Abstract
Body weight is an important economic trait for sheep meat production, and its genetic improvement is considered one of the main goals in the sheep breeding program. Identifying genomic regions that are associated with growth-related traits accelerates the process of animal breeding through marker-assisted selection, which leads to increased response to selection. In this study, we conducted a weighted single-step genome-wide association study (WssGWAS) to identify potential candidate genes for direct and maternal genetic effects associated with birth weight (BW) and weaning weight (WW) in Baluchi sheep. The data used in this research included 13,408 birth and 13,170 weaning records collected at Abbas-Abad Baluchi Sheep Breeding Station, Mashhad-Iran. Genotypic data of 94 lambs genotyped by Illumina 50K SNP BeadChip for 54,241 markers were used. The proportion of variance explained by genomic windows was calculated by summing the variance of SNPs within 1 megabase (Mb). The top 10 window genomic regions explaining the highest percentages of additive and maternal genetic variances were selected as candidate window genomic regions associated with body weights. Our findings showed that for BW, the top-ranked genomic regions (1 Mb windows) explained 4.30 and 4.92% of the direct additive and maternal genetic variances, respectively. The direct additive genetic variance explained by the genomic window regions varied from 0.31 on chromosome 1 to 0.59 on chromosome 8. The highest (0.84%) and lowest (0.32%) maternal genetic variances were explained by genomic windows on chromosome 10 and 17, respectively. For WW, the top 10 genomic regions explained 6.38 and 5.76% of the direct additive and maternal genetic variances, respectively. The highest and lowest contribution of direct additive genetic variances were 1.37% and 0.42%, respectively, both explained by genomic regions on chromosome 2. For maternal effects on WW, the highest (1.38%) and lowest (0.41%) genetic variances were explained by genomic windows on chromosome 2. Further investigation of these regions identified several possible candidate genes associated with body weight. Gene ontology analysis using the DAVID database identified several functional terms, such as translation repressor activity, nucleic acid binding, dehydroascorbic acid transporter activity, growth factor activity and SH2 domain binding.
Similar content being viewed by others
Growth-related traits such as body weight are economically important traits in sheep. Body weight measurements of lambs are performed at predefined times, including birth and weaning, so that breeding programs for these traits can be considered. Birth weight is generally associated with growth-related traits and body weights at later ages1. Weaning weight is a criterion for animal selection. For example, the total weaning weight of lambs per parity for each ewe is one of the important economic traits that reflects several reproductive traits, including maternal abilities and offspring survival. The phenotypic expression of growth-related traits in lambs, such as birth weight and the early growth rate, specially until weaning age, are determined not only by offspring own genetic potential but also by the maternal environment to provide an appropriate environment in the form of suitable feeding. The environmental component may be divided into permanent and temporary environmental parts. It is strongly recommended that the direct additive, maternal additive, and maternal permanent environmental effects should be included in animal models when evaluating the genetic merit to obtain accurate estimates of variance–covariance components2.
With the rapid advancement of genome sequencing technologies and the advent of dense marker BeadChips, it is now possible to determine the genotype of tens of thousands of markers and to conduct association studies between genetic markers and phenotypic records. These types of studies, referred to as genome-wide association studies (GWAS), have become a common approach to precisely identify quantitative trait loci (QTL)3.
In several previous GWAS related to growth traits in sheep, associated genetic markers or candidate genes for body weight have been reported4,5,6,7,8,9. However, the genetic basis of sheep breeds or populations, the varied number of samples used for GWAS, and the different densities of genetic marker panels led to different reports for previous studies.
Among the various approaches proposed for GWAS, the weighted single-step GWAS (WssGWAS)10 is recently used in populations where a large number of individuals are phenotyped over several generations, but genotyped for a smaller number of individuals who belong usually to the more recent generations. In this method, using phenotypes, genotypes, and a combination of a pedigree-derived relationship matrix and genomic relationship matrix (H matrix), genome-wide estimated breeding values (GEBV) are obtained from a genome-wide single-step best linear unbiased prediction (ssGBLUP,11). In the next step, based on the equivalence between marker effect models and breeding value models, GEBVs are converted to marker effects, and SNP effects are consequently estimated. One of the attractive features of WssGWAS is that it is possible to consider unequal variances for SNPs which leads to improved accuracy in estimating SNP effects10. In livestock populations, with the small number of phenotypes and genotypes, for which QTLs with large effects play an important role in phenotypic variance of target traits, WssGWAS may perform better than traditional GWAS methods12. This method has recently been used to identify genomic regions affecting production and reproductive traits in livestock13,14,15. In the present study, we implemented WssGWAS to identify genomic regions and related candidate genes associated with birth and weaning weights in Baluchi sheep. To understand the genetic basis of birth and weaning weights in sheep, we also performed a post GWAS function analyses including GO and KEGG pathway analyses to identify the biological processes and functional terms significantly enriched with identified candidate genes.
Material and methods
Phenotypes, genotypes, and pedigree
The phenotypic data used in this research included 13,408 birth weight and 13,170 weaning weight data of Baluchi lambs that were collected at Abbas-Abad Baluchi Sheep Breeding Station located in Mashhad-Iran. The pedigree file encompassed 22,160 animals including 518 sires and 6,283 dams. Details of management and feeding for Baluchi sheep were previously reported by Gholizadeh et al.16. The birth weights of lambs were recorded after birth. Subsequently, the lambs were raised until 90 days of age to determine the waning weight of each lamb. Characteristics of the data structure are summarized in Supplementary Table 1.
A total of 94 lambs were genotyped by Illumina 50K SNP BeadChip for 54,241 markers. Genotype data were provided by the animal genetics group of Sari Agriculture Science and Natural Resource University-Iran17. SNPs with unknown positions on the Ovine genome, and SNPs located on sex chromosomes were initially excluded from the data. Further quality control for Autosome SNPs was performed by Plink18 using the following criteria: individual call rate ≥ 95%; SNP call rate ≥ 95%; minor allele frequency ≥ 0.05; strong Hardy–Weinberg equilibrium violation p-value ≥ 10−6. After quality control, 94 lambs, and 41,102 SNPs remained for further analysis. For WssGWAS analysis, missing genotypes were imputed using Beagle software version 4.119.
Statistical model
First, weaning weights were adjusted to 90 days of age as follows,
where BW and WW are the birth weight and weaning weight of lambs, respectively. The univariate animal model including maternal effects used for ssGBLUP was as follows:
where y is the vector of observations; b is the vector of significant fixed effects (sex, birth type, herd, birth year, and age of mother at lambing); \(\mathbf{a}\boldsymbol{ }\sim N(0,{\varvec{H}}{\sigma }_{\text{a}}^{2})\) is the vector of direct additive genetic effects; \(\mathbf{p}\boldsymbol{ }\sim N(0,{\varvec{I}}{\sigma }_{\text{p}}^{2})\) is the vector of maternal permanent environmental effects; \(\mathbf{m}\boldsymbol{ }\sim N(0,{\varvec{H}}{\sigma }_{\text{m}}^{2})\) is the vector of maternal genetic effects; \(\mathbf{e}\boldsymbol{ }\sim N(0,{\varvec{I}}{\sigma }_{\text{e}}^{2})\) is the vector of random residuals; where \({\sigma }_{\text{a}}^{2}\), \({\sigma }_{\text{p}}^{2}\), \({\sigma }_{\text{m}}^{2}\), and \({\sigma }_{\text{e}}^{2}\) are the additive genetic, maternal permanent environmental, maternal genetic and residual variances, respectively. X, Z, W and S are the incidence matrices of b, a, p, and m, respectively.
H is the hybrid matrix that combines pedigree and genomic information11,20, and I is an identity matrix. The inverse of the H matrix was calculated as follows,
where A is the numerator relationship matrix based on pedigree data for all individuals; A22 is the numerator relationship matrix corresponding to the genotyped individuals; Gω is the weighted genomic relationship matrix which was obtained using Gω = αG + βA22 where α and β are weighting factors, selected to be 0.95 and 0.05, respectively. These weights were used to make G positive-definite, improve convergence21,22 , scale the genomic information to be compatible with the pedigree information and to control bias23,24; G is the genomic relationship matrix21, obtained as follows:
where Z is a matrix of gene content adjusted for allele frequencies (with elements of 0-2p, 1-2p, and 2-2p representing genotypes AA, Aa, and aa, respectively; p is the minor allele frequency (MAF)), D represents a diagonal matrix carried the weights of SNP, pi is the MAF of the ith SNP and m implies the number of SNPs.
Variance components and heritability of the studied traits were estimated using average information restricted maximum likelihood (AI-REML) method25 via pedigree data. Marker effects and further weights required for constructing G matrix were calculated in an iterative way proposed by Wang et al.10,26. An iteration method with the steps described below was used for the association study.
-
1.
First iteration (t = 1),
$$\left( {{\text{t }} = { 1}} \right):{\mathbf{D}} = {\mathbf{I}};{\mathbf{G}}\left( {\text{t}} \right) \, = {\mathbf{ZD}}({\mathbf{t}}){\mathbf{Z}}\prime ,\;where\lambda = \frac{1}{{\mathop \sum \nolimits_{i = 1}^{M} 2p_{i} \left( {1 - p_{i} } \right)}} \left( {{\text{VanRaden}},{ 2}00{8}} \right)$$ -
2.
GEBV calculation,
In this step, GEBVs were calculated for the entire population using the ssGBLUP approach, considering:
$$\mathbf{H}^{{ - 1}} = \mathbf{A}^{{ - 1}} + \left| {\begin{array}{*{20}c} {\mathbf{0}} & {\mathbf{0}} \\ {\mathbf{0}} & {\left( {0.95\mathbf{G}_{{\left( t \right)}} + 0.05\mathbf{A}_{{22}} } \right)^{{ - 1}} - \mathbf{A}_{{22}}^{{ - 1}} } \\ \end{array} } \right|$$ -
3.
Calculation of marker effects,
Marker effects (\(\widehat{{\varvec{u}}}\)) were obtained via GEBV conversion as,\(\varvec{\hat{u}}(t) = \lambda \varvec{D}_{{(t)}} \varvec{Z}\prime \varvec{G}_{{(t)}}^{{ - 1}} \widehat{\varvec{a}}_{g}\) where \({\widehat{a}}_{g}\) is the GEBV of individuals that were genotyped.
-
4.
Calculation of SNP weights,
SNP weights for the next iteration were obtained using the \(\text{Non}\) linearA approach as follows, \({d}_{i}= {CT}^{\frac{ \left|{\widehat{a}}_{i}\right|}{sd(\widehat{a})}-2}\)21. where \(CT\) is a constant, (1.125,21), that determines the departure from normality; \(\left|{\widehat{a}}_{i}\right|\) is the estimated effect for marker j, and \(sd(\widehat{a})\) is the standard deviation of the estimated effects of SNP. The NonlinearA approach avoids extreme values for weights and provides good convergence properties27. This is achieved as the maximum variation in weights is limited by the minimum between 5 and the exponent of CT28.
-
5.
The normalization of SNP weights to keep the total genetic variance constant as follows:
$$D_{{\left( {t + 1} \right)}} = \frac{{tr\left( {D_{t} } \right)}}{{tr\left( {D_{t} + 1} \right)}} D_{{\left( {t + 1} \right)}}$$ -
6.
Weighted G calculation (\({G}_{(t+1}\)) as follows,
$${G}_{(t+1)}= \lambda Z{D}_{(t+1)}Z{\prime}$$ -
7.
t = t + 1 and loop to step 2.
The SNP effects were estimated by 3 iterations. The percentage of genetic variance explained by the ith SNP window was calculated as follows:
$$\frac{{var\left( {a_{i} } \right)}}{{\sigma_{a}^{2} }} \times 100\% = \frac{{var\left( {\mathop \sum \nolimits_{j = 1}^{x} Z_{j} \hat{u}_{j} } \right)}}{{\sigma_{a}^{2} }} \times 100\%$$where ai is the genetic value of the ith SNP window that consists of a region of consecutive SNPs; \({\sigma }_{a}^{2}\) is the total additive genetic variance; \({Z}_{j}\) is the vector of the gene content of the jth SNP for all individuals; and \({\widehat{u}}_{j}\) is the effect of the jth SNP within the ith window.
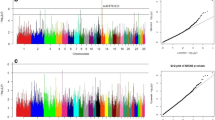
The iteration steps above were conducted via the BLUPF90 software family23for genomic analyses29. Initially, variance components were estimated using AIREMLF90 under a univariate animal model considering maternal effects. Estimated variance components then used in BLUPF90 for GEBVs prediction. PostGSf90 software was then used for SNP effects calculation. The proportion of variance explained by non-overlapping windows was estimated by summing the variance of SNPs within 1 megabase (Mb)30,31. SNP-density plot created by the CMplot package32 representing the number of quality-passed SNPs within each 1Mbp window size is given in Fig. 1. The Top 10 window regions explaining the highest percentages of additive and maternal genetic variance were selected as candidate QTL regions associated with body weights as used in previous studies in animal species31,33. Manhattan plots showing the distribution of marker effects over the genomic position were created using the CMplot package32 in R34,35.
Candidate gene identification and gene set enrichment analysis
Candidate genes associated with significant windows were retrieved from the NCBI Genome Data Viewer (https://www.ncbi.nlm.nih.gov/genome/gdv/) using OAR4 assembly. To identify Molecular functions and pathways, gene set enrichment analysis was conducted following wssGWAS analysis using DAVID (david.ncifcrf.gov/). GO terms and KEGG signaling pathways with a Fisher Exact P-value ≤ 0.01 were reported as significant.
Results
In this study, we identified window genomic regions associated with body weight at birth and weaning in the Baluchi sheep breed using WssGWAS.
Birth weight
The top 10 significant SNP windows that contributed to direct and maternal genetic effects for birth weight along with the identified potential candidate genes are listed in Table 1. The top 10 SNP windows explained a total of 4.30% of direct additive genetic variance. The additive genetic variance explained by the genomic window regions varied from 0.59 on chromosome 8 (37,942,516–38,934,945) to 0.31 on chromosome 1 (214,063,075–215,052,580) (Supplementary Tables 2–4). Further investigation of these regions on the NCBI Genome Data Viewer identified many genes in these areas.
For maternal effects on birth weight, a total of 4.62% of maternal genetic variance was explained by the top 10 SNP windows. The highest maternal genetic variance (0.84%) was explained by a genomic window on chromosome 10 (41,802,553- 42,753,634), and the lowest maternal genetic variance (0.32%) was explained by a genomic window on chromosome 17 (33,670,501–34,661,256). In these genomic window regions, several genes for maternal effects were identified (Table 1). Circle Manhattan plot from WssGWAS for direct and maternal genetic effects on birth weight is presented in Fig. 2. Results of gene ontology and KEGG signaling pathway analysis of candidate genes are presented in Table 2
The circular Manhattan plot from the WssGWAS for the direct additive and maternal genetic effects on birth weight explained by SNPs. The 2 circles from inside to outside occupy the maternal and direct additive genetic variance explained by SNPs (%), respectively. The outermost circle shows the SNP density in the 1 Mb window for each chromosome.
Weaning weight
Genomic windows identified for direct genetic and maternal effects that were significantly associated with weaning weight are listed in Table 3. For direct additive genetic effects, the top 10 genomic windows explained a total of 6.4% of the genetic variance. The contribution of explained genetic variance by genomic windows varied from 1.37% on chromosome 2 (235,006,576–235,981,086) to 0.42%, again on chromosome 2 (203,829,884–204,804,430) (Supplementary Tables 5–7). Chromosome 2 with 4 genomic window regions showed the most contribution of additive genetic variance. Several genes were identified for these genomic windows. For instance, the most significant window included LAPTM5, MATN1, SERINC2, FABP3, ZCCHC17, TRNA-CUG, SNRNP40, NKAIN1, PUM1 and SDC3 genes (Table3).
For maternal effects on WW, the highest and lowest maternal genetic variances were explained by genomic windows on chromosome 2. The top 10 window genomic regions explained 5.76% of the maternal genetic variation. The identified genes for the top genomic windows are listed in Table 3. Circle Manhattan plot from WssGWAS for direct and maternal genetic effects on WW is presented in Fig. 3. Significant GO terms and KEGG signaling pathways of candidate genes related to weaning weight are presented in Table 4.
The circular Manhattan plot from the WssGWAS for the direct additive and maternal genetic effects on weaning weight explained by SNPs. The 2 circles from inside to outside occupy the maternal and direct additive genetic variance, respectively. The outermost circle shows the SNP density in the 1 Mb window for each chromosome.
Discussion
In the present study, we identified the top 10 genomic windows that accounted for the highest explained genomic variance as candidate genomic regions for each trait. Studies have shown that the use of sliding windows for simultaneous analysis of multiple SNPs in ssGWAS is able to reduce the noise due to the estimation process36. The ssGWAS method combines all genotype, phenotype and pedigree data in single step10. Compared with single-marker regression GWAS, this method can use all markers simultaneously, resulting in greater power and precise estimate values36. Moreover, ssGWAS utilizes a large amount of phenotypic data of ungenotyped individuals which results in increasing the sample size to a certain extent, improving the accuracy of SNP effect estimation, and increasing the efficiency of SNP identification37. However, the ssGWAS model, considers equal variance for all marker effects and therefore may be constrained when traits are influenced by large QTL10. To overcome this challenge, the WssGWAS approach was proposed, in which SNP effects are weighted according to their importance in genetic variance of the trait, which improves the precision of QTL detection10.
Various studies have shown that growth traits such as body weight at birth and weaning in sheep are affected by direct genetic and maternal effects16,38. It has been proven that considering maternal effects in genetic evaluation models leads to a more accurate estimation of (co)variance, genetic parameters and, genetic evaluation of these traits39. By using the ssGWAS method, phenotypic and pedigree information of genotyped and non-genotyped animals together with the hybrid relationship matrix leads to a more reliable QTL detection and reducing the probability of spurious signals40. In this study, we performed WssGWAS to identify genomic regions and associated candidate genes responsible for genetic variation in BW and WW in Baluchi sheep. The identified genomic regions were associated with 108 and 97 possible candidate genes for BW and WW, respectively among which some of them were previously reported as candidate genes involved in body weight. Promising candidate genes found included AHCYL2, HILPDA, KCP, LEP, FZD9, MDH2, CLDN4, OSBPL2EIF5A2, RPL22L1, RPL22L1, TNIK, CYSTM1, DNAJC18, ECSCR and BBS12 for BW; and FABP3, MATN1, SDC3, FNDC5, PIKfyve, CCSER1, MMRN1, PDCL3, RRP15, TGFB2, RAPH1, ACSL6 and CSF2 for WW.
AHCYL2 gene has been reported to be associated with the growth, carcass traits, and the meat quality of sheep41. AHCYL2 gene has been reported as the main gene affecting the backfat thickness of beef cattle42. It has been documented that body weight in sheep could be controlled through up-regulation of AHCYL2 gene expression by regulating fat content and muscle development43. HILPDA is a small, lipid-droplet-associated protein expressed in several tissues that elevate lipid storage in hepatocytes, adipocytes, and macrophages44 through directly binding and inhibiting adipose triglyceride lipase45. The results of studies on muscle proteomic profiles show that body weight in sheep can be influenced by fat deposition43. KCP is a secreted protein that regulates the expression and function of BMPs46 which are multi-functional growth factors that belong to the transforming growth factor β (TGFβ) superfamily. The roles of BMPs in embryonic development have been extensively reported47. Leptin is principally produced in adipose tissue and is engaged in the regulation of body homeostasis, energy intake, storage and expenditure, fertility, and immune functions48. Haplotypes of LEP genes have been confirmed to be associated with body traits in sheep49. Leptin, with respect to its role in the regulation of energy metabolism, has been involved in the harmonious adjustment of maternal adaptations during pregnancy and lactation50. It has been reported that human breast milk leptin during the first stages of lactation may regulate processes that affect infant weight gain from the first to the sixth month51. FZD9 has been reported to be associated with lipid metabolism during lactation, quantity of milk produced52, pregnancy and, fertility53 which can support the role of this gene in the weight of lambs at birth. It has been reported that the MDH2 gene is less expressed in leaner pig breeds, and also, in general, this gene is expressed at higher levels in females than in males54, which could be concluded that this gene is related to body weight. It has been reported that the growth, health and, flexibility of low birth-weight pigs are compromised due to imperfect intestinal development. CLDN4 is a gene involved in the function of the intestinal barrier, which has a lower expression in pigs with low birth weight, which, along with the delay in the development of intestinal villi and crypts, could be a factor for the growth potential in female piglets with low birth weight55. OSBPL2 is one of the lipid transfer proteins that regulate intracellular cholesterol homeostasis and lipid droplet lipolysis in a way that OSBPL2 deficiency enlarges intracellular lipid droplets which may lead to obesity56. It has been reported that overexpression of EIF5A2 results in decreased growth rate and body weight in adult transgenic mice57. Lower expression of the RPL22L1 gene, involved in protein synthesis, has been confirmed to be associated with compromised bone formation and growth in the lambs with the most efficient body weight gain58. RPL22L1 is also reported as a candidate gene for birth weight in Holstein Friesian59,60. It has been demonstrated that TNIK has a critical role in the regulation of energy balance, glucose and fatty acid metabolism, lipid deposition, and insulin sensitivity, and TNIK knockout mice show a leaner phenotype. Studies demonstrated that CYSTM1 is significantly associated with the gestation length61,62. It has been confirmed that the gestation length is genetically in positive correlation with birth weight63. DNAJC18 and ECSCR contributed to maternal genetic effects for birth weight are associated with heat stress in cattle64,65. Heat stress during late gestation has been reported to reduce calf birth weight, which last significantly until 7 days of age (Trifkovic 2018). The BBS12 gene has been reported as a candidate gene for pure meat weight, foreshank weight, and silverside weight in beef cattle66.
FABP3 genes contributed to both direct additive and maternal genetic effects for weaning weight. FABP3 is involved in fatty acid transport from the cell membrane to the intracellular sites of fatty acid utilization and is mainly expressed in cardiac and skeletal muscle67. Clvo et al.68 reported a linkage disequilibrium between the FABP3 gene and a quantitative trait locus for milk fat contents in Manchega breed sheep. It has been reported that the composition of fatty acids in maternal milk fat has a positive effect on the live weight of lambs and the optimization of the growth potential of lambs until weaning69. Fatty acids that are produced in the liver and muscles have special functions in the body, and form the primary sources of energy stored in triglycerides, and participate in the formation of complex lipids, hormones and signaling compounds70. Cahyadi71 reported that FABP3 was significantly associated with body weights at birth, at 12 to 20 weeks, and slaughter in Korean Native Chickens. Kuzminska72 found significant associations between the FABP3 genotypes and the body weight at 210 days of age in Simmental cows. It was reported that there was a significant association between FABP3 gene variants and body weight and hock weight in swine73. The genetic polymorphisms of FABP3 were associated with early and late-stage stage body weights in the Landrace × Jeju black pig population74. It has been reported that the MATN1 gene plays an important role in muscle growth at different stages of development. The lower expression of this gene is associated with a decrease in muscle growth in low-birth-weight fetuses75. Feeding behavior and body weight are regulated by the SDC3 gene76. Srinivasa et al.77reported that expression of FNDC5 in skeletal muscle was associated with mRNA expression of IGF-I in obese individuals with reduced growth hormone showing a possible function for FNDC5 in skeletal muscle under a low growth hormone state. It was reported that the PIKfyve pathway is essential in mammary epithelial differentiation during pregnancy78 which supports its role in the maternal ability to grow lambs and their weaning weight. It has been reported that CCSER1 is associated with feed efficiency in beef cattle79 , growth and feed intake in sheep80, knuckle, biceps, and shank of beef carcass traits81. MMRN1 has been confirmed to be associated with feed efficiency, growth, and carcass traits in beef cattle82. It has been proved that NMS is involved in suckling-induced oxytocin release which is essential for milk ejection in mammals83. Lack of oxytocin or its receptor in mice results in deficient milk ejection response to the suckling stimulus84. It has been documented that PDCL3 variants are significantly associated with weight at 12 months of age, carcass weight, and loin eye area in an indigenous Korean cattle breed85. PDCL3 encodes a chaperone protein which through interacting with vascular endothelial growth factor receptor 2 is involved in angiogenesis86. RRP15 has been reported as a candidate gene for milk yield in dairy sheep which is expressed in either the milk transcriptome or the mammary gland87. TGFB2 gene is one of the members of the TGFB superfamily, which plays important roles in embryogenesis, cell differentiation, muscle development, growth, and reproductive regulation88,89. The RAPH1 gene has been confirmed to be associated with cell migration and nutrient absorption by rumen epithelia cells90. ACSL6 has been previously reported as a candidate gene associated with dry matter intake and mid-test body weight in the Angus population91 . In mammals, ACSL gene is essential for fatty acid degradation, remodeling of the phospholipid, and the long-chain acyl-CoA esters production that regulate different kinds of physiological, metabolism, and cell signaling processes92. CSF2 has been reported as one of the regulatory molecules that mediate maternal effects on embryonic development during the preimplantation period and enhance embryo competence for post-transfer survival93.
Conclusions
In the present study, we conducted a WssGWAS and identified many genomic regions and known Ovine candidate genes for birth weight and weaning weight in sheep which can provide new insights into the genetic basis of growth traits in sheep. These findings can be further investigated to search for causative mutations underlying body weights in lambs and for marker-assisted selection to improve the meat production in sheep. The genetic architecture of growth traits is very complex and these candidate genes may be under breed-specific effects. Therefore, it is recommended to study the genes identified in this study as candidate genes in different breeds of sheep under different environmental conditions. This leads to a better understanding of the complex pathways underlying growth traits in sheep. One limitation of our study is the sample size of genotyped individuals which is not large. Therefore, further high-resolution researches utilizing larger sample sizes may help validate the genomic regions and candidate genes associated with body weight in sheep identified in the present study.
Data availability
All data including genotypic data and phenotypes used in the current study that support the findings of this study are available from the corresponding author upon reasonable request. The phenotypes used in the current study were under legal restrictions and commercial ownership set by the Iran National Animal Breeding Center and Promotion of Animal Products and can be made available for academic purposes after signing a Material Transfer Agreement.
References
Boligon, A. A., de Albuquerque, L. G., Mercadante, M. E. Z. & Lôbo, R. B. Herdabilidades e correlações entre pesos do nascimento à idade adulta em rebanhos da raça Nelore. R. Brasil. Zootec. 38, 2320–2326 (2009).
Meyer, K. Variance components due to direct and maternal effects for growth traits of Australian beef cattle. Livest. Prod. Sci. 31, 179–204 (1992).
Hirschhorn, J. N. & Daly, M. J. Genome-wide association studies for common diseases and complex traits. Nat. Rev. Genet. 6, 95–108 (2005).
Almasi, M., Zamani, P., Mirhoseini, S. Z. & Moradi, M. H. Genome-wide association study of weaning traits in Lori-Bakhtiari sheep. Annals Anim. Sci. 20, 811–824 (2020).
Lu, Z. et al. Genome-wide association study of body weight traits in Chinese fine-wool sheep. Animals 10, 170 (2020).
Cao, Y. et al. Genome-wide association study of body weights in Hu sheep and population verification of related single-nucleotide polymorphisms. Front. Genet. 11, 588 (2020).
Yilmaz, O. et al. Genome-wide association studies of preweaning growth and in vivo carcass composition traits in Esme sheep. J. Anim. Breed. Genet. 139, 26–39 (2022).
Ghasemi, M., Zamani, P., Vatankhah, M. & Abdoli, R. Genome-wide association study of birth weight in sheep. Animal 13, 1797–1803 (2019).
Esmaeili-Fard, S. M., Gholizadeh, M., Hafezian, S. H. & Abdollahi-Arpanahi, R. Genes and pathways affecting sheep productivity traits: Genetic parameters, genome-wide association mapping, and pathway enrichment analysis. Front. Genet. 12, 710613 (2021).
Wang, H., Misztal, I., Aguilar, I., Legarra, A. & Muir, W. M. Genome-wide association mapping including phenotypes from relatives without genotypes. Genet. Res. 94, 73–83 (2012).
Aguilar, I. et al. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 93, 743–752 (2010).
Marques, D. B. D. et al. Weighted single-step GWAS and gene network analysis reveal new candidate genes for semen traits in pigs. Genet. Select. Evol. 50, 1–14 (2018).
Naserkheil, M., Bahrami, A., Lee, D. & Mehrban, H. Integrating single-step GWAS and bipartite networks reconstruction provides novel insights into yearling weight and carcass traits in hanwoo beef cattle. Animals 10, 1836 (2020).
Zhao, B. et al. Integration of a single-step genome-wide association study with a multi-tissue transcriptome analysis provides novel insights into the genetic basis of wool and weight traits in sheep. Genet. Select. Evol. 53, 1–14 (2021).
da Silva Neto, J. B. et al. Weighted genomic prediction for growth and carcass-related traits in Nelore cattle. Anim. Genet. 54, 271–283 (2023).
Gholizadeh, M. & Ghafouri-Kesbi, F. Estimation of genetic parameters for growth-related traits and evaluating the results of a 27-year selection program in Baluchi sheep. Small Rumin. Res. 130, 8–14 (2015).
Gholizadeh, M., Rahimi-Mianji, G., Nejati-Javaremi, A., De Koning, D. J. & Jonas, E. Genomewide association study to detect QTL for twinning rate in Baluchi sheep. J. Genet. 93, 489–493 (2014).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Browning, S. R. & Browning, B. L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097 (2007).
Legarra, A., Aguilar, I. & Misztal, I. A relationship matrix including full pedigree and genomic information. J. Dairy Sci. 92, 4656–4663 (2009).
VanRaden, P. M. Efficient methods to compute genomic predictions. J. Dairy Sci. 91, 4414–4423 (2008).
VanRaden, P. M. et al. Reliability of genomic predictions for North American dairy bulls. J. Dairy Sci 91, 305 (2008).
Misztal, I. et al. BLUPF90 and related programs (BGF90). in Proceedings of the 7th world congress on genetics applied to livestock production vol. 28 743 (Montpellier, 2002).
Fangmann, A. et al. Empirical comparison between different methods for genomic prediction of number of piglets born alive in moderate sized breeding populations. J. Anim. Sci. 95, 1434–1443 (2017).
Jensen, J., Mantysaari, E. A., Madsen, P. & Thompson, R. Residual maximum likelihood estimation of (co) variance components in multivariate mixed linear models using average information. J. Indian Soc. Agric. Stat. 49, 215–236 (1997).
Wang, H. et al. Genome-wide association mapping including phenotypes from relatives without genotypes in a single-step (ssGWAS) for 6-week body weight in broiler chickens. Front. Genet. 5, 134 (2014).
Garcia, A. L. S. et al. Development of genomic predictions for harvest and carcass weight in channel catfish. Genet. Select. Evol. 50, 1–12 (2018).
Buaban, S., Lengnudum, K., Boonkum, W. & Phakdeedindan, P. Genome-wide association study on milk production and somatic cell score for Thai dairy cattle using weighted single-step approach with random regression test-day model. J. Dairy Sci. 105, 468–494 (2022).
Aguilar, I., Misztal, I., Legarra, A. & Tsuruta, S. Efficient computation of the genomic relationship matrix and other matrices used in single-step evaluation. J. Anim. Breed. Genet. 128, 422–428 (2011).
Sweett, H. et al. Genome-wide association study to identify genomic regions and positional candidate genes associated with male fertility in beef cattle. Sci. Rep. 10, 20102 (2020).
Mohammadi, H. et al. Weighted single-step genome-wide association study uncovers known and novel candidate genomic regions for Milk production traits and somatic cell score in Valle Del Belice dairy sheep. Animals 12, 1155 (2022).
Yin, L. CMplot: Circle manhattan plot. R package version 3, (2020).
Yin, H. et al. Weighted single-step genome-wide association study of semen traits in Holstein bulls of China. Front. Genet. 10, 1053 (2019).
Team, R. D. C. R Development Core Team. R: A Language and Environment for Statistical Computing, 2019. Available in:< https://www.r-project.org (2019).
R Core Team, R. R: A language and environment for statistical computing. (2013).
Wang, H. et al. Genome-wide association mapping including phenotypes from relatives without genotypes in a single-step (ssGWAS) for 6-week body weight in broiler chickens. Front. Genet. 5, 87369 (2014).
Liu, H., Song, H., Liu, Y. & Ding, X. A single-step genome wide association study on body size traits using imputation-based whole-genome sequence data in Yorkshire pigs. Front. Genet. 12, 629049 (2021).
Pesántez-Pacheco, J. L. et al. Influence of maternal factors (weight, body condition, parity, and pregnancy rank) on plasma metabolites of dairy ewes and their lambs. Animals 9, 122 (2019).
Ghafouri-Kesbi, F., Mokhtari, M., Gholizadeh, M., Roudbar, M. A. & Abbasi, M. A. Parental imprinting effects on growth traits and Kleiber ratio in sheep. J. Agric. Sci. 160, 260–269 (2022).
Mancin, E., Lourenco, D., Bermann, M., Mantovani, R. & Misztal, I. Accounting for population structure and phenotypes from relatives in association mapping for farm animals: A simulation study. Front. Genet. 12, 642065 (2021).
Liu, Z. et al. Detection of selection signatures in South African Mutton Merino sheep using whole-genome sequencing data. Anim. Genet. 53, 224–229 (2022).
Martins, R. et al. Genome-wide association study and pathway analysis for fat deposition traits in nellore cattle raised in pasture–based systems. J. Anim. Breed. Genet. 138, 360–378 (2021).
Zhao, L. et al. Comparative proteomics reveals genetic mechanisms of body weight in Hu sheep and Dorper sheep. J. Proteomics 267, 104699 (2022).
Mattijssen, F. et al. Hypoxia-inducible lipid droplet-associated (HILPDA) is a novel peroxisome proliferator-activated receptor (PPAR) target involved in hepatic triglyceride secretion*. J. Biol. Chem. 289, 19279–19293 (2014).
Padmanabha Das, K. M. et al. Hypoxia-inducible lipid droplet-associated protein inhibits adipose triglyceride lipase. J. Lipid Res. 59, 531–541 (2018).
Ye, D. et al. Kielin/chordin-like protein deficiency causes cardiac aging in male mice. J. Mol. Med. 101, 731–742 (2023).
Kishigami, S. & Mishina, Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 16, 265–278 (2005).
Chilliard, Y., Delavaud, C. & Bonnet, M. Leptin expression in ruminants: Nutritional and physiological regulations in relation with energy metabolism. Domest. Anim. Endocrinol. 29, 3–22 (2005).
Shojaei, M. et al. Association of growth trait and Leptin gene polymorphism in Kermani sheep. J. Cell Mol. Res. 2, 67–73 (2010).
Woodside, B., Abizaid, A. & Walker, C.-D. Changes in leptin levels during lactation: Implications for lactational hyperphagia and anovulation. Horm. Behav. 37, 353–365 (2000).
Schuster, S., Hechler, C., Gebauer, C., Kiess, W. & Kratzsch, J. Leptin in maternal serum and breast milk: Association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr. Res. 70, 633–637 (2011).
Ahmad, S. M. et al. SNPs in Mammary gland epithelial cells unraveling potential difference in milk production between jersey and kashmiri cattle using RNA sequencing. Front. Genet. 12, 666015 (2021).
Neupane, M. et al. Loci and pathways associated with uterine capacity for pregnancy and fertility in beef cattle. PLoS One 12, e0188997 (2017).
Zhou, S. L., Li, M. Z., Li, Q. H., Guan, J. Q. & Li, X. W. Differential expression analysis of porcine MDH1, MDH2 and ME1 genes in adipose tissues. Genet. Mol. Res. 11, 1254–1259 (2012).
Ayuso, M., Irwin, R., Walsh, C., Van Cruchten, S. & Van Ginneken, C. Low birth weight female piglets show altered intestinal development, gene expression, and epigenetic changes at key developmental loci. FASEB J. 35, e21522 (2021).
Wang, T. et al. OSBPL2 is required for the binding of COPB1 to ATGL and the regulation of lipid droplet lipolysis. iScience 23, 101252 (2020).
Chen, M. et al. Overexpression of eIF-5A2 in mice causes accelerated organismal aging by increasing chromosome instability. BMC Cancer 11, 1–13 (2011).
Giráldez, F. J. et al. Fattening lambs with divergent residual feed intakes and weight gains: Unravelling mechanisms driving feed efficiency. Anim. Feed Sci. Technol. 273, 114821 (2021).
Zaborski, D., Grzesiak, W. & Pilarczyk, R. Detection of difficult calvings in the Polish Holstein-Friesian Black-and-White heifers. J. Appl. Anim. Res. 44, 42–53 (2016).
Cole, J. B., Waurich, B., Wensch-Dorendorf, M., Bickhart, D. M. & Swalve, H. H. A genome-wide association study of calf birth weight in Holstein cattle using single nucleotide polymorphisms and phenotypes predicted from auxiliary traits. J. Dairy Sci. 97, 3156–3172 (2014).
Purfield, D. C., Evans, R. D., Carthy, T. R. & Berry, D. P. Genomic regions associated with gestation length detected using whole-genome sequence data differ between dairy and beef cattle. Front. Genet. 10, 1068 (2019).
Fang, L. et al. Genetic and epigenetic architecture of paternal origin contribute to gestation length in cattle. Commun. Biol. 2, 100 (2019).
Coleman, L., Back, P., Blair, H., López-Villalobos, N. & Hickson, R. Sire effects on birth weight, gestation length, and pre-weaning growth of beef-cross-dairy calves: A case study in New Zealand. Dairy 2, 385–395 (2021).
Ahbara, A. et al. Genome-wide variation, candidate regions and genes associated with fat deposition and tail morphology in Ethiopian indigenous sheep. Front. Genet. 9, 699 (2019).
Peripolli, E. et al. Autozygosity islands and ROH patterns in Nellore lineages: Evidence of selection for functionally important traits. BMC Genomics 19, 1–14 (2018).
Chang, T. et al. A rapid and efficient linear mixed model approach using the score test and its application to GWAS. Livest. Sci. 220, 37–45 (2019).
Veerkamp, J. H. & Maatman, R. G. H. J. Cytoplasmic fatty acid-binding proteins: Their structure and genes. Prog. Lipid Res. 34, 17–52 (1995).
Calvo, J. H., Marcos, S., Jurado, J. J. & Serrano, M. Association of the heart fatty acid-binding protein (FABP3) gene with milk traits in Manchega breed sheep. Anim. Genet. 35, 347–349 (2004).
Ptáček, M. et al. Effect of milk intake, its composition, and fatty acid profile distribution on live weight of suckling Wallachian lambs until their weaning. Animals 9, 718 (2019).
Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 47, 39–48 (2006).
Cahyadi, M. et al. FABP3 and FABP4 genes are the potential candidates for body weights in Korean Native Chicken. Korean J. Poult. Sci. 40(2), 91–96 (2013).
Kuzminska, K. et al. FABP3 polymorphism in relation to growth traits in Simmental and Salers cows. Acta Sci. Polonorum. Zootech. 17, 1–6 (2018).
Ye, X., Robinson, J. A. B., Jiang, Z., Gibbins, A. M. V. & Gibson, J. P. Polymorphisms of histone deacetylase 1 and 3 genes and fatty acid binding protein 3 and 4 genes and their associations with economic traits in swine. in Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, August, 2002. Session 11 0–4 (Institut National de la Recherche Agronomique (INRA), 2002).
Han, S.-H. et al. Effects of ADCYP1R1, FABP3, FABP4, MC4R, MYL2 genotypes on growth traits in F 2 population between Landrace and Jeju native black pig. J. Anim. Sci. Technol. 50, 621–632 (2008).
García-Contreras, C. et al. Impact of genotype, body weight and sex on the prenatal muscle transcriptome of Iberian pigs. PLoS One 15, e0227861 (2020).
Huang, Y.-Z. et al. Genetic variants in SDC3 gene are significantly associated with growth traits in two Chinese beef cattle breeds. Anim. Biotechnol. 27, 190–198 (2016).
Srinivasa, S. et al. FNDC5 relates to skeletal muscle IGF-I and mitochondrial function and gene expression in obese men with reduced growth hormone. Growth Hormone IGF Res. 26, 36–41 (2016).
Ikonomov, O. C. et al. Unexpected severe consequences of Pikfyve deletion by aP 2-or Aq-promoter-driven Cre expression for glucose homeostasis and mammary gland development. Physiol. Rep. 4(11), e12812 (2016).
Abo-Ismail, M. K. et al. Development and validation of a small SNP panel for feed efficiency in beef cattle1. J. Anim. Sci. 96, 375–397 (2018).
Yurchenko, A. A. et al. High-density genotyping reveals signatures of selection related to acclimation and economically important traits in 15 local sheep breeds from Russia. BMC Genomics 20, 294 (2019).
Song, Y. et al. Genome-wide association study reveals the plag1 gene for knuckle, biceps and shank weight in simmental beef cattle. PLoS One 11, e0168316 (2016).
Smith, J. L. et al. Genome-wide association and genotype by environment interactions for growth traits in U.S. Red Angus cattle. BMC Genomics 23, 517 (2022).
Sakamoto, T. et al. Involvement of neuromedin S in the oxytocin release response to suckling stimulus. Biochem. Biophys. Res. Commun. 375, 49–53 (2008).
Nishimori, K. et al. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. 93, 11699–11704 (1996).
Lim, K.-S. et al. Identification of monoallelically expressed genes associated with economic traits in Hanwoo (Korean Native Cattle). Animals 12, 84 (2021).
Srinivasan, S., Meyer, R. D., Lugo, R. & Rahimi, N. Identification of PDCL3 as a novel chaperone protein involved in the generation of functional VEGF receptor 2. J. Biol. Chem. 288, 23171–23181 (2013).
Banos, G. et al. Genetic and genomic analyses underpin the feasibility of concomitant genetic improvement of milk yield and mastitis resistance in dairy sheep. PLoS One 14, e0214346 (2019).
Ingman, W. V. & Robertson, S. A. Defining the actions of transforming growth factor beta in reproduction. Bioessays 24, 904–914 (2002).
Lawrence, D. A. Transforming growth factor-beta: A general review. Eur. Cytokine Netw. 7, 363–374 (1996).
Terry, S. A., Basarab, J. A., Guan, L. L. & McAllister, T. A. Strategies to improve the efficiency of beef cattle production. Can. J. Anim. Sci. 101, 1–19 (2020).
Saatchi, M. et al. QTLs associated with dry matter intake, metabolic mid-test weight, growth and feed efficiency have little overlap across 4 beef cattle studies. BMC Genomics 15, 1–14 (2014).
Faergeman, N. J. & Knudsen, J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323, 1–12 (1997).
Loureiro, B. et al. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 150, 5046–5054 (2009).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.E.; Methodology: M.G. and M.E.; Formal analysis: H.K., M.G. and M.E.; Original draft preparation, H.F. and H.K.; Writing, review and editing, M.G.; supervision: M.G.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khazaei-Koohpar, H., Gholizadeh, M., Hafezian, S.H. et al. Weighted single-step genome-wide association study for direct and maternal genetic effects associated with birth and weaning weights in sheep. Sci Rep 14, 13120 (2024). https://doi.org/10.1038/s41598-024-63974-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63974-0
- Springer Nature Limited