Abstract
Despite the proven superiority of various luteal phase support protocols (LPS) over placebo in view of improved pregnancy rates in fresh cycles of IVF (in vitro fertilization) and ICSI (intracytoplasmic sperm injection) cycles, there is ongoing controversy over specific LPS protocol selection, dosage, and duration. The aim of the present study was to identify the optimal LPS under six core aspects of ART success, clinical pregnancy, live birth as primary outcomes and biochemical pregnancy, miscarriage, multiple pregnancy, ovarian hyperstimulation syndrome (OHSS) events as secondary outcomes. Twelve databases, namely Embase (OVID), MEDLINE (R) (OVID), GlobalHealth (Archive), GlobalHealth, Health and Psychosocial Instruments, Maternity & Infant Care Database (MIDIRS), APA PsycTests, ClinicalTrials.gov, HMIC Health Management Information Consortium, CENTRAL, Web of Science, Scopus and two prospective registers, MedRxiv, Research Square were searched from inception to Aug.1st, 2023, (PROSPERO Registration: CRD42022358986). Only Randomised Controlled Trials (RCTs) were included. Bayesian network meta-analysis (NMA) model was employed for outcome analysis, presenting fixed effects, odds ratios (ORs) with 95% credibility intervals (CrIs). Vaginal Progesterone (VP) was considered the reference LPS given its’ clinical relevance. Seventy-six RCTs, comparing 22 interventions, and including 26,536 participants were included in the present NMA. Overall CiNeMa risk of bias was deemed moderate, and network inconsistency per outcome was deemed low (Multiple pregnancy χ2: 0.11, OHSS χ2: 0.26), moderate (Clinical Pregnancy: χ2: 7.02, Live birth χ2: 10.95, Biochemical pregnancy: χ2: 6.60, Miscarriage: χ2: 11.305). Combinatorial regimens, with subcutaneous GnRH-a (SCGnRH-a) on a vaginal progesterone base and oral oestrogen (OE) appeared to overall improve clinical pregnancy events; VP + OE + SCGnRH-a [OR 1.57 (95% CrI 1.11 to 2.22)], VP + SCGnRH-a [OR 1.28 (95% CrI 1.05 to 1.55)] as well as live pregnancy events, VP + OE + SCGnRH-a [OR 8.81 (95% CrI 2.35 to 39.1)], VP + SCGnRH-a [OR 1.76 (95% CrI 1.45 to 2.15)]. Equally, the progesterone free LPS, intramuscular human chorionic gonadotrophin, [OR 9.67 (95% CrI 2.34, 73.2)] was also found to increase live birth events, however was also associated with an increased probability of ovarian hyperstimulation, [OR 1.64 (95% CrI 0.75, 3.71)]. The combination of intramuscular and vaginal progesterone was associated with higher multiple pregnancy events, [OR 7.09 (95% CrI 2.49, 31.)]. Of all LPS protocols, VP + SC GnRH-a was found to significantly reduce miscarriage events, OR 0.54 (95% CrI 0.37 to 0.80). Subgroup analysis according to ovarian stimulation (OS) protocol revealed that the optimal LPS across both long and short OS, taking into account increase in live birth and reduction in miscarriage as well as OHSS events, was VP + SCGnRH-a, with an OR 2.89 [95% CrI 1.08, 2.96] and OR 2.84 [95% CrI 1.35, 6.26] respectively. Overall, NMA data suggest that combinatorial treatments, with the addition of SCGnRH-a on a VP base result in improved clinical pregnancy and live birth events in both GnRH-agonist and antagonist ovarian stimulation protocols.
Similar content being viewed by others
Introduction
Normal luteal function is an essential component for pregnancy maintenance. In natural ovulatory cycles, the corpus luteum can produce adequate progesterone after ovulation until the placental function starts at seven weeks of gestation. Ovarian stimulation (OS) techniques, either with gonadotropin-releasing hormone (GnRH) agonist or antagonist protocols, often induce endocrine defects in the luteal phase with increasing evidence suggesting that the resulting luteal-phase dysfunction may lead to lower pregnancy rates in in vitro fertilization (IVF) and/or ICSI (intracytoplasmic sperm injection) cycles1,2. To counteract these effects, luteal-phase support (LPS) is a well-known intervention for almost all stimulated assisted reproductive technology (ART) cycles3. Progesterone is amongst the most commonly, exogenously supplemented compounds employed as support of the luteal phase; however, the route of progesterone administration remains controversial4. In addition to the route of progesterone supplementation, disparities across literature are also present, regarding LPS dosage, duration and its use as monotherapy or in the context of combinatorial treatment with compounds such as oestradiol, Dehydroepiandrosterone (DHEA), gonadotropin-releasing hormone agonist (GNRH-a) and/or human chorionic gonadotropin (hCG)2,4. A plethora of previous pairwise and network meta-analyses has been published in an effort to discern the optimal LPS protocol in fresh cycles5,6,7,8,9,10. However, significant modifiable limitations were recognised. Amongst the pairwise analyses, the one-to-one comparison of specific LPS protocols, dimmed the option of a holistic picture of LPS variability and efficacy to be provided. The homogenisation of LPS protocols under a single agent umbrella did not allow for the appreciation of combinatorial protocols whilst combination of patient populations undergoing both fresh and frozen embryo transfers introduced a significant degree of data bias. Lastly, the effect of LPS selection under different ovarian stimulation protocols had not been previously addressed despite the significant impact upon clinical outcomes11,12.
Given the significance of clinical implications of appropriate LPS selection upon pregnancy outcomes, the present network meta-analysis compared mono-and multi-compound LPS regimens for women undergoing fresh cycles of IVF or ICSI in respect to core aspects of IVF/ICSI success (live birth, clinical and biochemical pregnancy rate, miscarriage, multiple pregnancy and ovarian hyperstimulation events). Additionally, the optimal LPS protocol in both agonist and antagonist OS has been explored.
Methods
Search strategy and selection criteria. The present study was prospectively registered under the PROSPERO database CRD42022358986 and conducted according to the PRISMA-NMA checklist13. Twelve databases, namely Embase (OVID), MEDLINE (R) (OVID), GlobalHealth (Archive), GlobalHealth, Health and Psychosocial Instruments, Maternity & Infant Care Database (MIDIRS), APA PsycTests, ClinicalTrials.gov, CENTRAL, Web of Science, Scopus and HMIC Health Management Information Consortium and two prospective registers, MedRxiv, Research Square were searched from inception to August 1st 2023. Search strategy was as follows and adapted per requirements of each target database (luteal and (support or supplementation or addition) and (assisted reproduction or IVF or ICSI or in vitro fertilization) and fresh). mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kf, fx, dq, cw, ta, te, bt, nm, ox, px, rx, an, ui, sy, ux, mx]. To ensure that all previous meta-synthesised evidence have been identified and assessed, a snowball approach has also been implemented, where the search to the databases described above was also conducted with a limit to include only meta-analyses (N = 102). The original studies included in the relevant meta-analysis manuscripts and were extracted and deduplicated (N = 169). Those were compared to the manuscripts identified through the classical search (Fig. 1). All study designs were included in the initial search but only Randomised Control Trials (RCTs) met abstract selection criteria. No language or geographical restrictions were applied.
For both systematic review and network meta-analysis (NMA), RCTs comparing pharmacological treatments administered for luteal support, either as monotherapy or combinatorial therapy, against placebo or other active agents administered either as mono- or combinatorial therapy for women undergoing fresh IVF/ICSI cycles were included. Studies reporting outcomes from oocyte donation cycles, comparing dosage or timing of same compound, or including patients that had undergone Intrauterine insemination (IUI) or Gamete intrafallopian transfer (GIFT) and zygote intrafallopian transfer (ZIFT) and studies where the route or compound of LPS was not stated or ≥ 4 embryos transferred were excluded (Table S1). Non-blind, single and double-blind studies were included in the analysis. Two independent researchers (SLK, KS) independently selected the studies, reviewed the main reports and supplementary materials, extracted the relevant information from the included trials, and assessed the risk of bias. Any discrepancies were double-checked and resolved by discussion with other members of the review team (GG, NB, DM).
Data extraction
Events (%, N) of clinical pregnancy, live birth, biochemical pregnancy, miscarriage, multiple pregnancy and OHSS, as previously defined, and the total number of patients exposed per treatment were extracted. Patient demographics and treatment specific parameters were also collected to allow for NMA transitivity analysis and comprehensive exploration of employed treatments across studies. Crude demographic and clinical data were collected. Per study, the total percentage of fresh cycles, Day 3 ETs was calculated from the reported, individual study data (Figs. 2, 3, 4). Missing SD or IQR were calculated from p values, t values, and standard error (SE) to allow for data harmonisation. When mean and standard deviation values were recorded, Bland’s method was employed to calculate median and IQR (Wan et al., 2014). Additionally, treatment specific parameters, namely active compound (Progesterone, Estradiol, hCG, GNRH-a, DHEA), brand name, route of administration [O, IM, SC, PV, PR, Topical (Patch)] dose (Progesterone, Estradiol, DHEA and GnRH agonist in mg, hCG in IU, median day of treatment initiation and SD, median end of treatment (weeks) and SD, number of patients exposed to named compound (Table 1). Lastly, implantation and fertilisation rates (%) were extracted as reported per study, given the inclusion of ≥ 1 embryos per study and aggregate data analysed as descriptive statistics (Fig. S1).
Population percentage and crude numbers exposed to each luteal support regimen and baseline demographic characteristics. Percentage and number of participants exposed to each luteal support protocol (A), Comparison of median participant age [95% CrI] (B) and median BMI [95% CrI] (C) per luteal support intervention. Reference group was considered to be VP. One way ANOVA analysis was employed as data values abided by gaussian distribution. Two decimal p values and asterisk annotation of significance where p-value < 0.05, it is flagged with one star (*), p-value < 0.01, 2 stars (**), p-value < 0.001, three stars (***). placebo (no exposure), SCP (Subcutaneous progesterone), VP (vaginal progesterone), IMP + VP (intramuscular progesterone and vaginal progesterone), VP + OE (vaginal progesterone and oral estradiol), IMP (intramuscular progesterone), VP + PatchE (vaginal progesterone and patch oestrogen), IMP + OE (intramuscular progesterone and oral estradiol), IMHCG (intramuscular hCG), SCP + VP, Intranasal GnRH-a, OP (oral progesterone), IMP + IME (intramuscular progesterone and intramuscular estradiol), IMP + VP + OE (Intramuscular progesterone, vaginal progesterone and oral estradiol), IMP + VE (Intramuscular progesterone and vaginal estradiol), VP + SCGNRH-a [(Vaginal progesterone and subcutaneous GNRH agonist (GNRH-a)], VP + OE + SCGNRH-a (Vaginal progesterone, oral estradiol and subcutaneous GNRH-a), RP (Rectal progesterone), SCHCG (subcutaneous HCG), VP + DHEA (vaginal progesterone and oral DHEA), IMP + VP + SCGNRH-a (Intramuscular progesterone, vaginal progesterone and subcutaneous GNRH-a), OP + VP (oral progesterone and vaginal progesterone).
Comparison of clinical parameters [Median, 95% CrI] across treatment groups. Duration of infertility (A), Percentage of population diagnosed with primary infertility (B) or secondary infertility (C), Basal AMH ng/ml (D), basal LH IU/L (E), FSH IU/L (F). Only comparisons that reached statistical significance are depicted. The reference group was PVP. Two decimal p values and asterisk annotation of significance where p-value < 0.05, it is flagged with one star (*), p-value < 0.01, 2 stars (**), p-value < 0.001, three stars (***), p-value < 0.0001, four stars (****). LPS (luteal support), AMH (anti-mullerian hormone), FSH (Follicle stimulating hormone), LH (luteinising hormone), placebo (no exposure), SCP (Subcutaneous progesterone), VP (vaginal progesterone), IMP + VP (intramuscular progesterone and vaginal progesterone), VP + OE (vaginal progesterone and oral estradiol), IMP (intramuscular progesterone), VP + PatchE (vaginal progesterone and patch oestrogen), IMP + OE (intramuscular progesterone and oral estradiol), IMHCG (intramuscular hCG), SCP + VP, Intranasal GnRH-a, OP (oral progesterone), IMP + IME (intramuscular progesterone and intramuscular estradiol), IMP + VP + OE (Intramuscular progesterone, vaginal progesterone and oral estradiol), IMP + VE (Intramuscular progesterone and vaginal estradiol), VP + SCGNRH-a [(Vaginal progesterone and subcutaneous GNRH agonist (GNRH-a)], VP + OE + SCGNRH-a (Vaginal progesterone, oral estradiol and subcutaneous GNRH-a), RP (Rectal progesterone), SCHCG (subcutaneous HCG), VP + DHEA (vaginal progesterone and oral DHEA), IMP + VP + SCGNRH-a (Intramuscular progesterone, vaginal progesterone and subcutaneous GNRH-a), OP + VP (oral progesterone and vaginal progesterone).
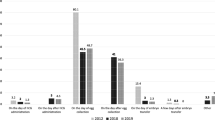
Comparison of clinical parameters [Median, 95% CrI], luteal support regimens duration and dosage across treatment groups. Progesterone levels on hCG trigger day (ng/ml) (A), Progesterone levels on embryo transfer (ET) day (ng/ml) (B), Endometrial thickness measurement on ET day (C), Ovarian stimulation protocol employed (D). Only comparisons that reached statistical significance are depicted. The reference group was PVP. Two decimal p values and asterisk annotation of significance where p-value < 0.05, it is flagged with one star (*), p-value < 0.01, 2 stars (**), p-value < 0.001, three stars (***), p-value < 0.0001, four stars (****). Abbreviations: LPS (luteal support), hCG (Human chorionic gonadotropin), hMG (human menopausal gonadotrophin), placebo (no exposure), SCP (Subcutaneous progesterone), VP (vaginal progesterone), IMP + VP (intramuscular progesterone and vaginal progesterone), VP + OE (vaginal progesterone and oral estradiol), IMP (intramuscular progesterone), VP + PatchE (vaginal progesterone and patch oestrogen), IMP + OE (intramuscular progesterone and oral estradiol), IMHCG (intramuscular hCG), SCP + VP, Intranasal GnRH-a, OP (oral progesterone), IMP + IME (intramuscular progesterone and intramuscular estradiol), IMP + VP + OE (Intramuscular progesterone, vaginal progesterone and oral estradiol), IMP + VE (Intramuscular progesterone and vaginal estradiol), VP + SCGNRH-a [(Vaginal progesterone and subcutaneous GNRH agonist (GNRH-a)], VP + OE + SCGNRH-a (Vaginal progesterone, oral estradiol and subcutaneous GNRH-a), RP (Rectal progesterone), SCHCG (subcutaneous HCG), VP + DHEA (vaginal progesterone and oral DHEA), IMP + VP + SCGNRH-a (Intramuscular progesterone, vaginal progesterone and subcutaneous GNRH-a), OP + VP (oral progesterone and vaginal progesterone).
Outcomes
The NMA primary outcomes were clinical pregnancy, defined as the presence of a gestational sac, with or without a fetal heartbeat on ultrasonography (US) and live birth, defined as the number of deliveries that resulted in live born neonate/s. Regarding live birth, singleton and non-singleton deliveries were considered as a single event. Secondary outcomes included biochemical pregnancy, defined as positive hCG test but without US verification 2 weeks following embryo transfer (ET), miscarriage defined as the spontaneous loss of a pregnancy before the 20th week, multiple pregnancy was defined as non-singleton clinical pregnancy and OHSS events. Crude events were collected per included study, and therefore no homogenisation of extracted data was required.
Data analysis
Effect estimates were calculated as odds ratios (ORs) for all outcomes, given that all were dichotomous, with respective 95% credibility intervals (95% CrIs) using Bayesian network and pair-wise meta-analysis14 (Fig. 5, Fig. S2–S10). Of note, a credibility interval is an interval within which an unobserved parameter value falls with a particular probability in Bayesian statistics comparable to the 95% Confidence interval commonly seen in frequentist statistics15. Network meta-analysis iterations were conducted with MetaInsight visual R package16. NMA was conducted using a fixed-effects model within a Bayesian setting, as unequal heterogeneity across all comparisons was assumed. Vaginal Progesterone (VP) was used as the reference treatment given its proven superiority over placebo and the NICE guideline recommendations17. A hierarchy of treatments was calculated for each outcome, based on the p-scores and SUCRA ratings. Summary of the rank distribution of LPS treatments, interpreted as the estimated proportion of treatments worse than the treatment of reference (VP) was displayed by Litmus Rank-O-Gram graphs and Radial SUCRA18 (Fig. S5–S6). Transitivity assumption was evaluated by comparing the distribution of key study characteristics across studies grouped by comparison (age and BMI). We assessed inconsistency between direct and indirect sources of evidence using global and local approaches. We assessed global inconsistency by using a design-by-treatment test19,20. Local inconsistency was evaluated by using the back calculation and separate indirect from direct design evidence methods, comparing direct and indirect evidence for each pairwise treatment comparison and node-splitting model21 (Table S2–S3; Fig. S3–S4). Possible heterogeneity of treatment effects and the robustness of findings was explored by subgroup network meta-analyses including only trials at overall low and medium risk of bias (Table 1, Fig. S7–S8, S10). Further subgroup analysis was conducted on trials using either standard (long) GnRH agonist or standard (short) GnRH antagonist protocol for ovarian stimulation to limit data heterogeneity. If mixed populations were included in the original publication, a cut-off of ≥ 65% of patients being treated with either of the protocols, was employed to categorise studies according to subgroup (Table S4). Mixmeta package in R v4.1.2 was employed for confounder exploration in a network meta-regression model. Gelman network convergence, network deviance and ranking analysis were conducted to quantify overall network discordance (Fig. S9–S10). Intergroup differences regarding demographic and treatment parameters were quantified, where appropriate by ANOVA (for parametric distributed variables e.g., Age, BMI) or Kruskal–Wallis test (non-parametric distribution of variables, e.g., all remaining variables). Multilevel network meta‐regression for the embryological parameters (number of transferred embryos, number of retrieved and mature oocytes, peak estradiol, % of day 3 embryos transferred) was undertaken for both primary and secondary outcomes22 (Table S11–S12).
Luteal support Bayesian fixed effect consistency forest plot (Odds ratio, 95% CrI) for Clinical Pregnancy (A) Live Birth (B) Biochemical Pregnancy (C) Miscarriage (D) and Multiple pregnancy (E) OHSS (F) outcomes). Graph generated by MetaInsight R package. Tabular results of design-by-treatment interaction model consistency depicted in Table S1–S2 per outcome. Node splitting model per comparison (direct and indirect effects) depicted in Table S.6–7. placebo (no exposure), SCP (Subcutaneous progesterone), VP (vaginal progesterone), IMP + VP (intramuscular progesterone and vaginal progesterone), VP + OE (vaginal progesterone and oral estradiol), IMP (intramuscular progesterone), VP + PatchE (vaginal progesterone and patch oestrogen), IMP + OE (intramuscular progesterone and oral estradiol), IMHCG (intramuscular hCG), SCP + VP, Intranasal GnRH-a, OP (oral progesterone), IMP + IME (intramuscular progesterone and intramuscular estradiol), IMP + VP + OE (Intramuscular progesterone, vaginal progesterone and oral estradiol), IMP + VE (Intramuscular progesterone and vaginal estradiol), VP + SCGNRH-a [(Vaginal progesterone and subcutaneous GNRH agonist (GNRH-a)], VP + OE + SCGNRH-a (Vaginal progesterone, oral estradiol and subcutaneous GNRH-a), RP (Rectal progesterone), SCHCG (subcutaneous HCG), VP + DHEA (vaginal progesterone and oral DHEA), IMP + VP + SCGNRH-a (Intramuscular progesterone, vaginal progesterone and subcutaneous GNRH-a), OP + VP (oral progesterone and vaginal progesterone).
Risk of bias assessment
Within-study bias was assessed with the Cochrane risk of bias tool RoB223 and the certainty of evidence using the GRADE Framework (Table 2). Overall network risk of bias was assessed with the Network Meta-Analysis framework (CINeMA)24 (Table S5–S10). Small-study effects and publication bias for each treatment pair was assessed using a contour-enhanced funnel plot.
Results
Included study design and quality of evidence assessment
From 1322 records initially retrieved, 76 RCTs, comparing 22 interventions of at least two arms comparing LPS protocols in fresh IVF/ICSI cycles, met the inclusion criteria (Fig. 1, Table 1)25,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,100. Overall risk-of-bias judgement was deemed “low” for 24 studies “some concerns” for 29 and “high” for 23 studies (Table 2). Overall, GRADE confidence in evidence was deemed “high” for 34 studies, “moderate” for 23 studies and “low” or “very low” for 19 studies (Table 2).
Participant and treatment characteristics
A total of 26,536 participants were randomly assigned to any of the following 22 treatments; placebo (no exposure)[N = 727], SCP (Subcutaneous progesterone) [N = 877], VP (vaginal progesterone) [N = 13862], IMP + VP (intramuscular progesterone and vaginal progesterone) [N = 475], VP + OE (vaginal progesterone and oral estradiol) [N = 898], IMP (intramuscular progesterone) [N = 2136], VP + PatchE (vaginal progesterone and patch estrogen) [N = 179], IMP + OE (intramuscular progesterone and oral estradiol) [N = 387], IMHCG (intramuscular hCG) [N = 592], SCP + VP [N = 78], Intranasal GnRH-a [N = 23], OP (oral progesterone) [N = 3693], IMP + IME (intramuscular progesterone and intramuscular estradiol) [N = 55], IMP + VP + OE (Intramuscular progesterone, vaginal progesterone and oral estradiol) [N = 249], IMP + VE (Intramuscular progesterone and vaginal estradiol) [N = 174], VP + SCGNRH-a [(Vaginal progesterone and subcutaneous GNRH agonist (GNRH-a)] [N = 1008], VP + OE + SCGNRH-a (Vaginal progesterone, oral estradiol and subcutaneous GNRH-a) [N = 386], RP (Rectal progesterone) [N = 168], SCHCG (subcutaneous HCG) [N = 160], VP + DHEA (vaginal progesterone and oral DHEA)[N = 104] and IMP + VP + SCGNRH-a (Intramuscular progesterone, vaginal progesterone and subcutaneous GNRH-a) [N = 213] and OP + VP (oral progesterone and vaginal progesterone) [N = 92] (Fig. 2A).
Median participant age across all treatment groups was 32 years [IQR 31.75, 33.85] (Fig. 2B) and the median BMI was 23.94 (kg/m2) [IQR 22.45, 26.8] (Fig. 2B,C). Duration of infertility was of a median of 4.96 years [IQR 3.98, 6.10] (Fig. 3A). The population percentage diagnosed with primary infertility was 29.6% [Range: 10.9 to 42.62%] and secondary infertility was 34.5% [Range: 16.1 to 84.65%] and were not found to significantly differ across comparator groups (Fig. 3A–C). Median values of basal AMH, LH, FSH, progesterone levels on HCG trigger, progesterone levels on embryo transfer (ET) day, and endometrial thickness on ET day, per LPS were not found to be significantly different in comparison to the VP group (Figs. 3D–F, 4A–C). Regarding OS protocol, 54.69% of the participants underwent ovarian stimulation with a standard (long) GnRH agonist while 18.17% with a standard (short) GnRH antagonist protocol. A 1.05% underwent OS via clomiphene and HMG, 0.96% via a microdose flare and 0.54% by an ultrashort GnRH protocol (Fig. 4D)101. The remaining 24.59% of the participants underwent either a standard long or short OS protocol however the distribution was not noted in the original studies. Characteristics of embryo transfers were not consistently reported across arms of included studies (Table S.11). Of note, 20 of the 76 studies, failed to report upon these variables.
Regarding LPS protocols, schemas were segregated by active compound to explore variations of dosage (median dosage and maximum dosage), initiation day, duration of LPS (weeks) as well route of administration (Tables 1, 3). The majority of LPS protocols were initiated on the oocyte pickup day (OPU), and duration of 8 weeks (SD = 2). No significant differences were noted amongst LPS protocols regarding implantation 24.55% [IQR 18.17, 28.9] or fertilisation 63.6% [IQR 61, 78.9] median rates (Fig. S1).
Data synthesis and network meta-analysis
VP was considered as the reference treatment as previously mentioned (NICE guidelines17. In NMA, effect size estimates suggested that all LPS protocols were consistently superior to placebo, employed as a negative control for both primary and secondary outcomes, regardless of risk of bias sensitivity analysis (Fig. 5, Fig. S2–S10, Tables S2–S4).
More specifically, regarding NMA primary outcomes:
-
1.
For clinical pregnancy events, reported by 74 studies, CiNeMa NMA RoB rating was deemed “moderate” and overall network incoherence was found to be moderate, χ2 7.02, 4 degrees of freedom, p-value: 0.005) (Table S5, Fig. 5A, Fig. S2A, Fig. S3A, Fig. S5A, Fig. S6A, Fig. S9A). All LPS protocols appeared to be equivalent to VP in respect to the clinical pregnancy events, except for VP + OE + SCGNRH-a, [OR 1.57 (95% CrI 1.11 to 2.22) (SUCRA: 80%; Npatients:386, “Moderate” GRADE] and VP + SCGNRH-a [OR 1.28 (95% CrI 1.05 to 1.55) (SUCRA: 80%; Npatients:583, “High” GRADE], which were found to be superior, with high SUCRA probability (Fig. 5A, Fig. S5A, S6A). Equally, VP + PatchE was also associated with higher clinical pregnancy probability, OR 1.73 (95% CrI 1.16, 2.58) (SUCRA: 79%; Npatients:179, “Moderate” GRADE). Treatments such as IMP + IME OR 2.68 (95% CrI 1.06, 7.72) (SUCRA: 90%; Npatients:55, “Low” GRADE) and were shown to be superior in comparison to VP however the certainty in evidence was deemed low given the small number of participants included and the high risk of subsequent heterogeneity.
-
2.
For the live pregnancy events, reported by 43 studies, CiNeMa NMA RoB rating was deemed “moderate” and overall network incoherence was found to be moderate, χ2 10.95 (5 degrees of freedom), p value: 0.052 (Table S6, Fig. 5B, Fig. S2B, Fig. S3B, Fig. S5B, Fig. S6B, Fig. S9B). The following interventions were found to improve live pregnancy events in comparison to the reference LPS, IMHCG [OR 9.67 (95% CrI 2.34 to 73.2) (SUCRA: 92%; Npatients:592, “Moderate” GRADE)], VP + OE [OR 4.57 (95% CrI 1.26 to 20) (SUCRA: 80%; Npatients:898, “Moderate” GRADE)], VP + OE + SCGNRH-a OR [OR 8.81 (95% CrI 2.35 to 39.1) (SUCRA: 95%; Npatients:386, “High” GRADE)], VP + SCGNRH-a [OR 1.76 (95% CrI 1.45 to 2.15) (SUCRA: 72%; Npatients:1008, “High” GRADE)] (Fig. 5B, Fig. S5B).
Regarding secondary outcomes:
-
3.
For biochemical pregnancy events, reported by 29 studies, CiNeMa NMA RoB rating was deemed “Moderate” and network incoherence was found to be moderate, χ2 6.60 (2 degrees of freedom), p value: 0.037 (Table S7, Fig. 5C, Fig. S2C, Fig. S3C, Fig. S5C, Fig. S6C, Fig. S9C). For VP versus all other LPS protocols. No LPS protocol appeared to result in a significantly higher biochemical pregnancy probability.
-
4.
Regarding miscarriage events, reported by 41 studies, CiNeMa NMA RoB rating was deemed “Moderate” and network incoherence was found to be moderate, χ2 11.30 (4 degrees of freedom), p value: 0.023 (Table S9, Fig. 5D, Fig. S2D, Fig. S4A, Fig. S5D, Fig. S6D, Fig. S9D). VP + SCGnRH-a was found to reduce miscarriage events in comparison to the reference LPS, [OR 0.54 (95% CrI 0.372 to 0.806), Npatients:1008, “Moderate” GRADE] with a SUCRA of 82.2% (Fig. 5D, Fig. S4A, Fig. S5D). Additionally, a similar finding was confirmed for IMP + IME [OR 0.08 (95% CrI 0.01 to 0.46), Npatients:55] however the certainty in evidence was deemed “Low”.
-
5.
For multiple pregnancy events, reported by 21 studies, CiNeMa NMA RoB rating was deemed “High” (Table S9). Overall network incoherence was found to be low, χ2. 0.115 (2 degrees of freedom), p value: 0.94 (Fig. 5E, Fig. S2E, Fig. S4B, Fig. S5E, Fig. S6E, Fig. S9E). All LPS protocols appeared to produce similar results to PVP, except for SCP [OR 0.09 (95% CrI 0.009 to 0.556); SUCRA 1.2%, Npatients:877, “High” GRADE] resulting to significantly lower multiple pregnancy events and IMP + VP + SCGNRH-a [OR 6.88 (95% CrI 2.42 to 30.4); SUCRA 81.2%, Npatients:213, “Low” GRADE] resulting in significantly higher multiple pregnancy events.
-
6.
For OHSS events, reported by 15 studies, CiNeMa NMA RoB confidence rating was deemed “Low” (Table S10). Overall network incoherence was found to be low, χ2.: 0.26 (2 degrees of freedom), p value: 0.015 (Fig. 5F, Fig. S2F, Fig. S5F, Fig. S6F, Fig. S9F). Pairwise analysis of included studies was not feasible due to the multitude of non-events (zero events of OHSS in either of the arms of the original study). All LPS protocols appeared to be associated with similar OHSS events to the reference LPS, except for OP [OR 1.87 (95% CrI 1.15 to 3.04); Npatients:3693, SUCRA 75%, “Low” GRADE] which was found to be associated with significantly higher OHSS events. The latter is likely to be a result of bias towards an OP LPS protocol selection in patients at high risk of ovarian hyperstimulation102,103.
Subgroup analysis of low and medium risk of bias studies (Figs. S7, S8, S10, Table 2) and node-splitting (Table S2–S3) did not significantly alter cumulative effects analysis or residual deviance (Fig. S10A–F). Optimal LPS per OS, long (Gonadotropin releasing hormone agonist) vs. short (GnRH antagonist) protocol, was explored to identify further sources of heterogeneity and to delineate whether a particular LPS appears to yield improved clinical outcomes in association with specific ovarian stimulation protocols (Table 4, Table S4). In view of live birth events, the following protocols were deemed optimal for participants that underwent OS by standard GnRH agonist protocol: (a) VP + OE + SCGNRH-a [OR 9.7 (95% CrI 3.73, 13.5)] (b) VP + OE [OR 4.58 (95% CrI 1.26, 20.3)], (c) VP + SCGNRH-a [OR 2.89 (95% CrI 1.46, 3.42)], and (d) IMHCG [OR 1.57 (95% CrI 2.24, 71.9)]. Of the aforementioned, the VP + OE, VP + SCGNRH-a and IMHCG comparators had a “High” GRADE rating while the VP + OE + SCGNRH-a protocol was also associated with a higher probability of miscarriage when used in combination with a GnRH agonist OS protocol, [OR 3.93 (1.69, 10.1)]. On the contrary, optimal luteal support protocols for standard GnRH antagonist OS were (a) IMHCG [OR 3.2 (95% CrI 1.54, 334.), “low” GRADE] and (b) VP + SCGNRH [OR 2.84 (95% CrI 1.35, 6.24), “High” GRADE] presenting the optimal LPS options across short protocols. Of note, IMHCG was also associated with a higher probability of miscarriage when used in conjunction with a short OS protocol [OR 2.11 (95% CrI 0.75, 6.40), high GRADE] while the opposite held true for VP + SCGNRH, which was associated with lower probability of miscarriage in short OS [OR 0.54 (95% CrI 0.37, 0.80), high GRADE]. Network meta-regression for all outcomes, according to embryological parameters, did not significantly alter effect sizes (Table S11–S12).
Overall, NMA data suggest that combinatorial treatments, with the addition of SCGNRH-a on a VP base results in improved clinical pregnancy and live birth events and reduced miscarriage events in participants undergoing OS either a standard GnRH antagonist or agonist protocol. However, participants undergoing a long GnRH protocol OS appear to benefit more from IMHCG as LPS while participants undergoing a short GnRH protocol OS appear to benefit more from VP + SCGNRH, considering the reduction of miscarriage events of these luteal support protocols in conjunction to OS.
Discussion
This study is based on 76 RCTs, including 26,536 participants randomly assigned to 22 LPS protocols including non-exposure. Given the plethora of previous data suggesting that any LPS protocol is superior to non-exposure, the most widely employed LPS, vaginal progesterone, was set as a reference treatment3,17. Overall, meta-synthesized data presented here, suggest that combinatorial treatments, those with the addition of SCGnRH on a VP base result in improved clinical pregnancy, OR 1.28 (95% CrI 1.05 to 1.55) and live birth events, OR 1.76 (95% CrI 1.45 to 2.15) with high confidence in evidence. Of note, addition of oral estradiol to a VP + SCGNRH-a LPS, resulted in further improvement of clinical pregnancy events by 29% and 44% increase of a clinical pregnancy and live birth odds respectively. Of note, participants undergoing a long GnRH protocol OS appeared to benefit more from progesterone free LPS such as IMHCG in view of increased live birth, OR 1.57 (95% CrI 2.24 to 71.9) and reduced miscarriage events, OR 1.57 (95% CrI 2.24 to 71.9). However, participants appeared to be at a higher risk of OHSS, OR 1.64 (95% CrI 0.74 to 3.73). On the other hand, participants undergoing a short GnRH OS protocol appeared to benefit more from VP + SCGNRH with a live birth OR 2.84 (95% CrI 1.35 to 6.26), however while the probability of miscarriage was significantly reduced, OR 0.55 (95% CrI 0.38 to 0.80), the probability of multiple pregnancy significantly increased, OR 8.34 (95% CrI 2.57 to 37.6).
Luteal support is a critical aspect of IVF/ICSI cycles as it aids in maintaining the endometrial lining, in turn promoting embryo implantation, and supporting early pregnancy. In fresh IVF cycles, luteal support management can pose several challenges, including timing and duration of administration, individual outcome variability and tolerability of LPSs that may impact upon the success rates of the cycle. The effectiveness of luteal support in achieving live birth and clinical pregnancy rates is dependent on the timing of its administration104,104,106. Various studies have examined the optimal timepoint to initiate LPS, with only two out of five RCTs reporting statistically significant results104. Earlier evidence had suggested that delayed administration of LPS [(24 h after ovum pick-up (OPU)] may be more advantageous than pre-OPU administration (12 h prior to OPU)106. Williams et al. found initiating LPS on day 3 post OPU to be significantly better than delaying it until day 6105. Overall, these studies suggest that the optimal time for LPS administration is from the evening of OPU up until 3 days post OPU. Present NMA evidence suggested that the majority of studies favoured LPS initiation on the day of OPU (within the 24 h timeframe following the procedure), including for LPS protocols generating superior results namely, VP + SCGNRH-a and VP + OE + SCGNRH-a. Equally important to the LPS initiation timing, is the duration of luteal support administration. A recent meta-analysis including 1297 participants, indicating that continuing progesterone for two weeks after a positive pregnancy test did not have any significant impact on miscarriage or delivery rates106. The same study suggested that it is unnecessary to continue LPS for up to 10 weeks of pregnancy with further studies reaching to the same conclusion107,107,109. However, ESHRE 2020 recommendations suggest that LPS should be administered up until, at least the day of the pregnancy test101. Aggregate evidence of the present study indicate that duration of administration is highly dependent upon the selected LPS regimen, with an overall median of 8 weeks [Range 2–12] coinciding with ultrasonographic evidence of fetal motion and the concept of the luteo-placental shift110,111.
In addition, while initiation and duration of LPS treatment may appear more standardised, the selection of optimal type and dose of luteal support is largely individualised and dependent upon participant factors such as age, BMI, and reproductive history. Regardless of clinical and demographic parameters, undoubedtly the most important parameter affecting LPS selection and duration of perscription, is fundamentaly influenced by patient preference, which is in turn heavily reliant upon LPS side effect profile and tolerability, patient compliance and cost. For example, in view of treatment acceptability, IM progesterone has been widely available prior to vaginal formulation becoming available, and has been shown to have superior absorption and achieve stable serum concentration shortly after administration109,112,113. Nonetheless, administration complications involving pain, higher risk of infection, sterile abscess formation, and even rarely eosinophilic pneumonia as well as practical impediments requiring daily visits and injections, have necessitated the exploration of alternative more convinient routes, such as the one offered by the vaginal preparation114. Currently, vaginal progesterone products are administered in various ways, including pessaries, capsules, tablets, gel, and inserts which can achieve maximum serum concentration of progesterone after 3–8 h of administration, and by daily doses of 300–600 mg may achieve adequate available plasma levels115. Evidence has also shown that a 300–600 mg of vaginal micronized progesterone daily can induce similar endometrial maturation as 100 mg intramuscular progesterone daily109. By enabling direct transport of "first uterine pass" progesterone from the vagina to the uterus, vaginal preparations achieve adequate tissue levels of progesterone with lower circulating levels, indicating acceptable bioavailability111.
Given the improved outcomes regarding clinical pregnancy and live birth, achieved by VP + SCGNRH-a and VP + OE + SCGnRH-a combinatorial treatments, shown in the present work, a mention to route and dosage of gonadotropin-releasing hormone agonist is warranted. The use of GnRH-a for LPS was suggested following accidental use of GnRH agonist during this phase which resulted in improved implantation rates116. The effect of GnRH agonist has been observed at three levels: support of the corpus luteum through pituitary LH secretion, direct effects on the embryo and implantation process, and the effect upon trophectoderm cells and endometrial GnRH receptors77,116,117. A meta-analysis showed that administering a single dose of GnRH-a increased the implantation rate in cycles with GnRH antagonist and long GnRH-a protocols, clinical pregnancy rate per transfer, and ongoing pregnancy rate118, whilst another revealed that the use of GnRH-a for LPS significantly improved live birth rate, clinical pregnancy rate, and ongoing pregnancy rate119. An additional study demonstrated that a single dose of GnRH-a had similar efficacy as three doses of hCG120. One can hypothesise that the addition of a GnRH agonist can bimodally support the corpus luteum by stimulating the release of gonadotrophins from the pituitary gland, and by directly influencing the endometrium through interaction with GnRH receptors. Furthermore, research suggests that administering a single dose of GnRH agonist during the luteal phase enhances rates of pregnancy, implantation, delivery, and birth among recipients of donated oocytes whose ovulation was suppressed and corpus luteum was absent, suggesting a potential direct impact of GnRH agonist on the embryo77,98,116,119. The present work has highlighted that a single SCGNRH administration in addition to a VP protocol, can positively impact on IVF/ICSI outcomes especially in patients undergoing GnRH antagonist OS and could be reserved for more challenging cycles to optimise results. Conversely, in view of the improved clinical pregnancy and live birth outcomes achieved by the addition of oral estradiol in the VP + SCGNRH-a protocol, exploration of the possible synergistic effects of this compound is necessitated. However, a Cochrane meta-analysis did not find evidence to support routinely administering estrogen with progesterone in IVF cycles9. In antagonist cycles, progesterone levels surge, leading to a rebound decrease in serum estradiol, which in turn has formulated the hypothesis that adding doses of 2–6 mg/day of estradiol could be beneficial111. However, contemporary systematic reviews failed to confirm the beneficial effects of oral or any route of estradiol addition to progesterone LPS upon pregnancy outcomes120,120,122. Of note, novel LPS regimens involving intranasal GnRH administration has been shown promising results regarding clinical pregnancy rates and treatment tolerability however given the scarse RCT evidence, further, adequately powered, RCTs would be required to allow recommendations regarding this LPS regimen117,123.
In addition, the present work has shown that progesterone free LPS protocols, such as intramuscular hCG, may be equal, if not more effective that progesterone-based LPS in view of live birth outcomes, especially in patients undergoing a GnRH agonist OS protocol. HCG, by mimicking LH pulsatility, was initially considered the primary choice for LPS as it stimulates the corpus luteum to produce progesterone continuously. However, this approach has drawbacks, as it can elevate the risk of OHSS, a hypothesis which was also confirmed by the present NMA, albeit lacking statistical significance, OR 1.64 [95% CrI 0.75, 3.71].
Limitations and future perspectives
The optimal protocol for luteal support is a constantly evolving field of research in artificial reproduction. In view of the plethora of available LPS protocols, NMA precision of estimates provides a more comprehensive understanding of the comparative effectiveness of different protocols. In the present work, only RCT data have been employed to reach meaningful conclusions limiting inherent bias of diverse participant populations, with add-on sensitivity analysis targeted at low and moderate risk of bias studies and ovarian stimulation protocol to further explore confounding factors and detect sources of heterogeneity. Given the anticipated diversity of measured outcomes, a bayesian meta-synthesis approach has been adopted to account for the expected heterogeneity and to incorporate modelling flexibility by allowing for posterior distributions interpreted as SUCRA probabilities with the later enabling crisper communication of the uncertainty in the treatment effects estimates.
On this note, in the present study, LPS protocols have been treated as unique comparator entities, allowing for assessment of selected outcomes on a protocol- rather than a compound-level. However, side effect and safety profile of combinatorial treatments has not been assessed and therefore a significant confounder in tolerability and in turn, compliance, especially in the context of combinatorial LPS, remains to be investigated. Additionally, cost-effectiveness analysis has not been undertaken, which needs to be factored in a joined patient and clinical decision-making. Moreover, while reported, the present study did not aim to clarify of optimal initiation/cessation timing of LPS or the optimal dosage and therefore to produce concrete recommendations regarding these LPS parameters, further studies with relevant designs should be implemented. Notably, included studies were significantly heterogeneous in terms of reporting the characteristics of embryo transfers. Abeit no statistical difference was reported for variables such as follicles retrieved, peak oestradiol levels and number of embryos transferred in individuals studies, confounding effects cannot be confidently excluded. Lastly, OHSS events were found to be considerably under-reported across RCTs with only 15 studies noting such events. Reflecting on the implications of OHSS upon both the patient clinical management as well as the success of the IVF/ICSI, it would be strongly recommended that future RCTs would thoroughly record OHSS events across study arms. Overall, luteal support management in fresh IVF cycles is a complex and dynamic process that calls for careful consideration and individualised LPS selection to achieve optimal outcomes.
Conclusion
Herein meta-synthesized data suggest that combinatorial treatments, with the addition of subcutaneous GnRH agonist, on a vaginally administered progesterone LPS base, results in improved clinical pregnancy and live birth events. However, the side-effect and tolerability profile of such combinatorial LPS protocols needs to be thoroughly investigated prior to their wide-scale adoption in clinical practice.
Data availability
All data associated with the present study are available in the main body or the supplementary material of the submission. Data regarding any of the subjects in the study has been published in the form of randomised control studies. Crude data were extracted and homogenized for the purposes of the present systematic review and network meta-analysis. All included studies have been referenced as required.
References
Suthaporn, S., Jayaprakasan, K., Maalouf, W., Thornton, J. G. & Walker, K. F. The strength of evidence supporting luteal phase progestogen after assisted reproduction: A systematic review with reference to trial registration and pre-specified endpoints. Eur. J. Obstet. Gynecol. Reprod. Biol. 1(245), 149–161 (2020).
Tomic, V., Kasum, M. & Vucic, K. The role of luteal support during IVF: A qualitative systematic review. Gynecol. Endocrinol. 35(10), 829–834 (2019).
Con, S. & Eftekhar, M. Luteal-phase support in assisted reproductive technology: An ongoing challenge. Int. J. Reprod. BioMed. 19(9), 761 (2021).
Wang, N. F., Bungum, L. & Skouby, S. O. What is the optimal luteal support in assisted reproductive technology?. Horm. Mol. Biol. Clin. Invest. 43(2), 225–233 (2022).
Liu, Y. et al. Single-dose versus multiple-dose GnRH agonist for luteal-phase support in women undergoing IVF/ICSI cycles: A network meta-analysis of randomized controlled trials. Front. Endocrinol. (Lausanne). 31(13), 802688. https://doi.org/10.3389/fendo.2022.802688 (2022).
Wu, H., Zhang, S., Lin, X., Wang, S. & Zhou, P. Luteal phase support for in vitro fertilization/intracytoplasmic sperm injection fresh cycles: A systematic review and network meta-analysis. Reprod. Biol. Endocrinol. 19(1), 1–1 (2021).
Conforti, A. et al. Luteal phase support using subcutaneous progesterone: A systematic review. Front. Reprod. Health 6(3), 634813 (2021).
Barbosa, M. W. P. et al. Oral dydrogesterone versus vaginal progesterone capsules for luteal-phase support in women undergoing embryo transfer: A systematic review and meta-analysis. JBRA Assist. Reprod. 22(2), 148–156. https://doi.org/10.5935/1518-0557.20180018 (2018).
van der Linden, M., Buckingham, K., Farquhar, C., Kremer, J. A. & Metwally, M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst. Rev. 2015(7), CD009154. https://doi.org/10.1002/14651858.cd009154.pub3 (2015).
Kyrou, D. et al. Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: A systematic review and meta-analysis. Hum. Reprod. 17(6), 734–740 (2011).
Yang, R., Guan, Y., Perrot, V., Ma, J. & Li, R. Comparison of the long-acting GnRH agonist follicular protocol with the GnRH antagonist protocol in women undergoing in vitro fertilization: A systematic review and meta-analysis. Adv. Ther. 38, 2027–2037 (2021).
Lambalk, C. B. et al. GnRH antagonist versus long agonist protocols in IVF: A systematic review and meta-analysis accounting for patient type. Hum. Reprod. 23(5), 560–579 (2017).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162(11), 777–784 (2015).
Efthimiou, O., Debray, T.P, van Valkenhoef, G., Trelle, S., Panayidou, K., Moons, K.G., Reitsma, J.B., Shang, A., Salanti, G., GetReal Methods Review Group. GetReal in network meta‐analysis: a review of the methodology. Res. Synth. Methods 7(3), 236–63 (2016).
Hespanhol, L., Vallio, C. S., Costa, L. M. & Saragiotto, B. T. Understanding and interpreting confidence and credible intervals around effect estimates. Braz. J. Phys. Ther. 23(4), 290–301. https://doi.org/10.1016/j.bjpt.2018.12.006 (2019).
Owen, R. K., Bradbury, N., Xin, Y., Cooper, N. & Sutton, A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res. Synth. Methods 10, 569–581 (2019).
NICE guidelines: Fertility problems: assessment and treatment Clinical guideline [CG156] Published: 20 February 2013 Last updated: 06 September 2017, https://www.nice.org.uk/guidance/cg156/chapter/recommendations. Accessed 9 Aug 2023.
Nevill, C. R., Cooper, N. J. & Sutton, A. J. A multifaceted graphical display, including treatment ranking, was developed to aid interpretation of network meta-analysis. J. Clin. Epidemiol. 157, 83–91 (2023).
Higgins, J.P., Altman, D.G. & Sterne, J.A. Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook. cochrane. org. 2011:243–96.
Higgins, J. P. et al. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 3(2), 98–110 (2012).
König, J., Krahn, U. & Binder, H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Statist. Med. 32(30), 5414–5429 (2013).
Phillippo, D. M. et al. Multilevel network meta-regression for population-adjusted treatment comparisons. J. R. Stat. Soc. Ser. A Stat. Soc. 183(3), 1189–1210. https://doi.org/10.1111/rssa.12579 (2020).
Deeks, J. J., Higgins, J. P. T., & Altman, D. G. (editors). Chapter 10: Analyzing data and undertaking meta-analyses. In: Higgins, J. P. T., Thomas, J., Chandler. J., Cumpston. M., Li. T., Page. M.J., & Welch, V.A. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). Available from www.training.cochrane.org/handbook
Nikolakopoulou, A. et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 17(4), e1003082 (2020).
Gawron, I. M., Chrostowski, B., Derbisz, K., Jach, R. & Pietrus, M. Comparison of dydrogesterone plus progesterone gel with subcutaneous aqueous progesterone plus progesterone gel for luteal phase supplementation of subsequent in vitro cycle in women after previous cycle failure. Ginekologia Polska https://doi.org/10.5603/GP.a2023.0062 (2023).
Kao, T. C. et al. Clinical use of aqueous subcutaneous progesterone compared with vaginal progesterone as luteal support in in vitro fertilization: A randomized controlled study in Taiwan. Taiwan. J. Obstet. Gynecol. 61(5), 863–867 (2022).
Razieh, D. F., Maryam, A. R. & Nasim, T. Beneficial effect of luteal-phase gonadotropin-releasing hormone agonist administration on implantation rate after intracytoplasmic sperm injection. Taiwan. J. Obstet. Gynecol. 48(3), 245–248 (2009).
Iwase, A. et al. Oral progestogen versus intramuscular progesterone for luteal support after assisted reproductive technology treatment: A prospective randomized study. Arch. Gynecol. Obstet. 277, 319–324 (2008).
Moini, A., Arabipoor, A., Zolfaghari, Z., Sadeghi, M. & Ramezanali, F. Subcutaneous progesterone (Prolutex) versus vaginal (Cyclogest) for luteal phase support in IVF/ICSI cycles: A randomized controlled clinical trial. Middle East Fertil. Soc. J. 27(1), 1–7 (2022).
Madkour, W. A. et al. Luteal phase support with estradiol and progesterone versus progesterone alone in GnRH antagonist ICSI cycles: A randomized controlled study. Hum. Fertil. 19(2), 142–149 (2016).
Kara, M., Aydin, T., Aran, T., Turktekin, N. & Ozdemir, B. Does dehydroepiandrosterone supplementation really affect IVF-ICSI outcome in women with poor ovarian reserve?. Eur. J. Obstet. Gynecol. Reprod. Biolog. 173, 63–65 (2014).
Serna, J. et al. Estradiol supplementation during the luteal phase of IVF-ICSI patients: A randomized, controlled trial. Fertil. Steril. 90(6), 2190–2195 (2008).
Fatemi, H. M., Popovic-Todorovic, B., Papanikolaou, E., Donoso, P. & Devroey, P. An update of luteal phase support in stimulated IVF cycles. Hum. Reprod. 13(6), 581–590 (2007).
Kleinstein J, Luteal Phase Study Group. Efficacy and tolerability of vaginal progesterone capsules (Utrogest™ 200) compared with progesterone gel (Crinone™ 8%) for luteal phase support during assisted reproduction. Fertil. Steril. 83(6):1641–1649 (2005).
Zegers-Hochschild, F. et al. Prospective randomized trial to evaluate the efficacy of a vaginal ring releasing progesterone for IVF and oocyte donation. Hum. Reprod. 15(10), 2093–2097 (2000).
Andersen, C. Y., Fischer, R., Giorgione, V. & Kelsey, T. W. Micro-dose hCG as luteal phase support without exogenous progesterone administration: Mathematical modelling of the hCG concentration in circulation and initial clinical experience. J. Assist. Reprod. Genet. https://doi.org/10.1007/s10815-016-0764-7 (2016).
Artini, P. G. et al. A comparative, randomized study of three different progesterone support of the luteal phase following IVF/ET program. J. Endocrinol. Investig. 18(1), 51–56 (1995).
Araujo, E., Bernardini, L., Frederick, J. L., Asch, R. H. & Balmaceda, J. P. Prospective randomized comparison of human chorionic gonadotropin versus intramuscular progesterone for luteal-phase support in assisted reproduction. J. Assist. Reprod. Genet. 11(2), 74–78 (1994).
Griesinger, G. et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: A randomized clinical trial. Hum. Reprod. 33(12), 2212–2221 (2018).
Goudge, C. S., Nagel, T. C. & Damario, M. A. Duration of progesterone-in-oil support after in vitro fertilization and embryo transfer: A randomized, controlled trial. Fertil. Steril. 94(3), 946–951 (2010).
Kohls, G. et al. Early progesterone cessation after in vitro fertilization/intracytoplasmic sperm injection: A randomized, controlled trial. Fertil. Steril. 98(4), 858–862 (2012).
Kyrou, D., Fatemi, H. M., Tournaye, H. & Devroey, P. Luteal phase support in normo-ovulatory women stimulated with clomiphene citrate for intrauterine insemination: Need or habit?. Hum. Reprod. 25(10), 2501–2506 (2010).
Prietl, G., Diedrich, K., van der Ven, H. H., Luckhaus, J. & Krebs, D. The effect of 17α-hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: A prospective and randomized controlled trail. Hum. Reprod. 7(1), 1–5 (1992).
Ceyhan, T. et al. Use of luteal estrogen supplementation in normal responder patients treated with fixed multi dose GnRH antagonist. Hum. Reprod. 22, I127–I128 (2007).
Farhi, J. et al. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil. Steril. 73(4), 761–766 (2000).
Engmann, L. et al. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: A prospective randomized study. Fertil. Steril. 89(3), 554–561 (2008).
Belaisch-Allart, J., De Mouzon, J., Lapousterle, C. & Mayer, M. The effect of HCG supplementation after combined GnRH agonist/HMG treatment in an IVF programme. Hum. Reprod. 5(2), 163–166 (1990).
Kupferminc, M. J. et al. A prospective randomized trial of human chorionic gonadotrophin or dydrogesterone support following in-vitro fertilization and embryo transfer. Hum. Reprod. 5(3), 271–273 (1990).
Aghahosseini, M. et al. Estradiol supplementation during the luteal phase in poor responder patients undergoing in vitro fertilization: A randomized clinical trial. J. Assist. Reprod. Genet. 28(9), 785–790 (2011).
Lin, H. et al. Oral estradiol supplementation as luteal support in IVF/ICSI cycles: A prospective, randomized controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 167(2), 171–175 (2013).
Yanushpolsky, E., Hurwitz, S., Greenberg, L., Racowsky, C. & Hornstein, M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization–embryo transfer cycles: A prospective randomized study. Fertil. Steril. 94(7), 2596–2599 (2010).
Elgindy, E. A., El-Haieg, D. O., Mostafa, M. I. & Shafiek, M. Does luteal estradiol supplementation have a role in long agonist cycles?. Fertil. Steril. 93(7), 2182–2188 (2010).
Isik, A. Z. et al. Single-dose GnRH agonist administration in the luteal phase of GnRH antagonist cycles: A prospective randomized study. Reprod. Biomed. 19(4), 472–477 (2009).
Yıldız, G. A., Şükür, Y. E., Ateş, C. & Aytaç, R. The addition of gonadotrophin releasing hormone agonist to routine luteal phase support in intracytoplasmic sperm injection and embryo transfer cycles: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 182, 66–70 (2014).
Dal Prato, L. et al. Vaginal gel versus intramuscular progesterone for luteal phase supplementation: A prospective randomized trial. Reprod. Biomed. 16(3), 361–367 (2008).
Propst, A. M. et al. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization-embryo transfer cycles. Fertil. Steril. 76(6), 1144–1149 (2001).
Chakravarty, B. N. et al. Oral dydrogesterone versus intravaginal micronized progesterone as luteal phase support in assisted reproductive technology (ART) cycles: Results of a randomized study. J. Steroid Biochem. Mol. Biol. 97(5), 416–420 (2005).
Friedler, S. et al. Luteal support with micronized progesterone following in-vitro fertilization using a down-regulation protocol with gonadotropin-releasing hormone agonist: A comparative study between vaginal and oral administration. Hum. Reprod. 14(8), 1944–1948 (1999).
Pouly, J. L. et al. Endocrinology: Luteal support after in-vitro fertilization: Crinone 8%, a sustained release vaginal progesterone gel, versus Utrogestan, an oral micronized progesterone. Hum. Reprod. 11(10), 2085–2089 (1996).
Salehpour, S., Tamimi, M. & Saharkhiz, N. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal-phase support in in vitro fertilization (IVF): A randomized clinical trial. Iran. J. Reprod. Med. 11(11), 913 (2013).
Bergh, C. & Lindenberg, S. A prospective randomized multicentre study comparing vaginal progesterone gel and vaginal micronized progesterone tablets for luteal support after in vitro fertilization/intracytoplasmic sperm injection. Hum. Reprod. 27(12), 3467–3473 (2012).
Doody, K. J. et al. Endometrin for luteal phase support in a randomized, controlled, open-label, prospective in-vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertil. Steril. 91(4), 1012–1017 (2009).
Tay, P. Y. S. & Lenton, E. A. The impact of luteal supplement on pregnancy outcome following stimulated IVF cycles. Med. J. Malays. 60(2), 151 (2005).
Abate, A. et al. Luteal phase support with 17α-hydroxyprogesterone versus unsupported cycles in in vitro fertilization: A comparative randomized study. Gynecol. Obstet. Investing. 48(2), 78–80 (1999).
Abate, A. et al. Intramuscular versus vaginal administration of progesterone for luteal phase support after in vitro fertilization and embryo transfer. A comparative randomized study. Clin. Exp. Obstet. Gynecol. 26(3–4), 203–206 (1999).
Aboulghar, M. A. et al. GnRH agonist plus vaginal progesterone for luteal phase support in ICSI cycles: A randomized study. Reprod. Biomed. 30, 52–56 (2015).
Aghsa, M. M., Rahmanpour, H., Bagheri, M., Davari-Tanha, F. & Nasr, R. A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest when used for luteal phase support in ICSI treatment. Arch. Gynecol. Obstet. 286, 1049–1054 (2012).
Ata, B., Yakin, K., Balaban, B. & Urman, B. GnRH agonist protocol administration in the luteal phase in ICSI-ET cycles stimulated with the long GnRH agonist protocol: A randomized, controlled double-blind study. Hum. Reprod. 23(3), 668–673 (2008).
Baker, V. L. et al. A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization. Hum. Reprod. 29(10), 12–20 (2014).
Ganesh, A. et al. Comparison of oral dydrogesterone with progesterone gel and micronized progesterone for luteal support in 1373 women undergoing in vitro fertilization: A randomized clinical study. Fertil. Steril. 95(6), 1961–1965 (2011).
Golan, A. et al. Human chorionic gonadotrophin is a better luteal support than progesterone in ultrashort gonadotrophin-releasing hormone agonist/menotrophin in-vitro fertilization cycles. Hum. Reprod. 8, 1372–1375 (1993).
Inamdar, D. B. & Majumdar, A. Evaluation of the impact of gonadotropin-releasing hormone agonist as an adjuvant in luteal-phase support on IVF outcome. J. Hum. Reprod. Sci. 5(3), 279–284 (2012).
Lockwood, G., Griesinger, G. & Cometti, B. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: A noninferiority randomized controlled study. Fertil. Steril. 101, 112–119 (2014).
Martinez, F. et al. Human chorionic gonadotropin and intravaginal natural progesterone are equally effective for luteal phase support in IVF. Gynaecological Endocrinol. 14, 316–320 (2000).
Patki, A. & Pawar, V. C. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecological Endocrinol. 23(Suppl 1), 68–72 (2007).
Stadtmauer, L., Silverberg, K. M., Ginsburg, E. S., Weiss, H. & Howard, B. Progesterone vaginal ring versus vaginal gel for luteal phase support with in vitro fertilization: A randomized comparative study. Fertil. Steril. 99, 1543–1549 (2013).
Tesarik, J., Mendoza-Tesarik, R. & Mendoza, N. Gonadotropin-releasing hormone agonist for luteal phase support: The origin of the concept, current experience, mechanism of action and future perspectives. Fertil. Steril. 106(2), 268–269 (2016).
Tournaye, H., Sukhikh, G. T., Kahler, E. & Griesinger, G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum. Reprod. 32(5), 1019–1027 (2017).
Michnova, L., Dostal, J., Kudela, M., Hamal, P. & Langova, K. Vaginal use of micronized progesterone for luteal support. A randomized study comparing Utrogestan® and Crinone® 8. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czech Republic. 161(1), 86–91 (2017).
Elgindy, E. A. et al. Towards an optimal luteal support modality in agonist-triggered cycles: A randomized clinical trial. Hum. Reprod. 33(6), 1079–1086 (2018).
Yang, D. Z. et al. A Phase III randomized controlled trial of oral dydrogesterone versus intravaginal progesterone gel for luteal phase support in in vitro fertilization (Lotus II): Results from the Chinese mainland subpopulation. Gynecological Endocrinol. 36(2), 175–183 (2020).
Tomic, V., Tomic, J., Klaic, D. Z., Kasum, M. & Kuna, K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 186, 49–53 (2015).
Gizzo, S. et al. Which luteal phase support is better for each IVF stimulation protocol to achieve the highest pregnancy rate? A superiority randomized clinical trial. Gynecological Endocrinol. 30(12), 902–908 (2014).
Kutlusoy, F. et al. Luteal phase support with estrogen in addition to progesterone increases pregnancy rates in in vitro fertilization cycles with poor response to gonadotropins. Gynecol. Endocrinol. 30(5), 363–366 (2014).
Ozer, G., Yuksel, B., Yucel Cicek, O. S. & Kahraman, S. Oral dydrogesterone versus micronized vaginal progesterone gel for luteal phase support in frozen-thawed single blastocyst transfer in good prognosis patients. J. Gynecol. Obstet. Hum. Reprod. 50(5), 102030. https://doi.org/10.1016/j.jogoh.2020.102030 (2021).
Saharkhiz, N. et al. A comparative study of dydrogesterone and micronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol. Endocrinol. 32(3), 213–217 (2016).
Horowitz, E. et al. A randomized controlled trial of vaginal progesterone for luteal phase support in modified natural cycle—Frozen embryo transfer. Gynecol. Endocrinol. 37(9), 792–797 (2021).
Belaisch-Allart, J., Testart, J., Fries, N., Forman, R. G. & Frydman, R. The effect of dydrogesterone supplementation in an IVF programme. Hum. Reprod. 2(3), 183–185 (1987).
Chi, H. et al. Vaginal progesterone gel is non-inferior to intramuscular progesterone in efficacy with acceptable tolerability for luteal phase support: A prospective, randomized, multicenter study in China. Eur. J. Obstet. Gynecol. Reprod. Biol. 1(237), 100–105 (2019).
Fusi, F. M. et al. GnRH agonists to sustain the luteal phase in antagonist IVF cycles: A randomized prospective trial. Reprod. Biol. Endocrinol. 17(1), 1–6 (2019).
Gorkemli, H., Ak, D., Akyurek, C., Aktan, M. & Duman, S. Comparison of pregnancy outcomes of progesterone or progesterone+estradiol for luteal phase support in ICSI-ET cycles. Gynecol. Obstet. Invest. 58(3), 140–144 (2004).
Ibrahem, M. A. Oral dydrogesterone versus vaginal micronized progesterone in luteal phase support after controlled ovarian stimulation using long gonadotropin-releasing hormone agonist in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Open J. Obstet. Gynecol. 9(12), 1558–1568 (2019).
Kapur, A., Prasad, S. & Kumar, A. Is luteal phase estradiol supplementation beneficial in long agonist IVF-ET cycles? First prospective randomised controlled study from Indian subcontinent. J. Clin. Diagn. Res. 12, 10 (2018).
Khrouf, M. et al. Progesterone for luteal phase support in in vitro fertilization: comparison of vaginal and rectal pessaries to vaginal capsules: A randomized controlled study. Clin. Med. Insights Womens Health 9, CMWH-S32156 (2016).
Kwon, S. K. et al. Luteal estradiol supplementation in gonadotropin-releasing hormone antagonist cycles for infertile patients in vitro fertilization. Clin. Exp. Reprod. Med. 40(3), 131 (2013).
Mele, D. et al. In vitro fertilization and psychological stress: New insight about different routes of progesterone administration. Ital. J. Gynaecol. Obstet. 32, 119–125. https://doi.org/10.36129/jog.32.02.04 (2020).
Zargar, M., Saadati, N. & Ejtahed, M. S. Comparison the effectiveness of oral dydrogesterone, vaginal progesterone suppository and progesterone ampule for luteal phase support on pregnancy rate during ART cycles. Int. J. Pharm. Res. Allied. Sci. 5(3), 229–236 (2016).
Pirard, C., Loumaye, E., Laurent, P. & Wyns, C. Contribution to more patient-friendly ART treatment: efficacy of continuous low-dose GnRH agonist as the only luteal support—Results of a prospective, randomized, comparative study. Int. J. Endocrinol. 5, 2015 (2015).
Var, T. et al. A comparison of the effects of three different luteal phase support protocols on in vitro fertilization outcomes: A randomized clinical trial. Fertil. Steril. 95(3), 985–989 (2011).
Humaidan, P. et al. The exogenous progesterone-free luteal phase: Two pilot randomized controlled trials in IVF patients. Reprod. Biomed. 42(6), 1108–1118 (2021).
ESHRE Guideline Group on Ovarian Stimulation, Bosch, E., Broer, S., Griesinger, G., Grynberg, M., Humaidan, P., et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020(2): hoaa009 (2020).
Yu, S. et al. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: A randomized controlled trial including 516 first IVF/ICSI cycles. Hum. Reprod. 33(2), 229–237 (2018).
Kuang, Y. et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 104(1), 62–70 (2015).
Connell, M. T. et al. Timing luteal support in assisted reproductive technology: A systematic review. Fertil. Steril. 103(4), 939–946 (2015).
Williams, S. C., Oehninger, S., Gibbons, W. E., Van Cleave, W. C. & Muasher, S. J. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: A randomized, prospective study. Fertil. Steril. 76(6), 1140–1143 (2001).
Sohn, S. H. et al. Administration of progesterone before oocyte retrieval negatively affects the implantation rate. Fertil. Steril. 71(1), 11–14 (1999).
Watters, M., Noble, M., Child, T. & Nelson, S. Short versus extended progesterone supplementation for luteal phase support in fresh IVF cycles: A systematic review and meta-analysis. Reprod. Biomed. 40(1), 143–150 (2020).
Fatemi, H. M. Simplifying luteal phase support in stimulated assisted reproduction cycles. Fertil. Steril. 110(6), 1035–1036 (2018).
Yanushpolsky, E. H. Luteal phase support in in vitro fertilization. Semin. Reprod. Med. 33(2), 118–127 (2015).
Murugan, V. A., Murphy, B. O., Dupuis, C., Goldstein, A. & Kim, Y. H. Role of ultrasound in the evaluation of first-trimester pregnancies in the acute setting. Ultrasonography 39(2), 178 (2020).
Tavaniotou, A., Smitz, J., Bourgain, C. & Devroey, P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum. Reprod. Update 6(2), 139–148 (2000).
Csapo, A. I., Pulkkinen, M. O., Ruttner, B., Sauvage, J. P. & Wiest, W. G. The significance of the human corpus luteum in pregnancy maintenance: I Preliminary studies. Am. J. Obstet. Gynecol. 112(8), 1061–1067 (1972).
Nillius, S. J. & Johansson, E. D. Plasma levels of progesterone after vaginal, rectal, or intramuscular administration of progesterone. Am. J. Obstet. Gynecol. 110(4), 470–477 (1971).
Zarutskie, P. W. & Phillips, J. A. A meta-analysis of the route of administration of luteal phase support in assisted reproductive technology: Vaginal versus intramuscular progesterone. Fertil. Steril. 92(1), 163–169 (2009).
Child, T., Leonard, S. A., Evans, J. S. & Lass, A. Systematic review of the clinical efficacy of vaginal progesterone for luteal phase support in assisted reproductive technology cycles. Reprod. Biomed. 36(6), 630–645 (2018).
Tesarik, J., Hazout, A. & Mendoza, C. Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Hum. Reprod. 19(5), 1176–1180 (2004).
Pirard, C., Donnez, J. & Loumaye, E. GnRH agonist as novel luteal support: Results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Hum. Reprod. 20(7), 1798–1804 (2005).
Oliveira, J. B. et al. Administration of single-dose GnRH agonist in the luteal phase in ICSI cycles: A meta-analysis. Reprod. Biol. Endocrinol. 8, 1–1 (2010).
Ma, X., Du, W., Hu, J., Yang, Y. & Zhang, X. Effect of gonadotrophin-releasing hormone agonist addition for luteal support on pregnancy outcome in vitro fertilization/intracytoplasmic sperm injection cycles: A meta-analysis based on randomized controlled trials. Gynecol. Obstet. Investig. 85(1), 13–25 (2020).
Pinheiro, L. M., da Silva, C. P., Moreto, T. C., Di Almeida, W. G. & de Castro, E. C. Estradiol use in the luteal phase and its effects on pregnancy rates in IVF cycles with GnRH antagonist: A systematic review. JBRA Assist. Reprod. 21(3), 247 (2017).
Munjal, R. & Gupta, S. Addition of oestradiol to progesterone for luteal phase support in GnRh antagonist IVF/ICSI cycles. Fertil. Sci. Res. 6(1), 35 (2019).
Scheffer, J. B. et al. A comparison of the effects of three luteal phase support protocols with estrogen on in vitro fertilization-embryo transfer outcomes in patients on a GnRH antagonist protocol. JBRA Assist. Reprod. 23(3), 239 (2019).
Buckett, W. M., Bentick, B. & Shaw, R. W. Induction of the endogenous gonadotrophin surge for oocyte maturation with intra-nasal GnRH analogue (buserelin): Effective minimal dose. Hum. Reprod. 13, 811–814 (1998).
Author information
Authors and Affiliations
Contributions
Study concepts: S.L.K., A.K.; Study design: S.L.K.; Data acquisition: S.L.K., K.S., G.G.; Quality control of data and algorithms: A.K., S.L.K.; Data analysis and interpretation: S.L.K., N.B., D.M.; Statistical analysis: S.L.K., A.K.; Manuscript preparation: S.L.K.; Manuscript editing: S.L.K., K.S., G.G., A.K., A.K., D.M.; Manuscript review: A.K., A.K., D.M., N.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kastora, S.L., Gkova, G., Stavridis, K. et al. Comparison of luteal support protocols in fresh IVF/ICSI cycles: a network meta-analysis. Sci Rep 14, 14492 (2024). https://doi.org/10.1038/s41598-024-64804-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64804-z
- Springer Nature Limited