Abstract
Over the years, obesity has become more commonplace and has had a substantial impact on several medical specialties, including reproductive medicine. The potential correlation between the visceral adiposity index (VAI) and infertility has yet to be determined. Women between the ages of 18 and 45 were included in this cross-sectional study, which was conducted as part of the National Health and Nutrition Examination Survey (NHANES) between 2015 and 2020. Three tertiles were used to group VAI levels. Subgroup analysis and weighted binary logistic regression were employed to investigate the independent relationship between VAI and infertility. Smooth curve fitting was used to explore nonlinear relationships. This cross-sectional study followed the criteria of the STROBE guidelines. Of the 1231 participants, 127 were infertile women aged 18–45 years. A higher VAI was associated with a higher prevalence of infertility (OR = 1.22, 95% CI:1.03–1.45), which remained consistent across all subgroups (p > 0.05 for all interactions). We demonstrated a positive nonlinear association between VAI and infertility using a smooth curve fit. A higher visceral adiposity index level is positively correlated with a higher incidence of infertility among women in the United States. Women who are infertile can be identified using the visceral obesity index, and controlling visceral obesity may help lower the chances of becoming infertile.
Similar content being viewed by others
Introduction
Infertility is a medical condition usually defined as the failure to conceive after 12 months of regular sexual intercourse1. About 7 to 15.5% of women in the US who are of reproductive age have infertility, and 8 out of 12 couples struggle with conception2. Infertility affects a sizable percentage of people worldwide (9.0%), in rich countries (3.5–16.6%), and in developing countries (6.9–9.3%)3. Even though infertility has gained international attention recently as a public health concern, the factors that contribute to it still need to be further investigated.
One prevalent issue among women who are fertile is obesity. It is generally regarded as an excessive build-up of body fat that has a detrimental impact on one’s health4,5. It is predicted that by 2025, more than 21% of women worldwide will be obese. This tendency may be linked to both rapid changes in lifestyle and economic development6. More gynecological disorders in women, such as excessive menstruation7, endometriosis and uterine fibroids (UF)8,9, polycystic ovary syndrome (PCOS)10,11, pregnancy complications like pre-eclampsia and eclampsia12, miscarriage13 and infertility14,15, are linked to higher body mass indices (BMI). Despite being a conventional and cost-effective approach, using body mass index (BMI) to evaluate obesity (defined as a BMI exceeding 25 kg/m2) lacks the ability to differentiate between lean and fat body mass16,17. Because BMI does not provide a good picture of obesity distribution, it is not appropriate to use it alone to assess obesity18. Waist circumference measures central obesity, but it may not adequately reflect the health hazards associated with abdominal obesity since it cannot distinguish between visceral and subcutaneous adipose tissue in the abdomen. A recently proposed mathematical model with a scientific design, the visceral obesity index (VAI) evaluates the quantity and function of visceral fat in a person’s body19. Increased visceral fat has been linked in studies to a number of diseases, including metabolic syndrome, diabetes, cardiovascular disease, and several forms of cancer20. Anthropometric information (waist circumference, BMI) and metabolic markers (triglycerides and HDL cholesterol) are combined in the VAI assessment approach. In essence, VAI offers a comprehensive evaluation of a person's visceral fat status and could be a helpful substitute for visceral CT scans due to its lower radiation risks and cost21.
This study investigated the connection between infertility and the visceral obesity index in American women between the ages of 18 and 45. The National Health and Nutrition Examination Survey (NHANES) will provide the data for the study. The goal is to shed light on the complex relationship between visceral fat and infertility in order to aid in the clinical development of treatments aimed at reducing the risk of infertility.
Materials and methods
Data source
The National Center for Health Statistics (NCHS) is the publishing organization for the National Health and Nutrition Examination Survey (NHANES), a national survey that evaluates Americans’ health and nutrition. To ensure that the sample was representative, a complicated, multistage probability design was used to perform the NHANES22. To gather information on the participants’ socioeconomic situation, health, and other aspects, a household interview was conducted. Both laboratory and physical examinations were conducted in a mobile examination center. The NCHS study Ethics Review Board authorized all NHANES study procedures, and all survey participants gave written informed consent. The public can access all the information regarding the NHANES study design and data at www.cdc.gov/nchs/nhanes/. This cross-sectional study followed the STROBE reporting standards23.
Study population
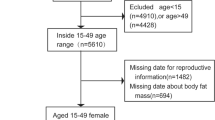
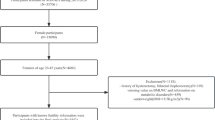
Data from the National Health and Nutrition Examination Survey (NHANES) covering the years 2015–2020 was utilized. We included in our research participants who provided comprehensive information on their visceral obesity index (VAI) and infertility. A total of 25,531 people were initially enrolled. After excluding male participants (n = 12,613), individuals without information on waist circumference (n = 143), triglycerides (n = 9141), BMI (n = 50), individuals without information on infertility (n = 1580), and female participants over 45 and under 18 (n = 773), our final analysis comprised 1231 eligible participants (Fig. 1).
Calculation of VAI
Amato et al. developed the gender-specific visceral obesity index (VAI) to account for physiological variations in visceral obesity between men and women24. VAI is a measure of anthropometric and metabolic characteristics, such as high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), waist circumference (WC), and body mass index (BMI). It is thought to be a sign of malfunction and buildup of visceral adipose tissue.
The VAI for each participant was calculated by using the following formulas. For males: VAI = WC/(39.68 + (1.88*BMI))*(TG/1.03)*(1.31/HDL-C); For females: VAI = WC/(36.58 + (1.89*BMI))*(TG/0.81)*(1.52/HDL-C). TG and HDL-C were calculated in mmol/L, and WC was calculated in cm in the formulas.
Assessment of infertility
The dependent variable for infertility was each woman’s self-report from the Reproductive Health Questionnaire (questionnaire variable name: RHQ074). Researchers prodded participants with questions like, “Have you tried to get pregnant for one year?”25. A “infertile” situation was indicated if the response was “yes,” and a “fertile” situation was indicated if it was no.
Covariates
Based on similar literature and practice26,27,28, factors included age, ethnicity, education level, marital status, poverty income ratio (PIR), diabetes, hypertension, alcohol and smoking patterns, waist circumference, BMI, triglycerides, and HDL cholesterol. There were five racial and ethnic groupings among the participants. Mexican Americans, Blacks, Whites, non-Hispanics, Hispanics, and Others (including Multiracial). Waist circumference, body mass index, triglycerides, HDL cholesterol, age in years, and poverty income ratio (PIR) were among the continuous variables that were measured. The definitions of the three degrees of education were as follows: high school graduate/GED or equivalent, above high school, and below high school29. The NHANES maintained its classification of marital status into five categories: cohabiting, single, widowed, divorced, married, and unmarried. Diabetes was described as a diagnosed illness30. Additionally, the measure of hypertension was self-reported31. Those who never smoked (less than 100 cigarettes in their lifetime), those who smoked in the past (at least 100 cigarettes in their lifetime, smokers, and no smokers at all), and those who now smoke (more than 100 cigarettes in their lifetime or smoked daily) were the three categories depending on their smoking status. A person's drinking status was ascertained by asking if they had more than 12 drinks in a year. Those who answered "yes" were deemed to be drinkers, while those who answered “no” were not.
Statistical analysis
All statistical analyses were performed with consideration for the intricate, multistage clustered surveys and with the appropriate NHANES sampling weights, following the recommendations of the Centers for Disease Control and Prevention.
In descriptive analyses, a weighted Student’s t-test (for continuous variables) or a weighted Chi-square test (for categorical data) was used to evaluate the two comparison groups based on their infertility status. For continuous data, proportions were utilized to represent categorical parameters, while averages and standard deviations were employed to describe them. Multivariate regression models using the NHANES complex sample design (sampling weights) were used to examine the link between VAI and infertility. In Model 1, covariates were left unchanged. Model 2 adjusted for age and race. Model 3 took into consideration the following variables: age, race, education level, marital status, number of cigarettes smoked in the past 100 days, number of beverages consumed annually, diabetes (yes/no), and hypertension (yes/no). We used smoothed curve fitting in addition to subgroup analyses to look at the nonlinear relationship between VAI and infertility.
We used R (http://www.r-project.org) and Empower software (http://www.empowerstats.com) for all statistical analyses, following the Centers for Disease Control and Prevention’s (CDC) instructions. The statistical significance criterion was established at p < 0.05.
Results
Baseline characteristics of study participants
Of the 1,231 participants, 127 were infertile women between the ages of 18 and 45, and 116 of the infertile women had regular menstruation over a 12 month period. The range of VAI for tertiles 1–3 were 0.1–0.65(≤ 0.65),0.65–1.75(≤ 1.75),and 1.75–19.69(≤ 19.69).Table 1 displays the characteristics of the study participants, categorized based on whether they were infertile or not. Women who were older, married or living together, drank alcohol, had a higher body mass index, and had a larger waist circumference were more likely to self-report being infertile. Furthermore, women with a higher VAI also had a higher prevalence of self-reported infertility, with a mean of 1.72 ± 1.91.
Relationship between visceral obesity index and infertility
The correlation between VAI and infertility is shown in Table 2. Our results imply that a higher risk of infertility is linked to a higher VAI. There was a positive correlation between VAI and infertility in Models 1, 2, and 3. According to the fully adjusted model (Model 3: OR = 1.22, 95% CI:1.03–1.45), there was a 22% increase in the probability of being infertile for every unit increase in VAI. The statistical significance of this link persisted even after dividing VAI into thirds. Those in the highest VAI tertile were at a 252% higher risk than those in the lowest VAI tertile (OR = 3.52, 95% CI:1.18–10.49; p = 0.02) (Table 2). Additionally, we used smoothed curve fitting to further examine the relationship between VAI and the risk of infertility, and the results indicated a positive nonlinear relationship (Fig. 2).
Subgroup analysis
We performed subgroup analyses to determine whether other factors altered the association between VAI and infertility. The results indicated that there was no dependence and that the subgroup between VAI and infertility was stable. The positive correlation between VAI and infertility was not significantly impacted by stratified factors such as age, ethnicity, education level, marital status, smoking at least 100 cigarettes, drinking alcohol at least 12 times a year, diabetes, and hypertension (p > 0.05), as shown in Table 3.
Discussion
We recruited 1231 female patients for this cross-sectional study, and the results showed a positive correlation between VAI and infertility, independent of age, race, education level, marital status, smoking, alcohol intake, diabetes, or hypertension. According to the findings of the investigation, lowering VAI levels may help decrease the likelihood of infertility.
This is the only study that we are aware of that assesses the correlation between VAI and female infertility. Obese women often experience irregular menstruation with poor ovulation, endometriosis, and infertility, which highlights the detrimental consequences of obesity on reproduction32,33. Numerous studies have demonstrated that obesity not only deteriorates metabolic status but also causes ovulatory dysfunction, increasing the incidence of infertility in obese women by three times compared to non-obese women34. Especially two investigations including sizable cohorts of Danish women who were considering becoming pregnant revealed an unfavorable relationship between higher BMI and fertility25. Notably, obese women still have low fertility even in the absence of ovulatory dysfunction. Gesink and colleagues examined a large American cohort of over 7000 women and discovered that the probability of spontaneous conception declined linearly with BMI > 29 kg/m2. Similar findings were found in a comparable study that involved over 3000 women in the Netherlands who had regular menstrual cycles35. Furthermore, it seems that participation in assisted conception programs reduces the fertility of obese women36. In fact, poor oocyte quality and reduced preimplantation have been linked to poor results in individuals undergoing in vitro fertilization (IVF) who are overweight or obese37. Losing weight is therefore highly advised in these women in order to enhance reproductive function38. Our latest research supports and validates the detrimental effect of visceral fat on infertility in women.
There is a connection between declining metabolism and visceral adiposity. In Yu Kang et al.’s study, patients who were metabolically unwell and obese (MUO) had a considerably greater VAI than patients who were healthy and obese (MHO). Furthermore, VAI and the frequency of conversion to the MUO phenotype correlated positively39. Insulin sensitivity did not correlate with waist circumference or BMI in a study by Amato et al., but there was a positive connection between VAI and cardiometabolic risk as well as visceral adipose tissue measured by magnetic resonance imaging19. An elevated VAI was linked to an increased cardiometabolic risk in a research involving 1764 hospitalized patients24. Studies have shown that TG/HDL-C is a strong predictor of metabolic syndrome and insulin resistance, playing a crucial role in the development of infertility40. The development of infertility and metabolic disorders is complex and diverse, and lipid metabolism disorders may play a key role in follicular development, egg maturation, and hormone secretion41. A large number of animal studies have confirmed that dyslipidemia can lead to a decrease in female reproductive capacity42. Therefore, VAI is able to indicate abnormal metabolic status in the body, thereby predicting infertility risk and serving as a basis for health promotion.
VAI has been shown to be a predictor of clinical severity and treatment outcome in patients with polycystic ovary syndrome21. The association between VAI and infertility is not well understood, but our findings suggest that increased VAI is associated with an increased risk of potential infertility, primarily because of neuroendocrine mechanisms that interfere with ovarian function and can affect ovulation rates and endometrial tolerance33. The circulating levels of gonadotropins, estradiol, and estradiol during the follicular phase are lower in obese women, even with normal menstrual cycles and apparently normal fertility. This suggests that the obesity condition itself has an inhibitory influence on the production of these hormones43. All systems involved in oocyte differentiation and maturation (including hormones, proteins, and soluble substances secreted by adipocytes) are dysregulated and impacted in their physiology because obesity is pathologically related to inflammation44. The reduction of women's fertility potential due to adipose tissue is therefore directly caused by malfunctioning of the primary molecular mechanisms that control the normal biological activity of the cellular components of their reproductive organs, which are also regulated by the hypothalamic-pituitary-ovarian axis11.
There are various benefits to our study. Initially, the NHANES database served as the foundation for our investigation, and every analysis considered the use of suitable NHANES sampling weights to increase the representativeness of the findings. Second, we investigated the nonlinear link between infertility and VAI by sensitivity analysis. This is the first study to look at the relationship between VAI and female infertility. Third, VAI is a quick and uncomplicated clinical tool that should be used to advise women of reproductive age about their higher risk of infertility at medical reviews. Our study is not without limits, though. First, we were unable to clearly determine a causal association among VAI and infertility because of the cross-sectional nature of our study. Secondly, an in-depth examination of additional markers was not feasible due to the restricted data present in the NHANES database.
Conclusion
According to our research, higher VAI is associated with a higher incidence of infertility. Therefore, high VAI levels may be associated with an increased risk of infertility, and VAI provides a practical and easily accessible method to assess metabolic and reproductive problems in infertile women. However, more large-scale prospective studies are needed in the future to confirm the results of this study.
Data availability
All data generated or analysed during this study are included in Supplementary Material.
References
Broughton, D. E. & Moley, K. H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 107, 840–847. https://doi.org/10.1016/j.fertnstert.2017.01.017 (2017).
Buzadzic, B. et al. New insights into male (in)fertility: The importance of NO. Br. J. Pharmacol. 172, 1455–1467. https://doi.org/10.1111/bph.12675 (2014).
Isbir, G. G. & Ozan, Y. D. Nursing and midwifery students’ experiences with the course of infertility and assisted reproductive techniques: A focus group study from Turkey. Nurse Educ. Pract. 28, 235–241. https://doi.org/10.1016/j.nepr.2017.10.002 (2018).
Catalano, P. M. & Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 356, j1. https://doi.org/10.1136/bmj.j1 (2017).
El Salam, M. A. A. Obesity, an enemy of male fertility: A mini review. Oman Med. J. 33, 3–6. https://doi.org/10.5001/omj.2018.02 (2018).
Jackson, S. S. et al. Anthropometric risk factors for cancers of the biliary tract in the biliary tract cancers pooling project. Cancer Res. 79, 3973–3982. https://doi.org/10.1158/0008-5472.Can-19-0459 (2019).
Wei, S., Schmidt, M. D., Dwyer, T., Norman, R. J. & Venn, A. J. Obesity and menstrual irregularity: Associations with SHBG, testosterone, and insulin. Obesity (Silver Spring) 17, 1070–1076. https://doi.org/10.1038/oby.2008.641 (2009).
Gallagher, C. S. et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 10, 4857. https://doi.org/10.1038/s41467-019-12536-4 (2019).
Yi, K. W. et al. Association of body mass index with severity of endometriosis in Korean women. Int. J. Gynaecol. Obstet. 105, 39–42. https://doi.org/10.1016/j.ijgo.2008.11.001 (2009).
Schuler-Toprak, S., Ortmann, O., Buechler, C. & Treeck, O. The complex roles of adipokines in polycystic ovary syndrome and endometriosis. Biomedicines https://doi.org/10.3390/biomedicines10102503 (2022).
Glueck, C. J. & Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 92, 108–120. https://doi.org/10.1016/j.metabol.2018.11.002 (2019).
Spradley, F. T. Metabolic abnormalities and obesity’s impact on the risk for developing preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R5–R12. https://doi.org/10.1152/ajpregu.00440.2016 (2017).
Lashen, H., Fear, K. & Sturdee, D. W. Obesity is associated with increased risk of first trimester and recurrent miscarriage: Matched case-control study. Hum. Reprod. 19, 1644–1646. https://doi.org/10.1093/humrep/deh277 (2004).
van der Steeg, J. W. et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum. Reprod. 23, 324–328. https://doi.org/10.1093/humrep/dem371 (2008).
Wise, L. A. et al. An internet-based prospective study of body size and time-to-pregnancy. Hum. Reprod. 25, 253–264. https://doi.org/10.1093/humrep/dep360 (2009).
Marquard, K. L. et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil. Steril. 95, 2146-2149.e2141. https://doi.org/10.1016/j.fertnstert.2010.10.026 (2011).
Low, N. et al. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. https://doi.org/10.1371/journal.pmed.1001356 (2012).
Stevens, J., McClain, J. E. & Truesdale, K. P. Selection of measures in epidemiologic studies of the consequences of obesity. Int. J. Obes. 32, S60–S66. https://doi.org/10.1038/ijo.2008.88 (2008).
Amato, M. C. & Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 730827. https://doi.org/10.1155/2014/730827 (2014).
Kahn, H. S. The, “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. https://doi.org/10.1186/1471-2261-5-26 (2005).
Zheng, S. H. & Li, X. L. Visceral adiposity index as a predictor of clinical severity and therapeutic outcome of PCOS. Gynecol. Endocrinol. 32, 177–183. https://doi.org/10.3109/09513590.2015.1111327 (2016).
Hackney, A. J., Klinedinst, N. J., Resnick, B. & Johantgen, M. Association of systemic inflammation and fatigue in osteoarthritis: 2007–2010 National Health and Nutrition Examination Survey. Biol. Res. Nurs. 21, 532–543. https://doi.org/10.1177/1099800419859091 (2019).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 12, 1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013 (2014).
Amato, M. C., Giordano, C., Pitrone, M. & Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 10, 183. https://doi.org/10.1186/1476-511X-10-183 (2011).
Wise, L. A., Palmer, J. R. & Rosenberg, L. Body size and time-to-pregnancy in black women. Hum. Reprod. 28, 2856–2864. https://doi.org/10.1093/humrep/det333 (2013).
Qin, Z. et al. Higher visceral adiposity index is associated with increased likelihood of abdominal aortic calcification. Clinics (Sao Paulo) 77, 100114. https://doi.org/10.1016/j.clinsp.2022.100114 (2022).
Wen, Z. & Li, X. Association between weight-adjusted-waist index and female infertility: A population-based study. Front. Endocrinol. (Lausanne) 14, 1175394. https://doi.org/10.3389/fendo.2023.1175394 (2023).
Zeng, Z. et al. Elevated visceral adiposity index linked to improved cognitive function in middle-aged and elderly Chinese: Evidence from the China health and retirement longitudinal study. Front. Aging Neurosci. 15, 1270239. https://doi.org/10.3389/fnagi.2023.1270239 (2023).
Francis, E. C., Zhang, L., Witrick, B. & Chen, L. Health behaviors of American pregnant women: A cross-sectional analysis of NHANES 2007–2014. J. Public Health (Oxf.) 43, 131–138. https://doi.org/10.1093/pubmed/fdz117 (2021).
Reinstatler, L., Khaleel, S. & Pais, V. M. Jr. Association of pregnancy with stone formation among women in the United States: A NHANES analysis 2007 to 2012. J. Urol. 198, 389–393. https://doi.org/10.1016/j.juro.2017.02.3233 (2017).
Sun, C., Wang, R., Li, Z. & Zhang, D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 246, 627–632. https://doi.org/10.1016/j.jad.2018.12.114 (2019).
Faulkner, J. L. Obesity-associated cardiovascular risk in women: Hypertension and heart failure. Clin. Sci. (Lond.) 135, 1523–1544. https://doi.org/10.1042/CS20210384 (2021).
Ibanez, L. & de Zegher, F. Adolescent PCOS: A postpubertal central obesity syndrome. Trends Mol. Med. 29, 354–363. https://doi.org/10.1016/j.molmed.2023.02.006 (2023).
Legro, R. S. et al. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 98, 4565–4592. https://doi.org/10.1210/jc.2013-2350 (2013).
Gesink Law, D. C., Maclehose, R. F. & Longnecker, M. P. Obesity and time to pregnancy. Hum. Reprod. 22, 414–420. https://doi.org/10.1093/humrep/del400 (2007).
Norman, R. J., Chura, L. R. & Robker, R. L. Effects of obesity on assisted reproductive technology outcomes. Fertil. Steril. 89, 1611–1612. https://doi.org/10.1016/j.fertnstert.2007.02.065 (2008).
Steegers-Theunissen, R. et al. Pre-conception interventions for subfertile couples undergoing assisted reproductive technology treatment: Modeling analysis. JMIR Mhealth Uhealth 8, e19570. https://doi.org/10.2196/19570 (2020).
Kasum, M. et al. The role of female obesity on in vitro fertilization outcomes. Gynecol. Endocrinol. 34, 184–188. https://doi.org/10.1080/09513590.2017.1391209 (2018).
Kang, Y. M. et al. Visceral adiposity index predicts the conversion of metabolically healthy obesity to an unhealthy phenotype. PLoS One 12, e0179635. https://doi.org/10.1371/journal.pone.0179635 (2017).
van der Ham, K. et al. Change in androgenic status and cardiometabolic profile of middle-aged women with polycystic ovary syndrome. J. Clin. Med. https://doi.org/10.3390/jcm12165226 (2023).
Jacewicz-Święcka, M. & Kowalska, I. Polycystic ovary syndrome and the risk of cardiometabolic complications in longitudinal studies. Diabetes Metab. Res. Rev. https://doi.org/10.1002/dmrr.3054 (2018).
Lindley, K. J. et al. Contraception and reproductive planning for women with cardiovascular disease. J. Am. Coll. Cardiol. 77, 1823–1834. https://doi.org/10.1016/j.jacc.2021.02.025 (2021).
Pasquali, R. Obesity and reproductive disorders in women. Hum. Reprod. Update 9, 359–372. https://doi.org/10.1093/humupd/dmg024 (2003).
Tchernof, A. & Despres, J. P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 93, 359–404. https://doi.org/10.1152/physrev.00033.2011 (2013).
Acknowledgements
We are grateful for the information provided by the National Health and Nutrition Examination Survey (2015–2020) of the United States, which is used in epidemiological research and health science research to aid in the development of sound public health policies, direct and design health plans and services, and advance health knowledge.
Funding
This study was supported by a grant from the Fund of Wuxi Health Commission (M202214), and supported by the Wuxi Taihu Lake Talent Plan, Support for Leading Talents in Medical and Health Professions (Mading academician, 4532001THMD).
Author information
Authors and Affiliations
Contributions
R.J.H and Y.B.W contributed to the conception of the study, J.R.Z performed the data analyses and wrote the manuscript, S.W and Y.W collected the data and helped to perform the data analysis. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuang, J., Wang, Y., Wang, S. et al. Association between visceral adiposity index and infertility in reproductive-aged women in the United States. Sci Rep 14, 14230 (2024). https://doi.org/10.1038/s41598-024-64849-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64849-0
- Springer Nature Limited