Abstract
In the current work, organic cyclopenta-thiophene (CPT) based derivatives (FICR and FICD1–FICD5) were designed by the modulation of end-capped acceptor group of the reference molecule i.e., FICR, to explore their nonlinear optical (NLO) response. The effect of terminal acceptor and donor groups in the tailored compounds was explored by using DFT based quantum calculations. The UV–Vis analysis, frontier molecular orbitals (FMOs), transition density matrix (TDM), natural bond orbitals (NBOs), density of states (DOS), nonlinear optical (NLO) analyses were performed at M06/6-311G(d,p) functional. The LUMO–HOMO band gaps of FICD1–FICD5 were found to be smaller (1.75–1.92 eV) comparative to FICR (1.98 eV). Moreover, the global reactivity parameters (GRPs) were correlated with the results of other analyses. FICD2 and FICD5 with lowest band gap 1.73 and 1.75 eV showed less hardness (0.86 and 0.87 eV, respectively), high softness (0.58 and 0.57 eV−1), and larger absorption spectrum (815 and 813 nm) in gaseous phase and (889 and 880 nm) in solvent phase among all entitled compounds. All the designed chromophores (FICD1–FICD5) demonstrated a significant NLO response as compared to FICR. Particularly, FICD2 and FICD5 exhibited the highest average linear polarizability (<α>) [2.86 × 10−22 and 2.88 × 10−22 esu], first hyperpolarizability (βtot) (8.43 × 10−27 and 8.35 × 10−27 esu) and second hyperpolarizability (γtot) (13.20 × 10−32 and 13.0 × 10−32 esu) values as compared to the other derivatives. In nutshell, structural modeling of CPT based chromophores with extended acceptors, can be significantly utilized to achieve potential NLO materials.
Similar content being viewed by others
Introduction
The most demanding task in present-day research is the structural modeling of sophisticated materials to generate specific non-linear optical (NLO) responses. There is an accelerating demand for the production of high-speed NLO materials owing to their enormous use in material sciences, chemical dynamics, surface interface sciences, optical computing1, fiber optics, data transformation and dynamic image processing2,3. The production and design of NLO materials has been a prominent area of theoretical and experimental research4.
Solid inorganic materials like LiNbO3 and KH2PO4 were the primary research area in NLO, but the attention was shifted to organic systems due to several significant reasons. Following the invention of simple harmonic generation (SHG) in organic compounds in 1965, the chemists involved many fruitful studies to create novel organic compounds with distinctive NLO characteristics5,6. Over the past two decades, organic materials have emerged as promising candidates for NLO applications, owing to their refractive index dispersion, high structural versatility, swift response rates and optical spectra in comparison to inorganic compounds7. In addition, they display great stability in the visible spectrum, natural softness, exceptional optical transparency, flexibility of their chemical structure due to diverse functional groups, wide variety of production processes, electro-optic switching, the generation of terahertz (THz) waves and frequency doubling8. Donor-π-acceptor (D-π-A) architecture plays a crucial role in achieving remarkable NLO responses9. There is a spatial separation of electrons in D-π-A systems, wherein, rich (D) and electron-deficient (A) moieties are interlinked by a π-conjugated bridge. The π-bridge between the donor and acceptor moieties provides a pathway for delocalized π-electrons10. Extending the conjugation length increases the probability of electronic transitions. This architecture exhibits intramolecular charge transfer (ICT) upon photoexcitation, where an electron is transferred from the donor to the acceptor through the π-bridge. This ICT process leads to significant changes in the molecular dipole moment, resulting in large second and third order NLO effects11. An effective push–pull mechanism has been established by earlier researchers using of D-π-A compounds tailored with different acceptors12,13. These push–pull arrangements affect charge distribution, broaden the wavelength range, intensify asymmetric electron distribution and narrow the energy band gap (ELUMO-EHOMO), hence increasing the NLO response14,15. This architecture is also utilized in the design of organic molecules, polymers, and materials as used in photonics and optoelectronics16. Thiophene-based compounds are considered as promising candidates, particularly when engineered with a push–pull architecture17. This structural design involves electron-donating and electron-withdrawing groups strategically positioned along the molecular backbone, creating a strong ICT effect. Such a configuration facilitates efficient delocalization of electron density, leading to NLO responses18,19,20,21. In current era, non-fullerene (NF) based compounds received a significant attention in the area of photonics due to (i) isotropic electron transference (ii) effective electron delocalization between D and A species, (iii) completely conjugated structure, and (iv) electronegativity22. Additionally, NFs materials, characterized by planar structures and tunable energy gaps, demonstrate significantly enhanced stability than other organic systems23,24. By utilizing the stronger D and A components in NFs, can enhance the NLO characteristics by developing an appropriate π-conjugated system.
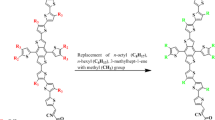
Le and coauthor have designed and synthesized a fused tris-thienothiophene (3TT) building units having molecular packing ability and strong electron-donating effect to achieve efficient NLO properties. The chromophores having three thieno[3,2-b]thiophene (TT) units combined with two cyclopentadienyl (CP) rings, resulting structures exhibit greater planarity, along with an extended electron delocalization length compared to a single thiophene ring. Moreover, increasing the length of conjugation can enhance the NLO characteristics, and a more rigid backbone can prevent bond rotation, thereby improving molecular packing25. The structure–property relationship exploited that the choice of ideal length of π-conjugation enhanced the NLO response26. Therefore, an elongated π-bridge improves the conjugation, which eventually raises the nonlinearity of the system. Moreover, its light harvesting ability results in red shifted absorption bands27. The incorporation of electronegative substituents such as cyano and nitro groups at the acceptor components of NF structures may also significantly influence their electronic characteristics28. Therefore, keeping in mind the prior discussion, in this study an organic fullerene free cyclopentathiophene (CPT) based compound is selected as parent compound (FOIC)29 comprising A–π–A framework. In the parent compound (FOIC), the bulky 1-hexyl-4-methyl groups attached to the π-spacer have been replaced with methyl (-CH3) groups to decrease computational expenses and eliminate steric hindrance arising from the large alkyl chains (Fig. 1). Hence, we designed five compounds (FICD1-FICD5) by altering its architecture from A-π-A to D-π-A and determined their NLO response. The aniline moiety has been selected as a donor owing to its simple, planar, and rigid structure and good electron-donating capabilitywhich enhances the optical nonlinearity of the system30. Literature data exploited that this is the first comprehensive DFT work to elaborate the NLO and electronic properties of FOIC based molecules. The main objective of this research article is to identify prospective materials which possess promising βtot and γtot amplitudes. It is anticipated that these FOIC based molecules would be utilized as significant NLO materials.
Computational procedure
Current quantum chemical calculations were executed in chloroform media using Gaussian 09 program31. For DFT based investigations; molecular structure input and visualizing output files of the entitled systems were generated by using the GaussView 6.032 and Chemcraft software33. The M0634 functional along with 6–311 G(d,p)35 basis set to accomplished current chemical calculations. The M06 functional, coupled with the 6-311G(d,p) basis set, has demonstrated excellent performance and shown a strong correlation with experimental data in numerous studies36,37. Therefore, we also used above mentioned functional and basis set in our study. At first, all the geometries were optimized in order to get true minima structures. The NBO 5.0 program38 was utilized for NBOs analysis i.e., calculating the energy of stabilization, find out intramolecular charge transfer (ICT) among various components (D, π, and A) of reference compound (FICR) and the investigated chromophores (FICD1-FICD5). The TD-DFT39 is one of the most widely used method for the calculation of excitation energies (UV–Vis) and FMOs analyses because of its reasonable agreement with experimental data. For this reasons, TD-DFT was used for (UV–Vis) and FMOs analyses in this study. The FMOs investigation was performed to calculate the energy gap between HOMOs and LUMOs40. The DOS analysis was also carried out to determine the charge density of the studied compounds by using PyMOlyze 2.0 program41. All the graphs were developed via using Origin 8.0 software42. The other software’s such as Multiwfn43, GaussSum44 and Avogadro45 were employed for the interpretation of results from output files.
Results and discussion
The present research is focused on designing a series of CPT based compounds (FICR and FICD1-FICD5) for NLO materials. For this purpose, already synthesized parent molecule (FOIC)29 with A–π–A type configuration is taken and by its some structural alteration the reference compound (FICR) is designed as illustrated in Fig. 1. To achieve a simple push–pull, D–π–A architecture, one of the peripheral acceptor of FOIC is exchanged with a donor moiety i.e., 4-methyl-N-phenyl-N-(p-tolyl)aniline, while other terminal acceptor is exchanged as with benzothiophene (BT) based acceptors in order to obtain FICR (Fig. 2). In the designed molecules (FICD1-FICD5), two chloro (-Cl), two nitro (-NO2), two trifluoromethyl (-CF3), two methyl acetate (-COOCH3) and two cyano (-CN) groups are substituted to BT acceptor moiety (Fig. 3). Cartesian coordinates are given in Tables S1-S6. The optimized structures of the entitled compounds are presented in Fig. 4. To study the impact of structural designing on NLO properties, DFT/TD-DFT computations are performed to calculate the transitions of NBOs, spectral absorption, HOMO/LUMO band gaps, ⟨a⟩, βtot and γtot. It is expected that this research will be significant in the field of NLO and will inspire experimental researchers to synthesize these chromophores with enhanced NLO responses.
Natural bond orbitals (NBOs) analysis
The electronic charge transfer and intramolecular charge delocalization from HOMOs to LUMOs in the π-conjugated network can be efficiently explained by NBOs investigation46,47. It demonstrate efficiency in interpreting orbital interactions within bonds, molecular stability, hyper-conjugative interactions, and hybridization of the studied compounds. Second-order perturbation theory is used to calculate the stabilization energy48 of the molecules by employing Eq. 1.
Here, i and j indicate donor and acceptor groups, while E(2) is energy of stabilization. Moreover, qi, εj, εi and Fi,j are donor orbital occupancy, diagonal and off-diagonal NBO Fock matrix elements between the NBOs. Table 1 displays some significant selected transitions while the detailed values of the investigated compounds are illustrated in Tables S7-S12.
Table 1 shows four important types of transitions such as π → π*, σ → σ*, LP → π* and LP → σ*. Among these π → π* are the most dominant transitions due to the presence of conjugation, while σ → σ* are the weak transitions due to sigma electrons. The most prominent π → π* transitions π(C21-C62) → π*(C22-C80), π(C21-C84) → π*(C22-C23), with 31.18 and 29.69 kcal/mol were observed in FICR and FICD1 and 31.64, 30.50, 29.64 and 31.36 were observed in transition π(C21-C84) → π*(C22-C23), for FICD2-FICD5, respectively. The least prominent π → π* transitions are π(C51-C53) → π*(C40-C42), for FICD1 and FICD4 having stabilization energies 0.5 kcal/mol, respectively and π(C93-N94) → π*(C82-C91), π(N103-O105) → π*(C72-N103), π(C51-C53) → π*(C40-C42), and π(C102-N103) → π*(C72-C102) in case of FICR, FICD2, FICD3 and FICD5 with stabilization energies values of 0.92, 0.93. 0.50 and 0.92 kcal/mol, respectively.
In case of σ → σ* transitions, the highest energies of stabilization were observed to be 8.58, 8.57, and 8.93 kcal/mol at σ(C10-C20) → σ*(C12-S61), σ(C10-C20) → σ*(C12-S83) and σ(C72-C102) → σ*(C102-N103) electronic transitions for compound FICR, FICD1 and FICD5, respectively. The transitions σ(C10-C20) → σ*(C12-S81) for compounds FICD2-FICD4 and having highest stabilization energies: 8.62, 8.62 and 8.58 kcal/mol, respectively. Moreover the transitions σ → σ* having the least stabilization energies (0.51 kcal/mol) were found as σ(C4-S57) → σ*(C13-S17) and σ(C4-S79) → σ*(C13-S17) transitions for FICR and FICD1, respectively. However in case of FICD2-FICD5 the transitions σ(C4-S77) → σ*(C13-S17) having least stabilization energies values of 0.51 kcal/mol, respectively.
Some important amplitudes of stability are also observed in lone pair transitions i.e., 28.29, 28.01, and 50.34 kcal/mol observed in LP2(S61) → π*(C11-C12), LP2(S83) → π*(C11-C12), LP2(O105) → π*(C103-O104), for FICR, FICD1 and FICD4. For compounds FICD2, FICD3 and FICD5 the transition LP2(S81) → π*(C11-C12) having the largest stabilization energies which are 28.18, 28.07 and 28.12 kcal/mol. Whereas, lowest stabilization energies 0.92, 0.62, 0.63, 0.51, 0.52 and 0.63 kcal/mol noted in electronic transitions LP1(S57) → σ*(C3-C4), LP1(S82) → σ*(C11-C84), LP1(S80) → σ*(C11-C82), LP1(F102) → π*(C71-C100), LP1(O105) → π*(C110-H111) and LP1(S80) → π*(C11-C82) for FICR, FICDI-FICD5 chromophores, respectively. Some other transitions with their stabilization energies can also be seen into the Tables S7-S12. Therefore, NBOs analysis of the molecules reveal that high ICT and extended hyperconjugation play a substantial role in the stability of investigated compounds.
Frontier molecular orbitals (FMOs) analysis
An FMO investigation was conducted to compare the electronic properties of the reference molecule (FICR) with its designed derivatives (FICD1-FICD5) along with their GRPs and DOS analyses. It is an outstanding method to determine the electronic transitions, chemical stability and reactivity of compounds49. Moreover, the energies of the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) were used to determine the GRPs of the studied compounds. The energy gaps of the designed compounds were computed in chloroform utilizing the M06/6-311G(d,p) functional. (Table 2). The other orbitals and their respective energy gaps are illustrated in Tables S13-S18.
Table 2 showed that EHOMOs in FICR and FICD1-FICD5 were found as −5.29, −5.26, −5.33, −5.29, −5.27 and −5.29 eV, respectively. Similarly, calculated values for ELUMOs are as −3.31, −3.36, −3.60,−3.44, −3.36 and −3.55 eV, respectively. In FICD5, HOMO is observed to be similar to FICR but LUMO is at lower energy (−3.55 eV), while with FICD2, both FICR and FICD5 showed HOMO at little bit higher energy (−5.29 eV) as compared to FICD2 (−5.33 eV). The Egap of all the designed compounds (FICD1-FICD5) is found lower than that FICR indicating easily ICT in derivatives. The least energy gap is observed in case of FICD2 (1.73 eV) and FICD5 (1.75 eV) might be due to the presence of strong electron withdrawing moieties (-NO2 and -CN groups, respectively) on the acceptors as compared to the other designed chromophores. In FICD1, the Egap was observed to be smaller than that of FICR but larger than FICD5 (1.92 > 1.75 eV). The reduction in FICD1 energy gap value as compared to FICR might be due to the replacement of terminal acceptor with benzothiophene acceptor having two -Cl groups. The increase in conjugation as well as inductive effect (-I) and resonating effect (-R) due to chloro group in FICD1 might be the reason of reduction of energy gap in FICD1. The further decreased in Egap in FICD5 might be due to the replacement of -Cl groups with strong electron with drawing -CN groups. In FICD3, the Egap (1.85 eV) was found smaller than FICD4 (1.91 eV) when the -COOCH3 group was replaced with trifluoro (-CF3) group at the acceptor unit resulting in lesser electron-withdrawing capability. Table 2 showed that, all the designed derivatives have smaller band gap as compared to the reference. The overall descending order of band gap in FICR and FICD1-FICD5 was found as : FICR > FICD1 > FICD4 > FICD3 > FICD5 > FICD2.
For the charge transfer, Egap is a crucial factor i.e., narrower the band gap, larger the charge transfer rate. The FICD2 and FICD5 exhibited the lowest energy gap due to the enhanced conjugation and electron withdrawing nature at acceptor caused by two -NO2 and two -CN strong electrons withdrawing groups resulting in the most efficient push–pull mechanism out of all the entitled compounds. The data of orbital energies can be used to depict the charge transfer analysis with the help of FMOs pictographs as shown in Fig. 5 and Figure S1. Figure 5 showed that HOMOs were primarily located over donor and π-spacer. In contrast, LUMOs exhibited a considerable charge density over acceptor unit across all the entitled compounds. The mesomeric effects on acceptor groups primarily involve the withdrawal of electron density from neighboring atoms through resonance delocalization. In case of FICD2, resonance stabilization involved the delocalization of electron density onto the oxygen atoms, resulting in a significant withdrawal of electron density from the adjacent atoms. Similarly, the mesomeric effect of cyano group was attributed to the resonance stabilization involving the carbon–nitrogen triple bond, leading to the withdrawal of electron density from neighboring atoms in FICD5. Overall, both -NO2 and -CN groups exert strong mesomeric effects, influencing the electronic properties and reactivity of molecules containing these acceptor groups.
Thus, a notable transfer of electronic charge is observed from donor to acceptor with the help of π-spacer moiety in the investigated molecules as illustrated in Fig. 5. For HOMO the charge is major located over the donor and π-spacer then in LUMO this charge is significantly moves towards the acceptors. The presence of π-spacer enhances ICT from electron-rich to electron-deficient molecules, demonstrating the effectiveness of these molecular systems as NLO materials.
UV–Visible analysis
In order to estimate the optoelectronic properties of the entitled chromophores (FICR and FICD1-FICD5), their UV–Visible analysis is carried out in chloroform and gaseous phase by employing TD-DFT computations at the above mentioned functional50. This analysis provides understanding of charge transference, contributing configurations and types of electronic transitions in the investigated compounds51,52. Moreover, the effect of the addition of a donor and modulation of terminal acceptor group on the optical properties of compounds is also studied. By executing TD-DFT computations, six lowest singlet–singlet allowed transitions have been calculated for FICR and FICD1-FICD5 and the results are tabulated in Tables S19-S24. The highest transition wavelengths (λmax) along with their major contributing percentages are shown in Table 3 and their UV–Vis spectra (gaseous phase) is depicted in Fig. 6.
Table 3 clearly depicts that all the chromophores showed highest absorption in the visible region of electromagnetic spectrum. In gaseous phase, the chromophore FICR exhibited the λmax with major H → L (97%) contribution at the range of 701 nm and oscillator strength (fos) of 1.77. All the designed chromophores FICD1-FICD5 showed greater λmax values at the range of 734–815 nm than FICR. The compound FICD2 and FICD5 showed the largest absorption wavelength as 815 and 813 nm and the least transition energy value of 1.52 and 1.53 eV with a smallest fos of 0.93 and 0.97, respectively and HOMO–LUMO contribution of H → L (97%). Both AICD2 and FICD5 were observed as the most-red shifted derivatives which might be due to the presence of -NO2 and -CN groups on the acceptor moiety that reduced the energy gap between molecular orbitals. The λmax (nm) of all the designed molecules is in the following ascending order: FICR > FICD4 > FICD1 > FICD3 > FICD5 > FICD2. The NLO efficiency of these materials was also calculated from excitation energy. The chromophores with lowest excitation energy exhibit bathochromic shift resulting in efficient NLO properties. The transition energy values (eV) gives the reverse order of λmax: FICR < FICD4 < FICD1 < FICD3 < FICD5 < FICD2. The UV–Visible graph in gaseous phase is displayed in Fig. 6.
In solvent phase (chloroform), the chromophore FICR exhibited the λmax with major H → L (94%) contribution at the range of 771 nm and oscillator strength of 2.20. All the designed chromophores FICD1-FICD5 showed greater λmax values at the range of 806–880 nm than FICR (Table 4). Like gas phase, in chloroform media both FICD2 and FICD5 chromophores showed the largest absorption wavelength as 889.29 and 879.95 nm with the least transition energy value of 1.39 and 1.41 eV, respectively and HOMO–LUMO contribution of H → L (94%). Both these compounds have strong electron withdrawing -NO2 and –CN groups on the acceptor moiety, hence, create a strong push–pull architecture that relatively lower the excitation energy and improved the absorption spectra towards bathochromic shift. The UV–Visible graph in solvent phase is displayed in Fig. 7. Literature study revealed that the polar medium aids in stabilizing the π–π* state alongside the n–π* state by employing a suitable electronic level53. Typically, the energy of compound interactions in polar media is governed by non-covalent interactions (NCIs) and polarity influences. This factor delineates dipolar interactions and hydrogen bonding, which play a significant role in stabilizing the molecules' first singlet electronic level. As solvent polarity increases, the molecule experiences a red shift54. The excited state is considered to be more polar than the ground state, resulting in greater stabilization of the excited state compared to the ground state in chloroform. Therefore, the current studied compounds showed wider absorption spectra in chloroform than that of gaseous phase which significantly improve the NLO properties of these compounds.
Global reactivity parameters (GRPs)
To explore the reactivity and stability of the designed compounds, the DFT approach is employed to estimate energy values, allowing for the examination of global reactivity parameters such as global hardness (η), global softness (σ), global electrophilicity index (ω), electronegativity (X), ionization potential (IP), electron affinity (EA), and chemical potential (μ)55. The GRPs for current study chromophores were calculated by utilizing Koopmans' theorem56 Equations: S1-S8 given in Table S29. Either donating or accepting capability of a molecule within a system is directly linked to its ionization potential (IP) and electron affinity (EA), respectively. Electronegativity (X) governs a compound's capacity to attract the electron cloud towards its own nucleus57. Furthermore, the energy gap is closely linked to the chemical potential, hardness, and stability of a compound, whereas it has an inverse relation with reactivity58.
Among all the designed compounds, FICD2 and FICD5 are regarded as the softer, less stable, and most reactive ones owing to their higher global softness value of 0.58 and 0.57 eV, respectively. The global softness of the designed compounds follows a descending order: FICD2(0.58 eV) > FICD5(0.57 eV) > FICD3(0.54 eV) > FICD1 = FICD4(0.52 eV) > FICR (0.50 eV) (Table 5). Nevertheless, the trends in global hardness values were noted to be opposite to those of global softness. The descending order of η is provided as follows: FICR (0.99 eV) > FICD4 (0.96 eV) > FICD1 (0.95 eV) > FICD3 (0.92 eV) > FICD5 (0.87 eV) > FICD2 (0.86 eV). The ΔNmax values reveal that the FICD2 and FICD5 compounds have the highest value at 5.18 and 5.06 eV, demonstrating a constant charge transfer throughout these molecules. The FICD2 and FICD5 were predicted to be the most favorable compound as compared to the others owing to their maximum softness value of 0.58 and 0.57 eV and lowest hardness value of 0.86 and 0.87 eV, respectively. Compounds (FICD2 and FICD5) with lower softness values exhibit higher polarizability with grater ICT might be consider as efficient materials for NLO applications.
Density of states (DOS)
The DOS analysis was accomplished to validate the findings obtained by FMO analysis for the studied compounds (FICR and FICD1-FICD5)59 are presented in Fig. 8. On graphs, the HOMOs also called the valence band are represented at right side, whereas the LUMOs known as conduction band are shown at left side. The x-axis depicts the energy gap, which represents the separation of HOMO and LUMO60. To explain the findings regarding the density of states (DOS), the compounds are categorized into three segments: donor (donating group), π-spacer (linker), and acceptor (end capped acceptor unit) as demonstrated by blue, green, and red lines, correspondingly. In the reference compound, donor shows electronic charge distribution of 24.60% toward HOMO and 0.50% to LUMO. Whereas π-spacer shows that electronic charge distribution provides 69.60% to HOMO and 57.50% to LUMO. Additionally, acceptor display electronic charge distribution pattern as 5.70% toward HOMO and 42.00% toward LUMO. On the other hand, in the designed compounds (FICD1-FICD5), donor display the electronic charge distribution pattern as 21.90, 24.10, 24.10, 23.10 and 26.10% to HOMO and 0.20% to LUMO. Similarly, for FICD1-FICD5, π-spacer distributes 72.50, 69.30, 69.70, 70.80 and 67.70% to HOMO and 30.10, 17.60, 20.70, 21.30 and 19.10% to LUMO. Likewise, acceptor shows the electronic charge distribution pattern as 5.60, 6.70, 6.20, 6.10 and 6.20% to HOMO and 69.70, 82.30, 79.10, 78.50 and 80.70% to LUMO. These findings showed that in the compounds, the electron density in the FMOs analysis of the studied compounds shows that the HOMO density is positioned over donor and π-spacer. While in the case of LUMO electron density is predominantly located over acceptor in all studied compounds.
Transition density matrix and exciton binding energy
The TDM investigations plays an essential part in determining the amount of electronic-charge transfer within reference and designed compounds61. The TDM is useful for assessing charge density excitation, electron–hole pair localization and delocalization, and excited state interactions between electron-accepting and electron-donating entities60.
Additionally, it is also helpful in understanding transition behavior from the ground (S0) to excited state (S1)62. The TDM of the studied compounds was calculated using the M06/6-311G(d, p) method. The influence of the hydrogen atom is ignored in this analysis owing to its small influence on electronic transitions. The TDM heat map of all the designed compounds demonstrate the nature of transition is illustrated in Fig. 9. In this visual representation, the lower horizontal axis and the left-side vertical axis depict the atom count, while the right-side vertical axis represents the transition coefficient and electron density resulting from interactions with light. Furthermore, the green dots on the dark blue background provide a pictorial illustration of charge transfer. To understand the distribution of charge density, we divided each designed compound into three components: π-spacer (linker), donor and acceptor. As indicated in the pictographs, the highest electronic cloud was apparent on the π -linker in FICR and FICD1 to FICD5. Although a little charge can be observed on the donor region, which successfully facilitate the shifting of electron density towards the acceptor despite trapping by incorporating electron coherence efficiently transferred from the donor to the π-linker.
Moreover, the binding energy (Eb) serves as a crucial parameter aiding researchers in evaluating the optoelectronic characteristics of material63. It facilitates researchers in computing the coulombic interaction forces between the hole (h) and electron (e). At excited state, exciton dissociation has an inverse relationship with the Eb, however there is a direct correlation between the Eb and the coulombic interaction between the h and e. Reduced Eb results in higher charge mobilities64. The Eb of designed chromophores FICR and FICD1-FICD5 was determined using Eq. 265.
In Eq. (4), \({\text{E}}_{{{\text{H}} - {\text{L}}}} { }\) signifies energy difference between HOMO/LUMO. \({\text{E}}_{{{\text{opt}}}} { }\) represents that the minimum energy required for the initial excitation from S0 to S1, resulting in generation of electron and hole66,67. Table 6 displays DFT computed findings for Eb of the above-mentioned chromophores.
The Eb value revealed that, FICD2 and FICD5 demonstrated a greater extent of charge separation when compared to other derivatives. Table 6 presents the calculated values of the HOMO–LUMO gap (EH-EL), the first single excitation energies (Eopt), and the excitation binding energies (Eb), combined with its high current charge density, makes FICD2 and FICD5 a highly promising candidate for achieving exceptional efficiency in NLO compounds. The observed correlation between binding energy and TDM outcomes demonstrates a remarkable degree of charge transfer within molecules. The following compounds' binding energy values are listed in decreasing order: FICD4 > FICD1 > FICR > FICD3 > FICD2 > FICD5.
Nonlinear optical (NLO) analysis
Linear polarizability (α) defines the potential of electric field to distort the molecular electron distribution68. The hyperpolarizability (βtot, γtot, etc.) is a key parameter that characterizes the nonlinear optical behavior of molecules and atoms, encompassing a wide range of complex phenomena within the field of nonlinear optics. The first hyperpolarizability (βtot) is ascertained through the finite field method, where the proximity of an applied electric field influences the system's molecular energy. The relationship between molecular structure and NLO effects is defined by the charge transfer capability and hyperpolarizability of a substance. When a material displays a substantial NLO response, it demonstrates an elevated dipole moment, significant linear polarization, and substantial hyperpolarizability values.
The dipole moment (µtot)69 of all the entitled compounds was determined by using Eq. 2.
Similarly, the linear polarizability ⟨a⟩70 was computed with the help of Eq. 3.
The magnitude of first hyperpolarizability βtot71 was measured with the help of Eq. 4.
where the βx = βxxx + βxyy + βxzz, βy=βyyy + βxxy + βyzz and βz = βzzz + βxxz + βyyz.
The second hyperpolarizability γtot 72 was calculated by using Eq. 5.
The calculated results for the electric dipole moment (µtot), linear polarizability (< α >), total first order hyperpolarizability (βtot), and total second-order hyperpolarizability (γtot), along with their respective tensors, have been presented in Tables S25-S28 for the FICR and FICD1-FICD5 systems. A summary of the key discoveries can be found in Table 7.
The dipole moment arises because of variations in electronegativity. A larger disparity in electronegativity corresponds to an increased dipole moment (µtot). In the context of the FICD5 molecule, it exhibited the highest total dipole moment (µtot) of 19.76 Debye (D) because of the notably high electronegativity cyano groups among all derivatives. The FICR and FICD1-FICD5 have shown the trend for dipole moment values as; FICD1 < FICD3 < FICD4 < FICR < FICD2 < FICD5. The increased electronegativity of atoms results in the attraction of charge density towards them, thereby leading to increase of linear polarizability. Consequently, the greatest linear polarizability, measured at 2.88 × 10−22 esu, was investigated in FICD5. This phenomenon can be associated with the incorporation of electronegative functional groups within the acceptor moiety. The observed linear polarizability trend of FICR and FICD1–FICD5 is FICR < FICD1 < FICD3 < FICD4 < FICD2 < FICD5. In the context of the FICD5 model, the dominant tensor component responsible for linear polarizability was oriented along the x-axis, denoted as αxx, with a magnitude of 5.94 × 10−22 esu. This specific tensor component plays a prominent role in determining the overall linear polarizability value.
Among the studied compounds, FICD2 exhibited the highest first hyperpolarizability at 8.43 × 10−27 esu. In contrast, FICD1 displayed the lowest first hyperpolarizability at 5.70 × 10−27 esu, attributed to minimal intramolecular charge transfer. Specifically, the component βxxx = 8.41 × 10−27 esu. contributes significantly to the overall first hyperpolarizability value in FICD2. Among all the compounds, the highest second hyperpolarizability value was observed in FICD2, measuring 13.2 × 10–32 esu. However, the trend for second hyperpolarizability is as follows: FICD1 < FICR < FICD4 < FICD3 < FICD5 < FICD2. Additionally, the second hyperpolarizability value, like linear polarizability and first hyperpolarizability, was contributed by the γX tensor, amounting to 1.32 × 10−32 esu. The calculated dipole moment, average polarizability, second-order and third-order polarizability values of the investigated chromophores (FICR, FICD1–FICD5) were significantly greater than that for para-nitroaniline (p-NA)73, a standard molecule for the analysis of the NLO response. The γtot values of FICR, FICD1, FICD2, FICD3, FICD4 and FICD5 were determined to be (8.16 × 10−32, 7.94 × 10−32, 1.33 × 10−31, 9.44 × 10−32, 8.24 × 10−32 and 1.30 × 10−31) larger than the γtot value of p-NA (7.29 × 10−36 esu)73, respectively.
In literature a lot of work has been done on the NLO study of both inorganic74 and organic compounds75, however, organic compounds showed significant NLO properties. Additionally, a comparative study was made between our designed derivatives with reported findings of compounds similar analogues (DFPPC and DCPPC)76. The findings of designed chromophores (FICR and FICD1-FICD5) showed remarkable results in terms of linear polarizability and second hyperpolarizability values as compared to DFPPC and DCPPC. Specifically, the linear polarizability values showed that designed chromophores (FICR and FICD1-FICD5) were 100, 103, 109, 105, 106 and 110 times greater than compound DFPPC (2.6116 × 10−23 esu) and 85, 87, 92, 88, 89 and 93 times greater than compound DCPPC (3.0772 × 10–23 esu). Similarly, the nonlinear second polarizability values of designed derivatives (FICR and FICD1-FICD5) were observed to be 25,096, 24,585, 40,615, 28,769, 25,015 and 40,000 times greater than DFPPC (3.2455 × 10−35 esu) and 26,506, 26,015, 43,042, 30,425, 26,579 and 42,339 times greater than DCPPC (3.0708 × 10–35 esu).
The analysis revealed that transitioning from the A–π–A configuration to the D–π–A configuration, coupled with an increased electron-withdrawing effect, induced pronounced charge transfer and a redshift in the spectral absorption, leading to enhanced optical nonlinearity in the derivatives. Notably, the narrowing of the band gap resulting in enhanced NLO property. Both the band gap and NLO property having inverse relation where compounds with narrower band gaps typically demonstrate superior NLO performance. It is noted that, both the compounds FICD2 and FICD5 having the lowest energy band gaps (1.73 and 1.75 eV respectively) and highest 2nd hyperpolarizability values (13.2 × 10−32 and 13.0 × 10−32 esu respectively). Due to their substantial polarizability and hyperpolarizability values, these designed compounds hold promise as prospective candidates for NLO materials. Among them, FICD2 and FICD5 emerge as a particularly outstanding constituent for nonlinear optics.
Conclusion
Herein, a series of CPT based derivatives (FICD1–FICD5) was designed from a NF molecule (FICR) by modifying the configuration from A–π–A to D–π–A. It is observed that acceptors pay a notable impact on electronic properties and to enhance the NLO response of the tailored compounds than the reference compound. Our performed analyses demonstrated that all proposed molecules experienced efficient charge transfer from donor to acceptor across a π-bridge, resulting in a lower energy gap (1.75–1.92 eV) than FICR (1.98 eV). The TDM and DOS data also supported the charge transfer process between HOMO and LUMO with an effective way. The FICD1-FICD5 showed greater absorption and less transitional energy values as compared to FICR. The broader red-shift spectrum value (λmax = 815 and 813 nm in gaseous phase and 889 and 880 nm in solvent phase) was exhibited by FICD2 and FICD5 as compared to all the other designed compounds. Moreover, NBOs analyses revealed that prolonged conjugation causes stability in the studied molecules. The significant NLO properties in terms of < α > (2.86 × 10−22 and 2.88 × 10−22 esu for FICD2 and FICD5, respectively), βtot (8.43 × 10−27 and 8.35 × 10−27 esu for FICD2 and FICD5, respectively) and γtot (13.20 × 10−32 and 13.00 × 10−32 esu) were investigated as compared to FICR and other designed compounds. The promising NLO results for FICD2 and FICD5 might be obtained due to the strong electron-withdrawing groups. Effective NLO responses of current study chromophores may serve as a driving force for researchers to synthesis such efficient NLO chromophores for photonic applications.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Yao, S., Zhou, X. & Hu, G. Experimental study on negative effective mass in a 1d mass-spring system. New J. Phys. 10(4), 043020 (2008).
Wada, O. Femtosecond all-optical devices for ultrafast communication and signal processing. New J. Phys. 6(1), 183 (2004).
Hu, X., Jiang, P., Ding, C., Yang, H. & Gong, Q. Picosecond and low-power all-optical switching based on an organic photonic-bandgap microcavity. Nat. Photon. 2(3), 185–189 (2008).
Marder, S. R., Sohn, J. E., Stucky, G. D., Eds.; Materials for Nonlinear Optics: Chemical Perspectives. ACS Symposium Series; American Chemical Society: Washington, DC, 1991; Vol. 455. https://doi.org/10.1021/bk-1991-0455.
Franken, P. A., Hill, A. E., Peters, C. W. & Weinreich, G. Generation of optical harmonics. Phys. Rev. Lett. 7(4), 118–119. https://doi.org/10.1103/PhysRevLett.7.118 (1961).
Williams, D. J. Organic polymeric and non-polymeric materials with large optical nonlinearities. Angew. Chem. Int. Ed. Engl. 23(9), 690–703. https://doi.org/10.1002/anie.198406901 (1984).
Naeem, J. et al. Assessment of alkali and alkaline earth metals doped cubanes as high-performance nonlinear optical materials by first-principles study. J. Sci. Adv. Mater. Devices 7(3), 100457 (2022).
Kim, S. et al. Highly nonlinear optical organic crystals for efficient terahertz wave generation, detection, and applications. Adv. Opt. Mater. 9(23), 2101019. https://doi.org/10.1002/adom.202101019 (2021).
Bureš, F. Fundamental aspects of property tuning in push-pull molecules. RSC Adv. 4(102), 58826–58851 (2014).
Hassan, A. U. & Li, Y. Convenient and facile theoretical design strategy to amplify NLO response of N-doped-indolo [3,2,1-jk]carbazole push-pull A-π-D-π-A switches. J. Chem. Tech. Biotech. 99(2), 355–369. https://doi.org/10.1002/jctb.7530 (2024).
Bano, R. et al. Face specific doping of janus all-Cis-1, 2, 3, 4, 5, 6-hexafluorocyclohexane with superalkalis and alkaline earth metals leads to enhanced static and dynamic NLO responses. J. Phys. Chem. Solids 160, 110361 (2022).
Khalid, M. et al. First theoretical probe for efficient enhancement of optical nonlinearity via structural modifications into phenylene based D–π–A configured molecules. RSC Adv. 12(48), 31192–31204 (2022).
Ciorba, S., Galiazzo, G., Mazzucato, U. & Spalletti, A. Photobehavior of the geometrical isomers of two 1,4-distyrylbenzene analogues with side groups of different electron donor/acceptor character. J. Phys. Chem. A 114(40), 10761–10768. https://doi.org/10.1021/jp105383e (2010).
Garza, A. J. et al. A computational study of the nonlinear optical properties of carbazole derivatives: theory refines experiment. Theor. Chem. Acc. 133, 1–8 (2014).
Halasyamani, P. S. & Zhang, W. Viewpoint: Inorganic materials for UV and deep-UV nonlinear-optical applications. Inorg. Chem. 56(20), 12077–12085. https://doi.org/10.1021/acs.inorgchem.7b02184 (2017).
Sivakumar, T., Vignesh, S., Jeyaperumal, K. S. & Muppudathi, A. L. Synthesis, growth and investigation of structural, optical, photoluminescence and thermal studies on the ZnSO4-doped DAST crystal for NLO applications. J. Mater. Sci. Mater. Electron. 32(13), 17936–17945 (2021).
Fernandes, S. S. et al. Theoretical and experimental studies of aryl-bithiophene based push-pull π-conjugated heterocyclic systems bearing cyanoacetic or rhodanine-3-acetic acid acceptors for SHG nonlinear optical applications. Dyes Pigments 149, 566–573 (2018).
Manuela, M. et al. Synthesis, fluorescence, and two-photon absorption properties of push-pull 5-arylthieno[3,2- b ]thiophene derivatives. Eur. J. Org. Chem. 2016(31), 5263–5273. https://doi.org/10.1002/ejoc.201600806 (2016).
Fernandes, S. S. et al. Synthesis and characterization of novel thieno [3, 2-b] thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dyes Pigments 136, 46–53 (2017).
Ivanou, D., Mendes, A., Fernandes, S. S., Belsley, M., Pereira, A. I., Justino, L. L., Burrows, H. D., Raposo, M. M. M. Push-Pull N, N-diphenylhydrazones bearing bithiophene or thienothiophene spacers as nonlinear optical second harmonic generators and as photosensitizers for nanocrystalline TiO2 dye-sensitized solar cells (2018).
Fernandes, S. S., Castro, M. C. R., Ivanou, D., Mendes, A. & Raposo, M. M. M. Push-pull heterocyclic dyes based on pyrrole and thiophene: Synthesis and evaluation of their optical, redox and photovoltaic properties. Coatings 12(1), 34 (2021).
Khalid, M. et al. Designing strategies towards non-fullerene DTCR1 based compounds for the exploration of non-linear optical behavior. J. Saudi Chem. Soc. 27(4), 101683 (2023).
Duan, L., Elumalai, N. K., Zhang, Y. & Uddin, A. Progress in non-fullerene acceptor based organic solar cells. Solar Energy Mater. Solar Cells 193, 22–65 (2019).
Cheng, P., Li, G., Zhan, X. & Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photon. 12(3), 131–142 (2018).
Cheng, Y.-J., Chen, C.-H., Lin, T.-Y. & Hsu, C.-S. Dithienocyclopentathieno [3, 2-b] thiophene hexacyclic arene for solution-processed organic field-effect transistors and photovoltaic applications. Chem. Asian J. 7(4), 818–825 (2012).
Berkovic, G., Shen, Y. R. & Shadt, M. The effect of conjugation length and electron donor groups on the second order nonlinear polarizability of cyano substituted aromatic molecules. Mol. Crystals Liq. Crystals Incorp. Nonlinear Opt. 150(1), 607–616. https://doi.org/10.1080/00268948708074818 (1987).
Duerto, I. et al. DSSCs based on aniline derivatives functionalized with a tert-butyldimethylsilyl group and the effect of the π-spacer. Dyes Pigments 148, 61–71 (2018).
Lee, D.-W., Kim, T. & Lee, M. An amphiphilic pyrene sheet for selective functionalization of graphene. Chem. Commun. 47(29), 8259–8261 (2011).
Li, T. et al. Fused tris (thienothiophene)-based electron acceptor with strong near-infrared absorption for high-performance as-cast solar cells. Adv. Mater. 30(10), 1705969 (2018).
González-Lainez, M. et al. Using functionalized nonlinear optical chromophores to prepare NLO-active polycarbonate films. Dyes Pigments 119, 30–40 (2015).
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. Gaussian 09, Revision D. 01, Gaussian, Inc., Wallingford CT. See also: URL: http://www.gaussian.com (2009).
Dennington, R., Todd, A., Keith, T. A., Millam, J. M. Semichem, Inc., Shawnee Mission, KS, GaussView, Version 6 (2016).
Zhurko, G. A., Zhurko, D. A. ChemCraft Program Version 1.6 Build 315 (2009).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120(1–3), 215–241. https://doi.org/10.1007/s00214-007-0310-x (2008).
Wong, M. W., Gill, P. M. W., Nobes, R. H. & Radom, L. 6–311G(MC)(d, p): A second-row analogue of the 6–311G(d, p) basis set: calculated heats of formation for second-row hydrides. J. Phys. Chem. 92(17), 4875–4880. https://doi.org/10.1021/j100328a015 (1988).
Andersson, M. P. & Uvdal, P. New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6–311+G(d, p). J. Phys. Chem. A 109(12), 2937–2941. https://doi.org/10.1021/jp045733a (2005).
Jawaria, R. et al. Exploration of key electronic and optical nonlinearity properties of synthesized salicylaldehyde based compounds via quantum chemical approach. Opt. Quantum Electron. 55(13), 1175 (2023).
Weinhold, F., Glendening, E. D. NBO 5.0 Program Manual: Natural Bond Orbital Analysis Programs. Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin, Madison, WI 53706 (2001).
Runge, E. & Gross, E. K. U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 52(12), 997–1000. https://doi.org/10.1103/PhysRevLett.52.997 (1984).
Khan, M. U. et al. Theoretical investigation of nonlinear optical behavior for rod and T-shaped phenothiazine based D-π-A organic compounds and their derivatives. J. Saudi Chem. Soc. 25(10), 101339 (2021).
Tenderholt, A. L. PyMOlyze, Version 2.0 (2007).
OriginPro, V. OriginLab Corporation. Northampton, MA, USA (2016).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012).
Gilowski, M. et al. Gauss sum factorization with cold atoms. Phys. Rev. Lett. 100(3), 030201 (2008).
Hanwell, M. D. et al. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 4(1), 1–17 (2012).
Nan, M. I. et al. Mono-and di-substituted pyrene-based donor-π-acceptor systems with phenyl and thienyl π-conjugating bridges. Dyes Pigments 181, 108527 (2020).
Goszczycki, P., Stadnicka, K., Brela, M. Z., Grolik, J. & Ostrowska, K. Synthesis, crystal structures, and optical properties of the π-π interacting pyrrolo [2, 3-b] quinoxaline derivatives containing 2-thienyl substituent. J. Mol. Struct. 1146, 337–346 (2017).
Costa, M. P., Prates, L. M., Baptista, L., Cruz, M. T. & Ferreira, I. L. Interaction of polyelectrolyte complex between sodium alginate and chitosan dimers with a single glyphosate molecule: A DFT and NBO study. Carbohydr. Polymers 198, 51–60 (2018).
Khalid, M. et al. NLO potential exploration for D–π–A heterocyclic organic compounds by incorporation of various π-linkers and acceptor units. Arab. J. Chem. 14(8), 103295 (2021).
Khan, M. U. et al. First theoretical framework of superalkali metals [M3X (M= Li, Na, k; X= O, S, F, N)] doped all-boron B38 nanocluster: A promising class of nonlinear optical materials for optoelectronic applications. J. Photochem. Photobiol. A Chem. 440, 114667 (2023).
Zhao, Z. et al. Ultraviolet narrowband photomultiplication type organic photodetectors with Fabry–Pérot resonator architecture. Adv. Funct. Mater. 32(29), 2203606 (2022).
Xu, C. et al. Efficient semitransparent layer-by-layer organic photovoltaics via optimizing wide bandgap and narrow absorption polymer layer thickness. Solar RRL 6(8), 2200308 (2022).
Rahmalia, W., Fabre, J.-F., Usman, T. & Mouloungui, Z. Aprotic solvents effect on the UV–visible absorption spectra of Bixin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 131, 455–460 (2014).
Adeoye, M. D. et al. Effect of solvents on the electronic absorption spectra of 9, 14 dibenzo (a, c) phenazine and tribenzo (a, c, i) phenazine. Sci. Res. Essays 4, 107–111 (2009).
Khalid, M. et al. A theoretical approach towards designing of banana shaped non-fullerene chromophores using efficient acceptors moieties: Exploration of their NLO response properties. Opt. Quantum Electron. 55(3), 258 (2023).
Koopmans, T. Über Die Zuordnung von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica 1(1–6), 104–113 (1934).
Parr, R. G., Donnelly, R. A., Levy, M. & Palke, W. E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 68(8), 3801–3807 (1978).
Khalid, M. et al. Exploration of noncovalent interactions, chemical reactivity, and nonlinear optical properties of piperidone derivatives: A concise theoretical approach. ACS Omega 5(22), 13236–13249. https://doi.org/10.1021/acsomega.0c01273 (2020).
Khalid, M. et al. Efficient tuning of small acceptor chromophores with A1-π-A2-π-A1 configuration for high efficacy of organic solar cells via end group manipulation. J. Saudi Chem. Soc. 25(8), 101305 (2021).
Ans, M. et al. Designing three-dimensional (3D) non-fullerene small molecule acceptors with efficient photovoltaic parameters. ChemistrySelect 3(45), 12797–12804. https://doi.org/10.1002/slct.201802732 (2018).
Khan, M. U., Fatima, A., Nadeem, S., Abbas, F., Ahamad, T. Impact of π-linkers modifications on tuning nonlinear optical amplitudes of pyridoquinazolinone-based aromatic dyes: A rational entry to novel D-π-A NLO materials. Polycyclic Aromatic Compd. 1–31 (2023).
Ahmad, M. S. et al. Synthesis and XRD, FT-IR vibrational, UV–Vis, and nonlinear optical exploration of novel tetra substituted imidazole derivatives: A synergistic experimental-computational analysis. J. Phys. Chem. Solids 115, 265–276 (2018).
ul Ain, Q., Shehzad, R. A., Yaqoob, U., Sharif, A., Sajid, Z., Rafiq, S., Iqbal, S., Khalid, M., Iqbal, J. Designing of benzodithiophene acridine based donor materials with favorable photovoltaic parameters for efficient organic solar cell. Comput. Theor. Chem. 1200, 113238 (2021). https://doi.org/10.1016/j.comptc.2021.113238.
Thompson, B. C. & Fréchet, J. M. J. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 47(1), 58–77. https://doi.org/10.1002/anie.200702506 (2008).
Köse, M. E. Evaluation of acceptor strength in thiophene coupled donor-acceptor chromophores for optimal design of organic photovoltaic materials. J. Phys. Chem. A 116(51), 12503–12509. https://doi.org/10.1021/jp309950f (2012).
Khan, M. U. et al. Prediction of second-order nonlinear optical properties of D–π–A compounds containing novel fluorene derivatives: A promising route to giant hyperpolarizabilities. J. Clust. Sci. 30(2), 415–430 (2019).
Khalid, M., Ali, A., Jawaria, R., Asghar, M. A., Asim, S., Khan, M. U., Hussain, R., ur Rehman, M. F., Ennis, C. J., Akram, M. S. First principles study of electronic and nonlinear optical properties of A–D–π–A and D–A–D–π–A configured compounds containing novel quinoline–carbazole derivatives. RSC Adv. 10(37), 22273–22283 (2020).
Sun, Y.-X. et al. Experimental and density functional studies on 4-(3, 4-dihydroxybenzylideneamino) antipyrine, and 4-(2, 3, 4-trihydroxybenzylideneamino) antipyrine. J. Mol. Struct. THEOCHEM 904(1–3), 74–82 (2009).
Kara Zaitri, L. & Mekelleche, S. M. Computational study of linear and nonlinear optical properties of substituted thiophene imino dyes using long-range corrected hybrid DFT methods. Mol. Phys. 118(4), 1618508 (2020).
Alparone, A. Linear and nonlinear optical properties of nucleic acid bases. Chem. Phys. 410, 90–98 (2013).
Plaquet, A. et al. In silico optimization of merocyanine-spiropyran compounds as second-order nonlinear optical molecular switches. Phys. Chem. Chem. Phys. 10(41), 6223–6232 (2008).
Lipkowitz, K. B. & Boyd, D. B. Three-dimensional structure database searches. Rev. Comput. Chem. 7, 67 (2009).
Muhammad, S. et al. Benchmark study of the linear and nonlinear optical polarizabilities in proto-type NLO molecule of para-nitroaniline. J. Theor. Comput. Chem. 18(06), 1950030 (2019).
Bano, R. et al. Superalkali (Li2F, Li3F) doped Al12N12 electrides with enhanced static, dynamic nonlinear optical responses and refractive indices. Mater. Sci. Semicond. Process. 143, 106518 (2022).
Khalil, K., Ludwig, R., Tighezza, A. M., Ayub, K., Mahmood, T. & Gilani, M. A. Unraveling the role of superalkalis in modulating the static and dynamic hyperpolarizabilities of emerging calix [4] arenes. Phys. Chem. Chem. Phys. (2024).
Hussain, S. et al. Efficient synthesis of nicotinaldehyde-based crystalline organic derivatives: Comparative analysis between experimental and DFT study. J. Mol. Struct. 1290, 135948 (2023).
Acknowledgements
Dr. Muhammad Khalid gratefully acknowledges the financial support of HEC Pakistan (project no. 20-14703/NRPU/R&D/HEC/2021). Authors are also thankful for cooperation and collaboration of A.A.C.B from IQ-USP, Brazil especially for his continuous support and providing computational lab facilities. The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R29), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Saadia Haq: Conceptualization; Formal analysis; Investigation; Writing - original draft; Validation; Visualization saadiahaq557@gmail.com Muhammad Khalid: Resources; software; Conceptualization; Formal analysis; Supervision; Validation. khalid@iq.usp.br Ajaz Hussain: Data curation; Visualization; Writing - review & editing drajazhussain@bzu.edu.pk Muhammad Haroon: Methodology; DFT data acquisition; Investigation; Resources; software. haroonm@miamioh.edu Saad M. Alshehri: Conceptualization; Formal analysis; Investigation; Funding acquisition; Visualization alshehri@ksu.edu.sa.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haq, S., Khalid, M., Hussain, A. et al. A first principles based prediction of electronic and nonlinear optical properties towards cyclopenta thiophene chromophores with benzothiophene acceptor moieties. Sci Rep 14, 13971 (2024). https://doi.org/10.1038/s41598-024-64700-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64700-6
- Springer Nature Limited