Abstract
Excessive and improper use of antibiotics causes antimicrobial resistance which is a major threat to global health security. Hospitals in sub-Saharan Africa (SSA) has the highest prevalence of antibiotic use. This systematic review and meta-analysis aimed to determine the pooled point prevalence (PPP) of evidence-based antimicrobial use among hospitalized patients in SSA. Literature was retrieved from CINAHL, EMBASE, Google Scholar, PubMed, Scopus, and Web of Science databases. Meta-analysis was conducted using STATA version 17. Forest plots using the random-effect model were used to present the findings. The heterogeneity and publication bias were assessed using the I2 statistics and Egger’s test. The protocol was registered in PROSPERO with code CRD42023404075. The review was conducted according to PRISMA guidelines. A total of 26, 272 study participants reported by twenty-eight studies published from 10 countries in SSA were included. The pooled point prevalence of antimicrobial use in SSA were 64%. The pooled estimate of hospital wards with the highest antibiotic use were intensive care unit (89%). The pooled prevalence of the most common clinical indication for antibiotic use were community acquired infection (41%). The pooled point prevalence of antimicrobial use among hospitalized patients were higher in SSA. Higher use of antibiotics was recorded in intensive care units. Community acquired infection were most common clinical case among hospitalized patients. Health systems in SSA must design innovative digital health interventions to optimize clinicians adhere to evidence-based prescribing guidelines and improve antimicrobial stewardship.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Global antibiotic consumption rates surged by 46%, indicating that the defined daily dose (DDD) per 1000 population per day rose from 9.8 to 14.3 between 2000 and 20181. In low- and middle-income countries (LMICs), antibiotic usage increased by 76% and is projected to continue rising by 20302. Hospitals in SSA have a higher prevalence of antibiotic usage (50%), including the use of broad-spectrum cephalosporins and penicillin3.
With improving economies and enhanced access to pharmaceuticals, many of LMICs now revealed antibiotic consumption rates comparable to or even surpassing those of high-income countries4. Sub-Saharan African countries are experiencing a similar trend in antibiotic consumption, which could be exacerbated by the region’s exceptionally high infectious disease burden5. This sharp rise in antibiotic usage with or without prescription, has become a pressing public health concern due to its strong association with the development of antimicrobial resistance in low resource clinical context6,7.
The misuse and overuse of antibiotics have led to increased rates of antimicrobial resistance, higher levels of morbidity and mortality, and escalated healthcare costs in low-income countries8,9. To address this issue, evaluating antibiotic prescribing patterns among patients in healthcare facilities is essential in identifying opportunities for antimicrobial stewardship to promote appropriate antibiotic use10,11.
Point prevalence studies have proven to be reliable and valid methods for measuring antibiotic use among hospitalized patients12. They provide crucial insights into the current state of antibiotic use within healthcare settings, aiding in the identification of patterns and deviations from recommended practices13. This data can inform targeted interventions to improve guideline adherence, optimize antibiotic selection, dosing, and duration, and reduce inappropriate prescriptions14,15. By promoting evidence-based clinical decisions, these studies contribute to the prevention of antibiotic overuse, the emergence of antimicrobial resistance, and the enhancement of patient outcomes, thus serving as a vital tool in advancing the quality and effectiveness of real-world healthcare practices16,17.
In sub-Saharan Africa, several point prevalence studies have reported a high rate of antibiotic use among hospitalized patients, along with inappropriate usage in healthcare facilities18. However, there is limited regional-level data available to describe the point prevalence of antibiotic use among hospitalized patients in SSA19. Understanding the epidemiology of antibiotic use in this context and assessing the quality of antibiotic prescribing are critical steps in designing effective antimicrobial stewardship interventions aimed at encouraging the rational use of antibiotics and improving clinical outcomes for patients20. Therefore, this systematic review and meta-analysis aimed to determine the pooled point prevalence of antibiotic use among hospitalized patients in sub-Saharan Africa.
Methods
Search strategy and selection of studies
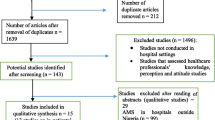
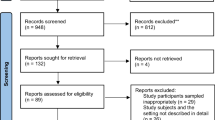
The search strategy aimed to find both published and unpublished literature. Initially, a preliminary search was conducted on the Google Scholar to identify indexed full texts or metadata of scholarly literature on the topic. We adapted key terms as needed for each database, utilizing a combination of MeSH terms and text words, employing Boolean operators “AND” and “OR” for searches in databases like CINAHL, PubMed, EMBASE, Scopus, and Web of Science (Appendix I). Additionally, we examined the reference lists of selected studies for potential additional sources. No restrictions were imposed based on language or publication year. After the search, all identified citations were organized and imported into EndNote version 15.0, with duplicates removed. Two independent reviewers (MTB and BH) screened titles and abstracts, and a third reviewer (ZEK) cross-checked them against the inclusion and exclusion criteria. Relevant studies meeting the criteria were obtained in full, along with their citation details. Studies reporting the point prevalence of antibiotic use among hospitalized patients in SSA, which were published from 2013 to 2023 were eligible for inclusion. Excluded were systematic reviews, Studies having participants sampled inappropriately and the setting not described in detail studies, data analysis not conducted with sufficient coverage of the identified sample, and literature from high-income countries. Two independent reviewers (MTB and BH) assessed the full text of selected citations against the inclusion criteria, with a third reviewer (LWT) conducting a double-check. Reasons for excluding studies failing to meet the inclusion criteria upon full text review were documented. Any disagreements between reviewers at each stage of the study selection process were resolved through discussion or by consulting a third reviewer. The PRISMA checklist (Appendix II) and flow chart was used to describe the matching pages in the manuscript with the number of articles identified, included, and excluded with justifications. The results of the search were fully reported in the final systematic review and presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram (Fig. 1)21.
PRISMA flow diagram of included studies: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Operational definitions
Point prevalence survey of antimicrobial use
Is a structured assessment done in healthcare settings to determine the percentage of patients receiving antimicrobial treatment at a particular moment22. Its goal is to assess the appropriateness of antimicrobial use, including choice, dosage, and duration, to enhance antimicrobial stewardship practices and combat antimicrobial resistance, ensuring effective and sustainable use of these essential medications23,24.
Evidence-based antimicrobial stewardship practice
Refers to healthcare professionals utilizing scientific evidence, clinical guidelines, and patient data to guide decisions on selecting, dosing, and timing antimicrobial treatment. Its objective is to enhance patient outcomes by reducing antimicrobial resistance and adverse effects, ensuring optimal treatment effectiveness25,26,27,28.
Data extraction
The data were extracted from included studies using the data extraction tool prepared by MTB. The tool includes variables such as the name of the author, publication year, study design, data collection period, sample size, study area, and the point prevalence of antimicrobial use. The data extraction tool contains information on the indication for antibiotic use; prevalence of antibiotic use in different wards, classes of antibiotics used, types of antibiotics used, and AWaRe classification. BH extracted the data, and LWT and MTB cross-checked the extracted data for its validity and cleanness. Authors of papers were contacted to request missing or additional data.
Data quality and risk of bias assessment
Eligible studies were critically appraised by two independent reviewers (MTB and BH). Full texts screening including the methodological quality assessment were examined using the JBI’s critical appraisal instrument for prevalence studies29. Studies that fulfill at least seven out of the nine domains of the JBI criteria questions were eligible for meta-analysis. The results of the critical appraisal were reported in narrative form and a table. A lower risk of bias (94%) observed after assessment (Appendix III). Studies with inadequate sample size, inappropriate sampling frame and poor data analysis were excluded. Articles were reviewed using titles, abstracts, and full text screening.
Data analysis
Included studies were pooled in a statistical meta-analysis using STATA version 17.0. Effect sizes were expressed as a proportion with 95% confidence intervals around the summary estimate. Heterogeneity was assessed using the standard chi-square I2 test. A random-effects model was used. As pooled proportions from individual cross-sectional design point-prevalence studies are prone to variance instability and can violate the assumption of normality. Therefore, to address this, we did the double arcsine transformation method to stabilize variances, ensuring our meta-analysis results to be more reliable30. Sensitivity analyses were conducted to test decisions made regarding the included studies. Visual examination of funnel plot asymmetry (Appendix IV) and Egger’s regression tests were used to check for publication bias31. A Forest plot with 95% CI was computed to estimate the pooled point prevalence of evidence-based antimicrobial use among hospitalized patients in SSA.
Protocol registration
The review protocol has been registered in PROSPERO with protocol registration number CRD42023404075.
Ethical approval
Not applicable. Unlike primary studies, systematic reviews do not include the collection of deeply personal, sensitive, and confidential information from the study participants. Systematic reviews involve the use of publicly accessible data as evidence and are not required to seek an institutional ethics approval before commencement.
Results
Search
A total of 2260 articles were obtained from CINAHL, EMBASE, Google Scholar, PubMed, Scopus, and Web of Science databases. Following the removal of 605 duplicates, at the title/abstract screening phase (n = 2016) and during the full-article screening (n = 212) articles were excluded. Accordingly, 32 studies were eligible for quality assessment. Finally, 28 studies were included in this meta-analysis (Fig. 1).
Study characteristics
The total sample size of this systematic review was 26, 272, ranging from 113 in Malawi32 to 4, 407 in South Africa33. Nine studies were reported from Nigeria34,35,36,37,38,39,40,41,42. Six articles were published from Ghana43,44,45,46,47,48. Four studies were reported from Kenya49,50,51,52. Equally two studies were reported from South Africa33,53 and Tanzania54,55. Bennin56, Botswana57, Ethiopia58, Malawi32, and Uganda59 reported only one study respectively (Table 1).
Antibiotic use by wards among hospitalized patients in sub-Saharan Africa
The use of antibiotics from highest to lowest were surgical (5764), medical (5440), intensive care (4676), obstetrics and gynecology (2410), neonatal (830), oncology (207), and orthopedic (30) wards respectively (Table 2).
Most commonly used antibiotics among hospitalized patients in sub-Saharan Africa
Ceftriaxone32,33,34,37,39,40,41,45,46,47,52,54,55,60,61, metronidazole32,34,37,39,40,42,43,44,46,47,52,54,55,59, gentamicin33,34,37,39,46,47,52,54,55,59, ampicillin33,38,46,54,55,60, and cefuroxime37,40,42,44,45,46 were the most commonly used antibiotics (Table 3). Six studies equally reported ciprofloxacin32,34,37,39,44,46 and amoxicillin-clavulanate33,34,39,42,61,62. Only three studies reported ampicillin-cloxacillin combination39,54,59 and amoxicillin32,38,46 as antibiotics used in hospitals in SSA (Table 3).
WHO AWARE classification of antibiotics used by hospitalized patients in sub-Saharan Africa
Only five studies reported antibiotics used based on the WHO’s access, watch, and reserve (AWaRe) classification33,37,49,53,59 (Table 4). The most commonly used antibiotics were the access group and ranged between 46.3 and 97.9%33,37,49,53,59, followed by the watch and reserve group that accounted for 1.8–53.5%33,37,49,53,59, and 0.0–5.0%33,37,49,53,59 respectively (Table 4).
Indications for antibiotic prescription among hospitalized patients in SSA
Community-acquired infection ranged from 27.7 to 61%, surgical antibiotic prophylaxis ranged from 14.6 to 45.3%, hospital-acquired infections ranged from 1.2 to 40.3%, and, medical prophylaxis ranged from 0.5 to 29.1% were the most common clinical indications (Table 5). Antibiotic prescription for 938 inpatients were done for unknown clinical indications (Table 5).
Pooled point prevalence of evidence-based use of antibiotics in SSA
The pooled point prevalence of evidence-based use of antimicrobials were 64.15% (95%CI: 58.31–69.79%) (Fig. 2).
The pooled prevalence of evidence-based antibiotic use in different wards in hospitals of SSA
Only seven studies from four countries reported the use of antibiotics in intensive care units41,49,50,51,52,55,58, ranging from 179 (66.5%) to 1565 (85.9%) (Table 3). The pooled point prevalence of antibiotics use in ICU were 87.90% (95% CI: 77.93–95.19%) (Fig. 3).
The uptake of antimicrobials in medical wards ranged from 63 (19.6%) to 236 (73.5%) as reported by thirteen studies34,36,37,41,43,49,50,51,52,54,55,58,61 from five countries (Table 3). The pooled prevalence of use of antibiotics in medical wards were 54.01% (95% CI: 47.24–60.71%) (Fig. 4).
Antibiotic use in obstetrics and gynecology wards ranges from 22 (6.9%) to 234 (72.9%)The pooled prevalence of antibiotics use in obstetrics and gynecology wards obtained from data extracted from eight studies published from Ethiopia58, Ghana45, Kenya49,50,51,52, and Nigeria34,37 (Table 3), were 45.70% (95% CI: 33.04–58.64) (Fig. 5).
Five counties from hospitals in sub-Saharan Africa, including Ethiopia58, Ghana61, Kenya49,50,51,52, Nigeria34,37,41, and Tanzania54,55, produced twelve articles that revealed the antimicrobials uptake in surgical wards with the lowest 74 (23%) to the highest 781 (82.4%) (Table 3). The pooled prevalence of antibiotics use in surgical wards were 57.74% (95% CI: 48.64–66.58) (Fig. 6).
The pooled prevalence of clinical indications for evidence-based antibiotic use in SSA
Twenty studies from seven countries in SSA such as, Botswana57, Ethiopia58, Nigeria35,37,39,40,41,42,63, Ghana43,46,47,48,61, Kenya49,50,52, Tanzania54,55, and Uganda59, reported that community- and hospital acquired infections were the most common clinical indications for antibiotics use (Table 5). The pooled prevalence of community- and hospital acquired infections for point of care antibiotics use were 40.99% (95% CI: 35.28–46.82%) (Fig. 7) and 11.15% (95% CI: 6.02–17.56%) (Fig. 8) respectively.
Seven countries including Botswana57, Ethiopia58, Nigeria34,35,37,39,40,41, Ghana45,47,61,64,65, Kenya49,50,52, Tanzania54,66, Malawi32, and Uganda59 conducted eighteen studies which reported medical and surgical prophylaxis were the second most common clinical indications for evidence-based uptake of antimicrobials (Table 5). The pooled prevalence of medical—and surgical prophylaxis for antibiotics use were 11.86% (95% CI: 8.02–16.33%) (Fig. 9) and 28.54% (95% CI: 25.29–31.91%) (Fig. 10) respectively.
The pooled prevalence of the use of antibiotics at point of care for unknown clinical indications reported from 15 articles conducted in five countries Ethiopia58, Ghana46,47,48,62,64, Kenya49,50, Nigeria34,35,37,39,40,41, and Tanzania54 (Table 5) were 7.67% (95% CI: 4.55–11.33%) (Fig. 11).
Visual funnel plots asymmetry examination and Egger’s regression tests revealed that there was no publication bias67.
Discussion
This systematic review and meta-analysis aimed to determine the pooled point prevalence of evidence-based antimicrobial use among hospitalized patients in sub-Saharan Africa. A total of 26, 272 patients admitted to twenty-eight hospitals of ten countries in SSA were included. The pooled point prevalence of antimicrobial use at point of care was 64%. The finding of this study is higher than the antibiotic use in hospitals of Middle East (28.3%)68 and Europe (30.5%)69. This could be attributed to misuse and overuse of antibiotics70,71, poor infection and disease prevention and control72, and, water, sanitation and hygiene practice in health-care facilities73, and poor surveillance of antimicrobial resistance in SSA74,75. The pooled point prevalence of antibiotic use in intensive care unit of hospitals in SSA were 89%. This finding is higher than a point prevalence of use of antimicrobials in ICUs in the United States 62.2% 76 and Poland 59.6%77.
The uses of antimicrobials at point of care in surgical and medical wards were 58% and 54% in SSA. The overuse or inappropriate use of antimicrobials at the point of care in medical and surgical wards can lead to antibiotic resistance8, which can make infections harder to treat. Moreover, unnecessary antimicrobial use can disrupt the balance of the microbiome, leading to complications like Clostridium difficile infections78. The pooled estimate of antibiotics used by inpatients admitted to obstetrics and gynecology wards of the hospitals in SSA were 46%. The finding of this study was higher than the antibiotic consumption in obstetrics and gynecology departments of Peruvian hospital 31%79. Higher antibiotic use in obstetrics and gynecology wards in SSA can be attributed to factors such as a higher prevalence of surgical procedures80, which often require prophylactic antibiotics to prevent post-operative infections81. Additionally, cases of infections related to childbirth, such as postpartum infections or complications following gynecological procedures, may necessitate antibiotic treatment in SSA82,83.
The pooled prevalence of community and hospital acquired infections in SSA were 41% and 11.15% respectively. The pooled estimate of this review was higher than a study in East Africa that reported 34% CAI84. This could be due to non-standardized antibiotic use in SSA. Our review result revealed that HAI in SSA were lower than the finding from LMICs 17.9%85.
The misuse of antibiotics in both community and hospital-acquired infections has far-reaching consequences86. In the community, inappropriate antibiotic use contributes to the development of antibiotic-resistant bacteria, rendering infections harder to treat and increasing healthcare costs87,88. Patients may experience treatment failures, longer hospital stays, and increased mortality rates89. Moreover, the continued misuse of antibiotics fuels the global crisis of antibiotic resistance, jeopardizing the effectiveness of these essential drugs for future generations90,91. In hospital settings, similar consequences are exacerbated by the potential for widespread outbreaks of antibiotic-resistant infections among vulnerable patients92. The resulting challenges in managing infections can strain healthcare systems, diminish the success of medical interventions, and underscore the critical need for stringent antibiotic stewardship practices to preserve the efficacy of antibiotics.
The pooled prevalence of the most common clinical indications for antibiotic use in hospitals of SSA were community acquired infection (40.99%), surgical prophylaxis (28.54%), medical prophylaxis (11.86%), and hospital acquired infection (11.15%).
This study revealed that the pooled prevalence of HAI (11.15%) is lower than the global estimate (14%)93. This could be attributed to inadequate infection control measures94, limited resources95, overcrowding96, and a higher burden of infectious diseases97. Poor sanitation and healthcare infrastructure can contribute to the increased risk of infections within healthcare facilities in SSA98.
According to this study, the pooled estimate of surgical prophylaxis is higher than Europe (16.8%)99 and the global surgical antibiotic prophylaxis at point of care (22.8%)17. The surgical prophylaxis in SSA is lower than a study reported in Myanmar (34.3%)100. Higher surgical antibiotic prophylaxis may be attributed to surgeon’s overuse of antibiotics to mitigate infection risks in environments with higher prevalence of surgical site infections and limited access to post-operative care in SSA101,102,103. Surgeons may also lack awareness of appropriate guidelines, and patients may expect antibiotics due to a perception of their effectiveness103.
The pooled point prevalence of medical prophylaxis in this study is lower than European region (24.9%)69 and Indonesia (47.1%)104. A lower point prevalence of medical prophylaxis in SSA suggests limited access and utilization of preventative medical interventions105. This may be indicative of healthcare system challenges, resource constraints, or insufficient awareness and education106,107. It can result in a higher disease burden, increased healthcare costs, and potentially poorer clinical and public health outcomes for the population10,108.
This review indicated that the pooled prevalence of community acquired infection is higher than a study conducted in the Middle East (16.8%)68. Community acquired infection in SSA according to this study were lower than Northern Ireland (66.2%)109. Higher prevalence of CAI could be due to lack of essential medical supplies, suboptimal sterilization procedures, and inadequate training in infection control110,111. High patient-to-nurse ratios and frequent patient turnover can further hinder the implementation of rigorous infection prevention measures, increasing the risk of infections spreading within healthcare settings112,113.
Antibiotic use for unknown clinical indications in SSA hospitals may occur due to inadequate training on antibiotic stewardship and a lack of access to timely microbiological testing3,114. Clinicians may resort to broad-spectrum antibiotics as a precautionary measure in the absence of specific diagnostic information, contributing to antibiotic misuse and resistance114.
Conclusion
The pooled point prevalence of antimicrobial use among hospitalized patients were higher in SSA. Higher use of antibiotics in intensive care unit, surgical, medical, and obstetrics and gynecology wards of hospital in SSA were recorded. Community acquired infection, surgical and medica prophylaxis, and hospital acquired infection were clinical indications reported to have the highest to lowest pooled point prevalence of antibiotics used. Health systems in SSA must design innovative interventions to optimize clinicians adhere to evidence-based prescribing guidelines and improve antimicrobial stewardship.
Implications for evidence-informed policy and clinical practice
A higher pooled point prevalence of antimicrobial use in sub-Saharan Africa implies a need for immediate policy and clinical practice interventions. Policymakers should prioritize allocation of scarce resources for antimicrobial stewardship programs and infection control measures. Innovative intervention must be in place to optimize clinicians adhere to evidence-based prescribing guidelines to combat antimicrobial resistance, reduce adverse effects, and improve patient outcomes.
Health systems in sub-Saharan Africa must emphasize the importance of leveraging clinical decision support digital health interventions to augment evidence-based antimicrobial stewardship. This evidence synthesis informs the policy decision makers to encourage the implementation of such tools to guide clinicians in evidence-based antimicrobial prescribing, reducing inappropriate use, combating resistance, and improving patient care in the context of resource constrained health system. Clinicians can benefit from real-time patient information, aiding in evidence-based prescribing and infection control efforts, significantly improving patient care. Collaboration between policymakers, clinicians, and healthcare facilities is crucial to mitigate the impact of these issues on public health.
Data availability
The datasets are available from the corresponding author on reasonable request.
Abbreviations
- AMR:
-
Antimicrobial resistance
- AHRI:
-
The Armauer Hansen Research Institute
- DDD:
-
Defined daily dose
- JBI:
-
The Joanna Briggs Institute
- LMICs:
-
Low- and Middle-Income Countries
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO:
-
International prospective registry of systematic reviews
- SDG:
-
Sustainable development goal
- SSA:
-
Sub-Saharan Africa
- WHO:
-
The World Health Organization
References
Browne, A. J. et al. Global antibiotic consumption and usage in humans, 2000–18: A spatial modelling study. Lancet Planet. Health 5, e893–e904 (2021).
Klein, E. Y. et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. U. S. A. 115, E3463-e3470 (2018).
Siachalinga, L. et al. Current antibiotic use among hospitals in the sub-Saharan Africa region; findings and implications. Infect. Drug Resist. 2023, 2179–2190 (2023).
Versporten, A. et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 6, e619–e629 (2018).
Belachew, S. A., Hall, L. & Selvey, L. A. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 10, 13 (2021).
Bell, B. G., Schellevis, F., Stobberingh, E., Goossens, H. & Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 14, 1–25 (2014).
Organization, W. H. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation (Springer, 2018).
Ayukekbong, J. A., Ntemgwa, M. & Atabe, A. N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 6, 47 (2017).
Founou, R. C., Founou, L. L. & Essack, S. Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PloS one 12, e0189621 (2017).
Godman, B. et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life 11, 528 (2021).
Majumder, M. A. A. et al. Antimicrobial stewardship: Fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020, 4713–4738 (2020).
Sartelli, M. et al. Ten golden rules for optimal antibiotic use in hospital settings: The WARNING call to action. World J. Emerg. Surg. 18, 50 (2023).
Katyali, D., Kawau, G., Blomberg, B. & Manyahi, J. Antibiotic use at a tertiary hospital in Tanzania: Findings from a point prevalence survey. Antimicrob. Resist. Infect. Control 12, 112 (2023).
Levy Hara, G. et al. Point prevalence survey of antibiotic use in hospitals in Latin American countries. J. Antimicrob. Chemother. 77, 807–815 (2022).
Moulin, E. et al. Point prevalence study of antibiotic appropriateness and possibility of early discharge from hospital among patients treated with antibiotics in a Swiss University Hospital. Antimicrob. Resist. Infect. Control 11, 66 (2022).
Charani, E. et al. Optimising antimicrobial use in humans–review of current evidence and an interdisciplinary consensus on key priorities for research. Lancet Reg. Health-Europe 2021, 7 (2021).
Porto, A. M., Goossens, H., Versporten, A., Costa, S. F. & Group, B. G. P. W. Global point prevalence survey of antimicrobial consumption in Brazilian hospitals. J. Hospit. Infect. 104, 165–171 (2020).
Bahta, M. et al. Dispensing of antibiotics without prescription and associated factors in drug retail outlets of Eritrea: A simulated client method. PLoS One 15, e0228013 (2020).
Ayalew, M. B. Self-medication practice in Ethiopia: A systematic review. Patient Preference Adherence 2017, 401–413 (2017).
Acam, J., Kuodi, P., Medhin, G. & Makonnen, E. Antimicrobial prescription patterns in East Africa: A systematic review. Syst. Rev. 12, 18 (2023).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906 (2021).
Zingg, W. et al. Antimicrobial use in acute care hospitals: National point prevalence survey on healthcare-associated infections and antimicrobial use, Switzerland, 2017. Eurosurveillance 24, 1900015 (2019).
Vandael, E. et al. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: Results of the Global-PPS and ECDC-PPS 2017. Antimicrob. Resist. Infect. Control 9, 1–13 (2020).
Gugliotta, C. et al. Prevalence study on health-care associated infections and on the use of antimicrobials carried out with the light protocol of the European Centre for Disease Prevention and Control. Ann. Ig 32, 357–367 (2020).
Glasziou, P., Dartnell, J., Biezen, R., Morgan, M. & Manski-Nankervis, J. A. Antibiotic stewardship: A review of successful, evidence-based primary care strategies. Aust. J. Gen. Pract. 51, 15–20 (2022).
Magin, P., Davey, A. & Davis, J. Evidence-based strategies for better antibiotic prescribing. Austral. J. Gener. Practition. 51, 21–24 (2022).
Borek, A. J. et al. Development of an intervention to support the implementation of evidence-based strategies for optimising antibiotic prescribing in general practice. Implement. Sci. Commun. 2, 1–16 (2021).
Mar, C. D., Hoffmann, T. & Bakhit, M. How can general practitioners reduce antibiotic prescribing in collaboration with their patients?. Austral. J. Gener. Pract. 51, 25–30 (2022).
Migliavaca, C. B., Stein, C., Colpani, V., Munn, Z. & Falavigna, M. Quality assessment of prevalence studies: A systematic review. J. Clin. Epidemiol. 127, 59–68 (2020).
Barker, T. H. et al. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 21, 189 (2021).
Iddagoda, M. T. & Flicker, L. Clinical systematic reviews—a brief overview. BMC Med. Res. Methodol. 23, 226 (2023).
Bunduki, G. K., Feasey, N., Henrion, M. Y., Noah, P. & Musaya, J. Healthcare-associated infections and antimicrobial use in surgical wards of a large urban central hospital in Blantyre, Malawi: A point prevalence survey. Infect. Prevent. Practice 3, 100163 (2021).
Skosana, P. et al. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev. Anti-infect. Therapy 19, 1353–1366 (2021).
Abubakar, U. Antibiotic use among hospitalized patients in northern Nigeria: A multicenter point-prevalence survey. BMC Infect. Dis. 20, 86 (2020).
Umeokonkwo, C. D. et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: A call for improved antibiotic stewardship. J. Glob. Antimicrob. Resist. 17, 291–295 (2019).
Manga, M. et al. Empirical antibiotherapy as a potential driver of antibiotic resistance: Observations from a point prevalence survey of antibiotic consumption and resistance in Gombe, Nigeria. Afr. J. Clin. Exp. Microbiol. 22, 273–278 (2021).
Aboderin, A. O. et al. Antimicrobial use among hospitalized patients: A multi-center, point prevalence survey across public healthcare facilities, Osun State, Nigeria. Germs 11, 523 (2021).
Chijioke, A. N. et al. Prevalence of antimicrobial use in major hospitals in Owerri, Nigeria. EC Microbiol. 3, 522–527 (2016).
Fowotade, A. et al. Point prevalence survey of antimicrobial prescribing in a Nigerian Hospital: Findings and implications on antimicrobial resistance. West Afr. J. Med. 37, 216–220 (2020).
Nnadozie, U. U. et al. Patterns of antimicrobial use in a specialized surgical hospital in Southeast Nigeria: Need for a standardized protocol of antimicrobial use in the tropics. Niger. J. Med. 30, 187–191 (2021).
Oduyebo, O. et al. A point prevalence survey of antimicrobial prescribing in four Nigerian Tertiary Hospitals. Ann. Trop. Pathol. 8, 42 (2017).
Ogunleye, O. O. et al. A multicentre point prevalence study of antibiotics utilization in hospitalized patients in an urban secondary and a tertiary healthcare facilities in Nigeria: Findings and implications. Expert Rev. Anti-infect. Therapy 20, 297–306 (2022).
Labi, A.-K. et al. Antibiotic use in a tertiary healthcare facility in Ghana: A point prevalence survey. Antimicrob. Resist. Infect. Control 7, 1–9 (2018).
Labi, A.-K. et al. Antimicrobial use in hospitalized patients: A multicentre point prevalence survey across seven hospitals in Ghana. JAC-Antimicrob. Resist. 3, 087 (2021).
Labi, A.-K. et al. Antibiotic prescribing in paediatric inpatients in Ghana: A multi-centre point prevalence survey. BMC Pediatr. 18, 1–9 (2018).
Amponsah, O. K. O. et al. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC Antimicrob. Resist. 3, 008 (2021).
Bediako-Bowan, A. A. et al. Antibiotic use in surgical units of selected hospitals in Ghana: A multi-centre point prevalence survey. BMC Public Health 19, 1–10 (2019).
Ankrah, D. et al. Point prevalence survey of antimicrobial utilization in Ghana’s premier hospital: Implications for antimicrobial stewardship. Antibiot. Basel 2021, 10 (2021).
Kamita, M. et al. Point prevalence survey to assess antibiotic prescribing pattern among hospitalized patients in a county referral hospital in Kenya. Front. Antibiot. 1, 993271 (2022).
Okoth, C. et al. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Hosp. Pract. 1995(46), 128–136 (2018).
Omulo, S. et al. Point-prevalence survey of antibiotic use at three public referral hospitals in Kenya. Plos one 17, e0270048 (2022).
Momanyi, L. et al. Antibiotic prescribing patterns at a leading referral hospital in Kenya: A point prevalence survey. J. Res. Pharm. Pract. 8, 149–154 (2019).
Skosana, P. et al. A national, multicentre, web-based point prevalence survey of antimicrobial use and quality indices among hospitalised paediatric patients across South Africa. J. Glob. Antimicrob. Resist. 29, 542–550 (2022).
Horumpende, P. G. et al. Point prevalence survey of antimicrobial use in three hospitals in North-Eastern Tanzania. Antimicrob. Resist. Infect. Control 9, 1–6 (2020).
Seni, J. et al. Antimicrobial use across six referral hospitals in Tanzania: A point prevalence survey. BMJ Open 10, e042819 (2020).
Ahoyo, T. A. et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: Results of the first nationwide survey in 2012. Antimicrob. Resist. Infect. Control 3, 17 (2014).
Anand Paramadhas, B. D. et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev. Anti Infect. Ther. 17, 535–546 (2019).
Fentie, A. M. et al. Multicentre point-prevalence survey of antibiotic use and healthcare-associated infections in Ethiopian hospitals. BMJ Open 12, e054541 (2022).
Kiggundu, R. et al. Point prevalence survey of antibiotic use across 13 hospitals in Uganda. Antibiotics 11, 199 (2022).
Kiggundu, R. et al. Point prevalence survey of antibiotic use across 13 hospitals in Uganda. Antibiot. Basel 2022, 11 (2022).
Labi, A. K. et al. Antimicrobial use in hospitalized patients: A multicentre point prevalence survey across seven hospitals in Ghana. JAC Antimicrob. Resist. 3, 087 (2021).
Labi, A. K. et al. Antibiotic use in a tertiary healthcare facility in Ghana: A point prevalence survey. Antimicrob. Resist. Infect. Control 7, 15 (2018).
Abubakar, U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob. Resist. Infect. Control 9, 63 (2020).
Labi, A. K. et al. Antibiotic prescribing in paediatric inpatients in Ghana: A multi-centre point prevalence survey. BMC Pediatr. 18, 391 (2018).
Amponsah, O. K. O. et al. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC-Antimicrob. Resist. 3, 008 (2021).
Seni, J. et al. Multicentre evaluation of significant bacteriuria among pregnant women in the cascade of referral healthcare system in North-western Tanzania: Bacterial pathogens, antimicrobial resistance profiles and predictors. J. Glob. Antimicrob. Resist. 17, 173–179 (2019).
Lin, L. & Chu, H. Quantifying publication bias in meta-analysis. Biometrics 74, 785–794 (2018).
Alothman, A. et al. Prevalence of infections and antimicrobial use in the acute-care hospital setting in the Middle East: Results from the first point-prevalence survey in the region. Int. J. Infect. Dis. 101, 249–258 (2020).
Plachouras, D. et al. Antimicrobial use in European acute care hospitals: Results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill 2018, 23 (2018).
Mallah, N., Orsini, N., Figueiras, A. & Takkouche, B. Income level and antibiotic misuse: A systematic review and dose–response meta-analysis. Eur. J. Health Econ. 23, 1015–1035 (2022).
Mallah, N., Orsini, N., Figueiras, A. & Takkouche, B. Education level and misuse of antibiotics in the general population: A systematic review and dose–response meta-analysis. Antimicrob. Resist. Infect. Control 11, 24 (2022).
Barrera-Cancedda, A. E., Riman, K. A., Shinnick, J. E. & Buttenheim, A. M. Implementation strategies for infection prevention and control promotion for nurses in Sub-Saharan Africa: A systematic review. Implement. Sci. 14, 111 (2019).
Bouzid, M., Cumming, O. & Hunter, P. R. What is the impact of water sanitation and hygiene in healthcare facilities on care seeking behaviour and patient satisfaction? A systematic review of the evidence from low-income and middle-income countries. BMJ Glob. Health 3, e000648 (2018).
Moyo, P. et al. Prevention of antimicrobial resistance in sub-Saharan Africa: What has worked? What still needs to be done?. J. Infect. Public Health 16, 632–639 (2023).
Kariuki, S., Kering, K., Wairimu, C., Onsare, R. & Mbae, C. Antimicrobial resistance rates and surveillance in sub-saharan africa: Where are we now?. Infect. Drug Resist. 15, 3589–3609 (2022).
Magill, S. S. et al. Antimicrobial Use in US Hospitals: Comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin. Infect. Dis. 72, 1784–1792 (2020).
Trejnowska, E. et al. Surveillance of antibiotic prescribing in intensive care units in Poland. Can. J. Infect. Dis. Med. Microbiol. 2018, 14 (2018).
Patangia, D. V., Anthony Ryan, C., Dempsey, E., Paul Ross, R. & Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiol. Open 11, e1260 (2022).
Arteaga-Livias, K. et al. Compliance with antibiotic prophylaxis in obstetric and gynecological surgeries in two peruvian hospitals. Antibiotics 12, 808 (2023).
Eddy, K. E. et al. Factors affecting the use of antibiotics and antiseptics to prevent maternal infection at birth: A global mixed-methods systematic review. Plos one 17, e0272982 (2022).
Vippadapu, P. et al. Choice of antimicrobials in surgical prophylaxis - overuse and surgical site infection outcomes from a tertiary-level care hospital. Front. Pharmacol. 2022, 13 (2022).
Ngonzi, J. et al. Incidence of postpartum infection, outcomes and associated risk factors at Mbarara regional referral hospital in Uganda. BMC Pregn. Childbirth 18, 270 (2018).
Abdel Jalil, M. H. et al. Surgical site infections following caesarean operations at a Jordanian teaching hospital: Frequency and implicated factors. Sci. Rep. 7, 12210 (2017).
Beletew, B., Bimerew, M., Mengesha, A., Wudu, M. & Azmeraw, M. Prevalence of pneumonia and its associated factors among under-five children in East Africa: A systematic review and meta-analysis. BMC Pediatr. 20, 254 (2020).
Murni, I. K. et al. Risk factors for healthcare-associated infection among children in a low-and middle-income country. BMC Infect. Dis. 22, 406 (2022).
Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 12, 3903–3910 (2019).
Prestinaci, F., Pezzotti, P. & Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathogens Glob. Health 109, 309–318 (2015).
Erku, D. A., Mekuria, A. B. & Belachew, S. A. Inappropriate use of antibiotics among communities of Gondar town, Ethiopia: A threat to the development of antimicrobial resistance. Antimicrob. Resist. Infect. Control 6, 112 (2017).
Chinemerem Nwobodo, D. et al. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 36, e24655 (2022).
Aslam, B. et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug rResist. 2018, 1645–1658 (2018).
Adebisi, Y. A. Balancing the risks and benefits of antibiotic use in a globalized world: The ethics of antimicrobial resistance. Globaliz. Health 19, 27 (2023).
Sukhum, K. V. et al. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Commun. Med. 2, 62 (2022).
Raoofi, S. et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS One 18, e0274248 (2023).
Lowe, H. et al. Challenges and opportunities for infection prevention and control in hospitals in conflict-affected settings: A qualitative study. Conflict Health 15, 94 (2021).
Spencer, S. A. et al. A health systems approach to critical care delivery in low-resource settings: A narrative review. Intens. Care Med. 49, 772–784 (2023).
Improta, G. et al. A case study to investigate the impact of overcrowding indices in emergency departments. BMC Emerg. Med. 22, 143 (2022).
Daw, M. A., Mahamat, M. H., Wareg, S. E., El-Bouzedi, A. H. & Ahmed, M. O. Epidemiological manifestations and impact of healthcare-associated infections in Libyan national hospitals. Antimicrob. Resist. Infect. Control 12, 122 (2023).
Taye, Z. W., Abebil, Y. A., Akalu, T. Y., Tessema, G. M. & Taye, E. B. Incidence and determinants of nosocomial infection among hospital admitted adult chronic disease patients in University of Gondar Comprehensive Specialized Hospital, North-West Ethiopia, 2016–2020. Front. Public Health 11, 1087407 (2023).
Zarb, P. et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 17, 20316 (2012).
Oo, W. T. et al. Point-prevalence surveys of antimicrobial consumption and resistance at a paediatric and an adult tertiary referral hospital in Yangon, Myanmar. Infect. Prevent. Pract. 4, 100197 (2022).
Mehtar, S. et al. Implementation of surgical site infection surveillance in low- and middle-income countries: A position statement for the International Society for Infectious Diseases. Int. J. Infect. Dis. 100, 123–131 (2020).
Sefah, I. A. et al. Appropriateness of surgical antimicrobial prophylaxis in a teaching hospital in Ghana: Findings and implications. JAC-Antimicrob. Resist. 2022, 4 (2022).
Mwita, J. C. et al. Key issues surrounding appropriate antibiotic use for prevention of surgical site infections in low-and middle-income countries: A narrative review and the implications. Int. J. Gener. Med. 2017, 515–530 (2021).
Karmila, A. et al. The prevalence and factors associated with prophylactic antibiotic use during delivery: A hospital-based retrospective study in Palembang, Indonesia. Antibiot. Basel 2021, 10 (2021).
Alemu, A. Y. et al. Healthcare-associated infection and its determinants in Ethiopia: A systematic review and meta-analysis. PLoS One 15, e0241073 (2020).
Ayed, H. B. et al. Prevalence and risk factors of health care–associated infections in a limited resources country: A cross-sectional study. Am. J. Infect. Control 47, 945–950 (2019).
Saleem, Z. et al. Antibiotic utilization patterns for different wound types among surgical patients: Findings and implications. Antibiotics 12, 678 (2023).
Ataiyero, Y., Dyson, J. & Graham, M. Barriers to hand hygiene practices among health care workers in sub-Saharan African countries: A narrative review. Am. J. Infect. Control 47, 565–573 (2019).
Alahmadi, Y. et al. Point-prevalence surveys of antibiotic use and HAIs. Hospital Pharm. Europe Pharm. Pract. 84, 27–29 (2016).
Fraser, J. L., Mwatondo, A., Alimi, Y. H., Varma, J. K. & Vilas, V. J. D. R. Healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018: A review. Int. J. Infect. Dis. 103, 469–477 (2021).
Abubakar, U., Amir, O. & Rodríguez-Baño, J. Healthcare-associated infections in Africa: A systematic review and meta-analysis of point prevalence studies. J. Pharm. Policy Pract. 15, 99 (2022).
Gidey, K., Gidey, M. T., Hailu, B. Y., Gebreamlak, Z. B. & Niriayo, Y. L. Clinical and economic burden of healthcare-associated infections: A prospective cohort study. Plos one 18, e0282141 (2023).
Igunma, A. & Adebudo, O. Healthcare-associated infections and control strategies. Niger. J. Med. Dental Educ. 5, 81–87 (2023).
Sono, T. M. et al. Current rates of purchasing of antibiotics without a prescription across sub-saharan Africa; rationale and potential programmes to reduce inappropriate dispensing and resistance. Expert Rev. Anti-infective Therapy 21, 1025–1055 (2023).
Acknowledgements
We would like to acknowledge the Ethiopian Evidence Based Health Care and Development Centre, A JBI Centre of Excellence, and the Armauer Hansen Research Institute for proving the training on comprehensive systematic review, meta-analysis, and access to databases.
Author information
Authors and Affiliations
Contributions
MTB and SM was involved in a principal role in the conception of ideas, developing methodologies, and writing the article. MTB, SM, MW, SWG, VS, YS, BH and ZEK were involved in the analysis, interpretation and writing. All authors involved in proofreading and writing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boltena, M.T., Wolde, M., Hailu, B. et al. Point prevalence of evidence-based antimicrobial use among hospitalized patients in sub-Saharan Africa: a systematic review and meta-analysis. Sci Rep 14, 12652 (2024). https://doi.org/10.1038/s41598-024-62651-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62651-6
- Springer Nature Limited