Abstract
The present cross-sectional study aimed to explore the relationship between systemic inflammatory indices (SIIs) and anthropometric measures, metabolic, and liver function biomarkers in patients with non-alcoholic fatty liver disease (NAFLD). This study was carried out on 238 NAFLD patients with overweight or obesity, aged 18–55 years. Anthropometric measurements were done and body mass index (BMI), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) were estimated. Metabolic factors including serum glucose, lipid profile, liver function biomarkers, and complete blood cell count were assessed after a 24-h fasting state. SIIs including the ratios of neutrophil to lymphocyte (NLR), monocytes to lymphocyte (MLR), platelet to lymphocyte (PLR), and monocytes to high-density lipoprotein cholesterol (MHR) were calculated. Results indicate that apart from PLR, all of the SIIs significantly changed by increasing steatosis severity (all p < 0.05). Moreover, changes in NLR showed a significant association with anthropometric indices including waist circumference (p = 0.032), BMI (p = 0.047), and WHtR (p = 0.002), as well as levels of fasting blood sugar (p = 0.045), triglycerides, (p = 0.025) and low-density lipoprotein cholesterol (p = 0.006). The findings also indicate the relations between lipid profile and all studied SIIs, notably MHR and MLR. All of the SIIs exhibited associations with some liver function indices as well. MHR was positively correlated with the metabolic risk factors of NAFLD while, oppositely, PLR was considered as a preventive marker of NAFLD.
Similar content being viewed by others
Introduction
Chronic low-grade inflammation is considered as a main driver of liver-related metabolic disturbances1. Both systematic intra- and extra-hepatic inflammatory consequences trigger the progression of a cluster of pathological entities including hepatic steatosis, insulin resistance, oxidative stress, and gut dysbiosis, and consequently, emerged as non-alcoholic fatty liver disease (NAFLD)2,3. In 2020, metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed as a more appropriate term than NAFLD, because this nomenclature better defines the pathophysiology of the disease and its related metabolic disorders. Chronic inflammation and fibrosis are considered to be the main factors in the pathogenesis of MAFLD4.
NAFLD, the most common hepatic disease, is the cornerstone of a plethora of metabolic disorders including type 2 diabetes mellitus (T2DM), metabolic syndrome (Mets), and cardiovascular disease (CVD) that are inter-related through systemic inflammation3. Accordingly, the sustained activation of pro-inflammatory signaling pathways accelerates NAFLD progression to steatohepatitis, liver fibrosis, and even, carcinoma1.
Complete blood count (CBC) compartments including platelets, lymphocytes, monocytes, and neutrophils as prominent immune cells, simultaneously mediate inflammatory and metabolic processes2,5. Monocytes, as a part of the phagocytic immune system, exert critical functions in inflammation, while platelets and neutrophils are involved in cytokines and chemokines production2,6. Systemic inflammatory indices (SIIs) including monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-high-density lipoprotein cholesterol (HDL-C) ratio (MHR) have recently achieved great attention in clinical settings6. Notwithstanding the feasibility of these blood routine tests, SIIs are simple, non-invasive, and useful markers of the prognosis, diagnosis, and risk assessment for metabolic conditions2,6,7. In the case of NAFLD, due to problems in their sensitivity, validation, and expensiveness, SIIs could be preferred to current screening and diagnostic tools such as biopsy, imaging techniques, and even liver-related indices (e.g. fatty liver index, NAFLD fibrosis score, and hepatic steatosis index)8,9. The current body of evidence suggests the relation between SIIs, especially MHR, and NLR, with T2DM, Mets, and NAFLD outcomes2,10,11. Indeed, PLR has been even suggested as a protective factor of NAFLD12. To our knowledge, there is limited research on correlations between SIIs and NAFLD metabolic outcomes as well as the severity8. Therefore, the current cross-sectional study aimed to investigate the relationship between SIIs and anthropometric indices, metabolic and liver function biomarkers in patients with NAFLD.
Materials and methods
Study design
The present cross-sectional study was done on 238 NAFLD patients with overweight or obesity (body mass index (BMI) ≥ 25 kg/m2) (both genders and ages ranged from 18 to 55 years). The diagnosis of NAFLD was done by ultrasonography based on Hamaguchi et al.13 definition. The patients who were pregnant, lactating, menopause, athletes, bariatric surgery, suffering from other chronic diseases and malignancies, receiving antihypertensive, anti-hyperlipidemic, and anti-diabetic medications as well as oral contraceptives, nutritional supplements three months before the study were excluded. The study protocol was approved by the Ethics Committee of Tabriz University of Medical Science (IR.TBZMED.REC.1401.689). Moreover, informed consent was fulfilled for each subject after considering the study criteria and full explanation regarding study objectives and protocol.
Sample size
The sample size was estimated as 238 according to the prevalence rate of NAFLD in Iran, reported by Moghaddasi far et al.14 and by considering a 95% confidence level and 6% error using G- power software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). The calculation resulted in a total of 231 sample size. Considering the possiblity of nonrespodents during the data collection process, the number of participants was increased by 15%, leading to a total of 264 participants.
Data collection
Personal details including demographic and socioeconomic status as well as behavioral details e.g. smoking, alcohol drinking, medical history, and taking supplements were obtained through a face-to-face interview by a trained nutritionist. Weight and height were measured in fasting state using a calibrated stadiometer (Seca, Hamburg, Germany) with light cloths and no shoes to the nearest 100 g and 5 mm, respectively. Waist circumference (WC) was measured at the midpoint between the lower border of the rib cage and iliac crest with 0.1 cm precision whereas hip circumference (HC) was assessed around the widest part of the buttocks to the nearest 0.1 cm using a non-stretchable anthropometric tape. Then, anthropometric indices including BMI, WC to HC ratio (WHR), and WC to height ratio (WHtR) were estimated.
Laboratory assay
After 12–14 h of overnight fasting, 10 ml blood samples were collected and divided into two tubes; 5 ml whole blood in EDTA-containing vacuum blood collection tubes (Vacutainer K2E) and 5 ml vacuumed gel separator tubes to be centrifuged for sera and then stored at -80 ◦C until the end of the study. Automated and daily-calibrated Coulter CBCH1 counter was used for assessing total white blood cell count, neutrophil, lymphocyte, monocyte count per mm3 as well as platelets count in whole blood and then, the ratios NLR, MLR, PLR and MHR were calculated as SIIs. In addition, the sera were used to determine metabolic parameters including fasting blood sugar (FBS), triglycerides (TG), total cholesterol (TC), and HDL-C using commercial kits (Pars-Azmoon Co., Tehran, Iran), and an auto-analyzer (Hitachi 902, Tokyo, Japan) as well as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) based on the International Federation of Clinical Chemistry procedure to calculate AST to ALT ratio (AST/ALT)15. Electrochemiluminescence immunoassay was applied to determine serum ferritin levels. Then, low-density lipoprotein cholesterol (LDL-C) was estimated using Friedewald formula as follows16:
Statistical analysis
Data was analyzed using Statistical Package for Social Analysis (SPSS Inc., ver. 20, Chicago, IL, USA). The Kolmogorov–Smirnov test was used to check the normality distribution of continuous variables whereas Chi-square test was used for the relationship between categorical variables. Differences in symmetric and asymmetric continuous variables were done using two-independent sample t- and Mann–Whitney U-tests between the two groups, respectively. For comparing the mean of the studied variables in more than two groups, analysis of variance was applied. The association between continuous variables was checked using Pearson and Spearman correlation coefficient for symmetric and asymmetric data, respectively. p < 0.05 was considered as statistically significant.
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Tabriz University of Medical Science (IR.TBZMED.REC.1401.689) and all eligible patients signed an informed written consent form.
Results
Table 1 shows participants' characteristics. More than half of the participants were female, married, and with university degrees. Overweight and mild steatosis were found in 71% and 58.8% of the studied patients, respectively. Most of the patients showed central obesity in terms of WC and WHtR, while around one-third of them had high WHR.
Between-gender comparisons in the studied parameters are shown in Table 2. There were significant differences in all anthropometric measures and indices between the genders, i.e. mean WC and WHR were significantly greater in males than females while mean BMI and WHtR were higher in women than men (p < 0.05). Among metabolic factors, significant between-gender differences in serum levels of HDL-C, AST, ALT, AST/ALT, and ferritin were found (p < 0.001). Mean platelets and monocytes were statistically different between genders (p < 0.001 and p = 0.012, respectively). Although MHR in males was significantly greater than females (p < 0.001), MLR and PLR were higher in women than men (p = 0.047 and p = 0.005, respectively).
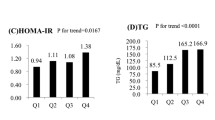
Table 3 demonstrates differences in anthropometric measures, metabolic factors, liver function, and SIIs by the severity of liver steatosis. By increasing the severity of liver steatosis, all of the anthropometric indices (except for HC) and metabolic parameters ( apart from TC and HDL-C) increased significantly (p < 0.05). Furthermore, liver function indices changed parallel with changes in NAFLD severity in terms of significant increases in ALT, AST, and ferritin as well as decreases in AST/ALT ratio. WBC (p < 0.001), monocytes (p = 0.035), and neutrophils (p < 0.001) also enhanced by the hepatosteatosis severity. Indeed, apart from PLR, all of the SIIs were significantly greater in grade II than in grade I liver steatosis (all p < 0.05) (Table 3).
As Table 4 demonstrates, MHR was significantly associated with HDL-C (p < 0.001), LDL-C (p < 0.001), and AST/ALT (p = 0.019) ratio whereas metabolic factors including serum TG (p = 0.037), TC (p = 0.011), and LDL-C (p < 0.001) were significantly different among tertiles of MLR.
Table 5 shows the association of NLR and PLR with studied factors. Anthropometric indices including WC (p = 0.032), BMI (p = 0.047), and WHtR (p = 0.002) as well as FBS (p = 0.045), TG (p = 0.025), and LDL-C (p = 0.006) levels were significantly different in NLR teritles. Serum HDL-C (p = 0.036) and AST/ALT ratio (p = 0.001) were markedly related with PLR tertiles, while ALT levels were marginally associated with the tertiles of PLR (p = 0.051).
The correlations between SIIs and anthropometric indices, metabolic and liver function biomarkers are presented in Table 6. Only NLR was significantly correlated with BMI (r = 0.160, p = 0.013) while MLR and PLR were significantly correlated with liver function biomarkers, particularly in females and moderate steatosis. In addition, there were significant correlations between serum ferritin and all SIIs, apart from PLR. Within-gender associations between SIIs and the studied factors were tested (Data not presented in Tables), i.e. among females, LDL-C was significantly correlated with MHR (r = 0.266, p = 0.002), MLR (r = 0.191, p = 0.003), and NLR (r = 0.176, p = 0.045) whereas PLR was found to be correlated with serum ALT (r = -0.245, p = 0.004), AST/ALT ratio (r = 0.229, p = 0.008) and ferritin (r = 0.194, p = 0.025).
Discussion
The present study investigated the association of a number of SIIs including MHR, MLR, NLR, and PLR with metabolic and anthropometric factors and also liver function in patients with NAFLD. The detection of chronic inflammation is considered a crucial goal for clinical specialists. Inflammatory biomarkers such as C-reactive protein and pro-inflammatory cytokines (e.g. interleukin 6 and tumor necrosis factor-α have been commonly studied to identify inflammatory states17. Furthermore, hepatosteatosis screening and monitoring are very important for the prevention of severe complications such as liver fibrosis and thereby finding reliable and simple markers of NAFLD in the blood seems to be necessary3. There is a growing recognition toward the critical role of systemic inflammation (intra- and extra-hepatic) assessment by relatively novel inflammatory indices such as NLR or MHR in NAFLD using a rapid, inexpensive, and routine blood test2.
Results of the present study demonstrated that apart from PLR, all of the SIIs significantly changed by increasing steatosis severity. Moreover, changes in NLR showed a significant association with anthropometric indices including WC, BMI, and WHtR, as well as levels of FBS, TG, and LDL-C. The findings also indicate the relations between lipid profile and all studied SIIs, notably MHR and MLR. All of the SIIs exhibited associations with some liver function indices as well. MHR was positively correlated with the metabolic risk factors of NAFLD while, oppositely, PLR was considered as a preventive marker of NAFLD. Our results also showed that anthropometric measures, liver function biomarkers, MHR, and MLR were significantly different between the genders. There is cumulative evidence indicating that inflammatory conditions more frequently occur in men whereas chronic inflammatory diseases with lower mortality rates mostly affect women18,19,20.
We documented significant increases in SIIs (in terms of MHR, MLR, and NLR) parallel to the changes of all anthropometric and liver function indices as well as lipidemia, by an increasing trend in hepato-steatosis severity. In this context, the association between inflammation, SIIs, and dyslipidemia has been well established in many conditions such as rheumatoid arthritis, neurological disease, depression, and mood disorders20,21,22,23. For example, Gong et al.24 in their study on patients with ischemic stroke showed that SIIs including NLR, PLR, and lymphocyte-monocyte ratio (LMR) were greater in those with neurological problems than controls. It is suggested that high PLR values could be used for the prediction of thrombosis and inflammatory disturbances as well as neurological outcomes in patients with acute ischemic stroke24. Additionally, the effectiveness of PLR in the prediction of all-cause and cardiovascular mortality in the maintenance of patients undergoing hemodialysis is also documented25. Moreover, SIIs have been implicated in the assessment of endocrine, metabolic, and CVDs, as well as cancers2,6,26.
Despite the relatively weak association found between anthropometric indices (BMI, WC, and WHtR) and serum FBS levels with changes in NLR values in this study, other SIIs failed to present any relation to those factors. The findings also indicate the relations between lipid profile and all studied SIIs, notably MHR and MLR. Although these associations are not well established by previous studies on obesity-related conditions such as Mets and NAFLD yet, patients with Mets have shown significantly higher MHR, NLR, and PLR whereas lower LMR compared with healthy subjects10,27,28,29,30. However, it should be noted that gender, BMI, and fasting hyperglycemia could influence SIIs and thereby, lead to variations in findings26. Our data shed light on the use of NLR, MHR, and MLR for the assessment of metabolic outcomes of NAFLD; namely by the hypothesis on the ability of MHR and MLR in the assessment of lipidemia as well as the application of NLR in obesity and glycemia assessment. Based on current evidence, MHR has been suggested for showing the complex interplay between pro-inflammatory/pro-atherogenic factors as well as lipid profile26. Therefore, it appears that high MHR might be a useful marker for the evaluation of lipidemia in NAFLD and other metabolic diseases.
Regarding the possible application of NLR for the assessment of glycemia, in line with our results, several studies have reported the relationship between higher NLR and poor glycemic control, macro- and micro-vascular diabetic complications in T2DM patients31,32. It appears that NLR could be used as a predictive marker of glycemic status in metabolic disorders.
As NAFLD is the hepatic manifestation of metabolic dysregulations, it involves a complex interplay among factors including excessive body fat deposition, impaired glucose and lipid homeostasis, oxidative stress, and chronic inflammation33. The progression of pro-inflammatory signaling pathways leads to impairments in liver function characterized by hepatic histologic findings34. To date, limited studies have investigated the relationship between SIIs and NAFLD2,12,35,36. In the present study, all of the SIIs showed correlations with a number of liver function biomarkers. These findings suggest increased MHR and PLR values as the progressive and preventive markers of NAFLD, respectively. A recent study reported the relationship between NLR and PLR with NAFLD, after adjusting for potential confounders12. Similar to our findings, Zhao et al.2 reported that MHR was significantly higher in patients with NAFLD than in healthy controls and suggested that MHR is strongly associated with the risk of NAFLD compared with NLR, PLR, and LMR. Indeed, Huang et al.37 reported that MHR was significantly correlated with the high risk of NAFLD, even after adjusting for age, sex, BMI, WC, ALT, TG, TC, FBS, and blood pressure. MHR is calculated based on monocytes, which are considered an important component of the immune system and a vital mediator of both chronic and acute inflammation, and thereby, induces immune-inflammatory responses38. In addition, HDL-C by producing an anti-inflammatory response to macrophages and mediating cholesterol consumption could be necessary for the prevention of inflammatory diseases39. Therefore, increased MHR is associated with diabetic nephropathy, predicting Mets and NAFLD10,40.
Regarding PLR, several studies similarly suggested that PLR may be more useful as a marker in determining the increased thrombotic state and inflammatory response in morbid obesity, obstructive sleep apnea–hypopnea syndrome, overall survival in patients with NAFLD-related hepatocellular carcinoma5,36,41,42. Taken together, it seems that PLR has relatively higher diagnostic accuracy for liver fibrosis35. It appears that the combination of NLR and MLR (as the markers of anthropometric and lipid-related changes) with PLR and MHR (as the indicators of liver function and metabolic regulation) could present a comprehensive perspective of NAFLD.
Accordingly, these findings could be explained by the potent role of inflammation, in terms of peripheral blood leukocytes in the progression of NAFLD43. Stimulated hepatocyte neutrophil infiltration is followed by macrophage recruitment and cellular damage, which activates pro-inflammatory pathways as well as oxidative stress43. In addition, liver stellate cells facilitate the production of hepatic reactive oxygen species and the progression of liver fibrosis through increasing neutrophils survival. Current evidence also suggests an interplay between platelets and pro-inflammatory cells, which is followed by increased chemokine and immune-inflammatory mediator secretion, which subsequently induce NAFLD43. Taken together, changes in the balance and the total count of neutrophils, lymphocytes, and platelets in the blood could reflect a systemic inflammatory state and reflect the risk of NAFLD and its related outcomes43.
Some limitations should be acknowledged when interpreting the findings of this study. First, the nature of cross-sectional cannot make a judgment regarding the causal relationship between SIIs and NAFLD. Larger sample size with various spectrum of NAFLD severity in different age groups is needed to confirm these results. Furthermore, sarcopenia, which is a progressive and systemic skeletal muscle disease, has attracted considerable attention as one of the pathological conditions involved in the development and progression of NAFLD. Sarcopenia has been reported to be closely associated with NAFLD. It causes sarcopenia-related obesity, which increases the risk of nonalcoholic steatohepatitis, liver fibrosis, and CVD44,45,46. However, due to the lack of data on sarcopenia status in our patients, we were unable to explore the association of SIIs with sarcopenia in the current study, which is suggested to be evaluated in future studies. The current study has several strengths too. Subgroup comparison based on NAFLD severity, and considering not only metabolic but also anthropometric indices and the biomarkers of liver function in relation to SIIs are considered as the strengths of the present study.
In conclusion, SIIs, which can be easily measured in clinical practice, are related to the presence and severity of NAFLD. Moreover, we suggested that PLR and MHR by themselves or combined with other SIIs could be used in NAFLD monitoring. However, further research is required to confirm these findings.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- ANOVA:
-
Analysis of Variance
- AST:
-
Aspartate aminotransferase
- AST/ALT:
-
Aspartate aminotransferase to alanine aminotransferase ratio
- BMI:
-
Body mass index
- CBC:
-
Complete blood count
- FBS:
-
Fasting blood sugar
- HC:
-
Hip circumference
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- LMR:
-
Lymphocyte to monocytes ratio
- Mets:
-
Metabolic syndrome
- MHR:
-
Monocytes to High-density lipoprotein cholesterol ratio
- MLR:
-
Monocytes to lymphocyte ratio
- NAFLD:
-
Non-alcoholic fatty liver disease
- NLR:
-
Neutrophil to lymphocyte ratio
- PLR:
-
Platelet to lymphocyte ratio
- SIIs:
-
Systemic inflammatory indices
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- WC:
-
Waist circumference
- WHR:
-
Waist to hip ratio
- WHtR:
-
Waist to height ratio
References
Tutunchi, H. et al. The association of the steatosis severity, NAFLD fibrosis score and FIB-4 index with atherogenic dyslipidaemia in adult patients with NAFLD: A cross-sectional study. Int. J. Clin. Pract. 75(6), e14131 (2021).
Zhao, Y. et al. Diagnostic performance of novel inflammatory biomarkers based on ratios of laboratory indicators for nonalcoholic fatty liver disease. Front. Endocrinol. 13, 981196 (2022).
Powell, E. E., Wong, V.W.-S. & Rinella, M. Non-alcoholic fatty liver disease. The Lancet 397(10290), 2212–24 (2021).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73(1), 202–209 (2020).
Erdal, E. & İnanir, M. Platelet-to-lymphocyte ratio (PLR) and Plateletcrit (PCT) in young patients with morbid obesity. Rev. Assoc. Med. Bras. 65(9), 1182–7 (2019).
Platini, H. et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic markers for advanced non-small-cell lung cancer treated with immunotherapy: A systematic review and meta-analysis. Medicina 58(8), 1069 (2022).
Zubiaga, L. & Ruiz-Tovar, J. Correlation of preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with metabolic parameters in patients undergoing sleeve gastrectomy. Surg. Obes. Relat. Dis. 16(8), 999–1004 (2020).
Papatheodoridi, M. & Cholongitas, E. Diagnosis of non-alcoholic fatty liver disease (NAFLD): Current concepts. Curr. Pharm. Des. 24(38), 4574–4586 (2018).
Fedchuk, L. et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 40(10), 1209–1222 (2014).
Vahit, D., Akboga, M. K., Samet, Y. & Hüseyin, E. Assessment of monocyte to high density lipoprotein cholesterol ratio and lymphocyte-to-monocyte ratio in patients with metabolic syndrome. Biomark. Med. 11(7), 535–540 (2017).
Jia, J. et al. Monocyte to high-density lipoprotein cholesterol ratio at the nexus of type 2 diabetes mellitus patients with metabolic-associated fatty liver disease. Front. Physiol. 12, 762242 (2021).
Zhou, Y. et al. The correlation between neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with nonalcoholic fatty liver disease: A cross-sectional study. Eur. J. Gastroenterol. Hepatol. 34(11), 1158–1164 (2022).
Hamaguchi, M. et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. ACG 102(12), 2708–15 (2007).
Moghaddasifar, I. et al. Prevalence of non-alcoholic fatty liver disease and its related factors in Iran. Int. J. Organ Transplant. Med. 7(3), 149–160 (2016).
Schumann, G. et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37°C Part 9: Reference procedure for the measurement of catalytic concentration of alkaline phosphatase: International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Scientific Division, Committee on Reference Systems of Enzymes (C-RSE) 1. Clin. Chem. Lab. Med. 49(9), 1439–46 (2011).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18(6), 499–502 (1972).
Arefhosseini, S., Roshanravan, N., Asghari, S., Tutunchi, H. & Ebrahimi-Mameghani, M. Expression of inflammatory genes, WBC-derived inflammatory biomarkers and liver function indices: Effects of myo-inositol supplementation in obese patients with NAFLD. J. Funct. Foods 104, 105524 (2023).
GBD 2016Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100), 1211–59 (2017).
Fairweather, D., Cooper, L. T. Jr. & Blauwet, L. A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 38(1), 7–46 (2013).
Ma, J. et al. Urinary copper, systemic inflammation, and blood lipid profiles: Wuhan-Zhuhai cohort study. Environ. Pollut. 267, 115647 (2020).
Mena-Vázquez, N. et al. Postprandial hyperlipidemia: Association with inflammation and subclinical atherosclerosis in patients with rheumatoid arthritis. Biomedicines 10(1), 133 (2022).
Su, M., Ouyang, X. & Song, Y. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and monocyte to lymphocyte ratio in depression: A meta-analysis. J. Affect. Disord. 308, 375–383 (2022).
Marazziti, D. et al. Neutrophil/lymphocyte, platelet/lymphocyte, and monocyte/lymphocyte ratios in mood disorders. Curr. Med. Chem. 29(36), 5758–5781 (2022).
Gong, P. et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J. Neuroinflamm. 18(1), 51 (2021).
Mureșan, A. V. et al. The predictive value of NLR, MLR, and PLR in the outcome of end-stage kidney disease patients. Biomedicines 10(6), 1272 (2022).
Battaglia, S. et al. Gender, BMI and fasting hyperglycaemia influence Monocyte to-HDL ratio (MHR) index in metabolic subjects. PLoS ONE 15(4), e0231927 (2020).
Ganjali, S. et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 233(12), 9237–9246 (2018).
Gong, S. et al. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine 97(43), e12813 (2018).
Buyukkaya, E. et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin. Appl. Thromb./Hemostas. 20(2), 159–163 (2014).
Akboga, M. K. et al. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: A single center large-scale study. Platelets 27(2), 178–183 (2016).
Shiny, A. et al. Association of neutrophil-lymphocyte ratio with glucose intolerance: An indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol. Ther. 16(8), 524–530 (2014).
Hussain, M., Babar, M. Z. M., Akhtar, L. & Hussain, M. S. Neutrophil lymphocyte ratio (NLR): A well assessment tool of glycemic control in type 2 diabetic patients. Pak. J. Med. Sci. 33(6), 1366–1370 (2017).
Tutunchi, H., Arefhosseini, S. & Ebrahimi-Mameghani, M. Clinical effectiveness of α-lipoic acid, myo-inositol and propolis supplementation on metabolic profiles and liver function in obese patients with NAFLD: A randomized controlled clinical trial. Clin. Nutr. ESPEN 54, 412–420 (2023).
Aktas, G. et al. Liver steatosis is associated both with platelet distribution width, neutrophil/lymphocyte and monocyte/lymphocyte ratios. Primary Health Care 10(4), 1–4 (2020).
Michalak, A. et al. Towards an evaluation of alcoholic liver cirrhosis and nonalcoholic fatty liver disease patients with hematological scales. World J. Gastroenterol. 26(47), 7538–7549 (2020).
Chen, M. et al. The role of platelet-related parameters for the prediction of NAFLD in OSAHS patients. BMC Pulm. Med. 22(1), 487 (2022).
Huang, H. et al. Association between monocyte to high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: A cross-sectional study. Mediat. Inflamm. 2021, 6642246 (2021).
Mukherjee, R. et al. Non-Classical monocytes display inflammatory features: Validation in sepsis and systemic lupus erythematous. Sci. Rep. 5, 13886 (2015).
De Nardo, D. et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 15(2), 152–160 (2014).
Karatas, A., Turkmen, E., Erdem, E., Dugeroglu, H. & Kaya, Y. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomark. Med. 12(9), 953–959 (2018).
Purón-González, E. et al. Predictive markers of nonalcoholic fatty liver disease in lean patients. A multinomial regression model and a 2k factorial analysis. Eur. J. Gastroenterol. Hepatol. 33(10), 1316–21 (2021).
Safcak, D. et al. Nonalcoholic fatty liver disease-related hepatocellular carcinoma: Clinical patterns, outcomes, and prognostic factors for overall survival-a retrospective analysis of a slovak cohort. J. Clin. Med. 10(14), 3186 (2021).
Sun, W. et al. The association of systemic inflammatory biomarkers with non-alcoholic fatty liver disease: A large population-based cross-sectional study. Prev. Med. Rep. 37, 102536 (2024).
Iwaki, M. et al. Impact of sarcopenia on non-alcoholic fatty liver disease. Nutrients 15(4), 891 (2023).
Tarantino, G., Sinatti, G., Citro, V., Santini, S. J. & Balsano, C. Sarcopenia, a condition shared by various diseases: Can we alleviate or delay the progression?. Intern. Emerg. Med. 18(7), 1887–1895 (2023).
Zhao, J. et al. Higher systemic immune-inflammation index is associated with sarcopenia in individuals aged 18–59 years: a population-based study. Sci. Rep. 13(1), 22156 (2023).
Funding
This study was funded by the 'Research Vice-Chancellor' of Tabriz University of Medical Sciences, Tabriz, Iran (grant number.70895).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: S.A. and T.A. collected data and wrote the original paper; M.E.M. and H.T. contributed to the conception of the article; M.E.M. contributed to the statistical analysis; S.A. H.T. and M.E.M. contributed to the final revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arefhosseini, S., Aghajani, T., Tutunchi, H. et al. Association of systemic inflammatory indices with anthropometric measures, metabolic factors, and liver function in non-alcoholic fatty liver disease. Sci Rep 14, 12829 (2024). https://doi.org/10.1038/s41598-024-63381-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63381-5
- Springer Nature Limited