & Grady, W. M. Chapter Four - Methylation-specific droplet digital PCR (MS-ddPCR) for detection and absolute quantification of rare methylated alleles☆. in Epigenetics Methods (ed. Tollefsbol, T.) vol. 18 63–79 (Academic Press, 2020).
Stuppia, L., Antonucci, I., Palka, G. & Gatta, V. Use of the MLPA assay in the molecular diagnosis of gene copy number alterations in human genetic diseases. Int. J. Mol. Sci. 13, 3245–3276 (2012).
Li, X. et al. Paired box 5 is a novel marker of breast cancers that is frequently downregulated by methylation. Int. J. Biol. Sci. 14, 1686–1695 (2018).
Yang, J., Niu, H., Huang, Y. & Yang, K. A systematic analysis of the relationship of CDH13 promoter methylation and breast cancer risk and prognosis. PLoS ONE 11, e0149185 (2016).
Xu, J. et al. Methylation of HIN-1, RASSF1A, RIL and CDH13 in breast cancer is associated with clinical characteristics, but only RASSF1A methylation is associated with outcome. BMC Cancer 12, 243 (2012).
Marzese, D. M. et al. DNA methylation index and methylation profile of invasive ductal breast tumors. J. Mol. Diagn. 14, 613–622 (2012).
Moelans, C. B., Verschuur-Maes, A. H. & van Diest, P. J. Frequent promoter hypermethylation of BRCA2, CDH13, MSH6, PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast cancer. J. Pathol. 225, 222–231 (2011).
Qian, Z. R. et al. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod. Pathol. 20, 1269–1277 (2007).
Shiu, B.-H. et al. Interactive association between dietary fat and sex on CDH13 cg02263260 methylation. BMC Med. Genom. 14, 13 (2021).
Tuoya, A.-D. et al. Relationship between methylation of FHIT and CDH13 gene promoter region and liver cancer. Curr. Med. Sci. 40, 502–509 (2020).
Abudukadeer, A. et al. Clinical relevance of CDH1 and CDH13 DNA-methylation in serum of cervical cancer patients. Int. J. Mol. Sci. 13, 8353–8363 (2012).
Guo, Q. et al. Correlations of promoter methylation in WIF-1, RASSF1A, and CDH13 genes with the risk and prognosis of esophageal cancer. Med. Sci. Monit. 22, 2816–2824 (2016).
Jeong, Y. J., Jeong, H. Y., Bong, J. G., Park, S. H. & Oh, H. K. Low methylation levels of the SFRP1 gene are associated with the basal-like subtype of breast cancer. Oncol. Rep. 29, 1946–1954 (2013).
Fiegl, H. et al. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: Association with HER-2/neu status in primary breast cancer. Cancer Res. 66, 29–33 (2006).
Pang, J.-M.B. et al. Methylation profiling of ductal carcinoma in situ and its relationship to histopathological features. Breast Cancer Res. 16, 423 (2014).
Feng, W. et al. Correlation between CpG methylation profiles and hormone receptor status in breast cancers. Breast Cancer Res. 9, R57 (2007).
Ma, S. J. et al. Association of progesterone receptor status with 21-gene recurrence score and survival among patients with estrogen receptor-positive breast cancer. BMC Cancer 23, 330 (2023).
Acknowledgements
The authors wish to thank Zora Lasabova and the Department of molecular biology and genomics at Jessenius Faculty of Medicine in Martin, Comenius University for technical support.
Funding
This work was supported by the Slovak Research and Development Agency (grant number APVV-16–0021) and the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences (grant number VEGA 1/0286/22).
Author information
Authors and Affiliations
Contributions
IB, MS, DD, and AK: writing original draft; IB, MS, DD, and EL: conducting experiments, statistical analysis, review and editing of manuscript; IS, KJ, AH and IK: formal analysis, visualisation; ZD, KB, and EH: conceptualisation, editing and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baranová, I., Samec, M., Dvorská, D. et al. Droplet digital PCR analysis of CDH13 methylation status in Slovak women with invasive ductal breast cancer. Sci Rep 14, 14700 (2024). https://doi.org/10.1038/s41598-024-65580-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65580-6
- Springer Nature Limited