Abstract
Wolbachia bacteria are common endosymbionts of insects and have recently been applied for controlling arboviral vectors, especially Aedes aegypti mosquito populations. However, several medically important mosquito species in Sri Lanka were present with limited information for the Wolbachia infection status. Therefore, the screening of Wolbachia in indigenous mosquitoes is required prior to a successful application of Wolbachia-based vector control strategy. In this study, screening of 78 mosquito species collected from various parts of the country revealed that 13 species were positive for Wolbachia infection, giving ~ 17% infection frequency of Wolbachia among the Sri Lankan mosquitoes. Twelve Wolbachia-positive mosquito species were selected for downstream Wolbachia strain genotyping using Multi Locus Sequencing Type (MLST), wsp gene, and 16S rRNA gene-based approaches. Results showed that these Wolbachia strains clustered together with the present Wolbachia phylogeny of world mosquito populations with some variations. Almost 90% of the mosquito populations were infected with supergroup B while the remaining were infected with supergroup A. A new record of Wolbachia supergroup B infection in Ae. aegypti, the main vectors of dengue, was highlighted. This finding was further confirmed by real-time qPCR, revealing Wolbachia density variations between Ae. aegypti and Ae. albopictus (p = 0.001), and between males and females (p < 0.05). The evidence of natural Wolbachia infections in Ae. aegypti populations in Sri Lanka is an extremely rare incident that has the potential to be used for arboviral vector control.
Similar content being viewed by others
Introduction
Wolbachia bacteria widely spread among varieties of insect species and exhibit different effects on the host cells or immune systems. At present, Wolbachia plays an important role in many of the insect vector control programs due to its biosafety and environmentally friendly nature. Its application is enormous and promising for agricultural pests such as fruit flies; flea beetle, Aphthona spp., to control leafy spurge1; house fly, Musca domestica, and stable fly, Stomoxys calcitrans2. In addition, this approach has been practiced for medically important vector control programs including Aedes vectors3,4,5; and paratransgenesis of Wolbachia is being considered for use in mosquitoes to reduce the spread of malaria6 and in tsetse fly to reduce the spread of sleeping sickness7. Moreover, other mosquito species, such as Aedes polynesiensis (South Pacific)8, Aedes albopictus (Italy)9, and Culex quinquefasciatus (southwestern Indian ocean)10, have also been field tested to determine the feasibility of using Wolbachia-based population suppression technology in the near future.
Wolbachia are maternally inherited and favor infected females by inducing Cytoplasmic Incompatibility (CI). The CI caused developmental failure of offspring in the cross between uninfected females and Wolbachia-infected males. This increased the relative success of infected females in the populations. Along with this approach, Wolbachia induced CI was being applied to create sexually incompatible Ae. aegypti male mosquitoes to be used for Wolbachia-based population suppression strategies, i.e., Incompatible Insect Technique (IIT) and in combined with Sterile Insect Techniques (SIT) that ionizing irradiation was to ensure the male sterility and to avoid the release of fertile female mosquitoes. On the other hand, Wolbachia had been implemented with the population replacement strategy, that both Wolbachia infected males and females were introduced in the field to establish the Wolbachia infected population which significantly reduced the cost of mosquito production. In fact, attention on Wolbachia-based approach for Ae. aegypti mosquito control has been drawn in Sri Lanka on both replacement and suppression strategies11,12. Sri Lanka released Wolbachia-infected mosquitoes for the pilot replacement program (20 km2) for dengue vector control during year 2020–202111 and also research-scale trial with SIT12. This was supported by the present promising results recorded from several countries such as China4, Brazil13, Australia14, Vietnam15, and Thailand16. Therefore, there has been a strong requirement for screening of primarily major arboviral vectors and other mosquito species for autochthonous Wolbachia infection before implementing a large scale Wolbachia-infected Ae. aegypti release program in Sri Lanka. Identification of autochthonous Wolbachia infection in Ae. aegypti host was primarily important to reduce the risk in future failures due to generating sexually compatible mating.
In addition to CI induced by Wolbachia, this bacterium was also capable of inducing several other sex-related phenotypes, including male killing (MK), male feminization (MF) and thelytokous parthenogenesis (TP)17. An application of artificially Wolbachia transferring from a different host, such as Drosophila melanogaster into Ae. aegypti to suppress natural populations of dengue virus vectors, have been successfully demonstrated by many countries18. Therefore, the knowledge on Wolbachia-mosquito symbiosis in various autochthonous insects is a crucial factor. Identifying naturally occurring Wolbachia strains in mosquitoes is useful for two reasons. Firstly, the transfer of naturally occurring Wolbachia among closely related species is more promising than among distinct ones19,20, since there is an increased chance of a) not having negative effects on the host and b) successfully adapting and expressing the desired phenotype in the new host. Secondly, the dynamics of Wolbachia strains that are introduced into an insect population may be altered by the Wolbachia strains that already exist in that populations due to CI and/or competition among strains21.
Furthermore, besides reproductive host manipulations, Wolbachia can also affect nutritional and metabolic pathways of the hosts as well as can affect host development and lifespan. In addition, Wolbachia can provide protection of the hosts from pathogens and parasites, as well as affect host mating behavior and facilitate host speciation17,22. It also alters the competence of transinfected arthropod vectors for the transmission of arboviruses through competition for resources, immune-priming, induction of the phenoloxidase cascade and induction of microRNA-dependent immune pathways22. As Wolbachia provide various benefits, the application of mosquitoes control using Wolbachia bacteria is very promising.
Wolbachia autochthonous infection in Ae. aegypti has been recorded from time to time in several countries18,20,23, where the screening methods has been always doubtful either with possible contamination of the sample or false positivity due to inappropriate research practices. Therefore, our study adopted Multi Locus Sequencing Type (MLST), wsp gene, and 16S rRNA gene-based approaches, together with the quantification of Wolbachia infection densities in different hosts through the qPCR method to confirm the detection of Wolbachia in Ae. aegypti. In addition, the investigation of Wolbachia diversity and density in Sri Lanka mosquitoes should be vital information for future arboviral disease control programs applying Wolbachia bacteria which could reduce the public health and economic burdens from mosquito-borne diseases.
Results
Wolbachia incidence and prevalence in Sri Lankan mosquitoes
Out of 78 mosquito species screened, 13 were positive with both wsp and 16S rRNA PCR assays giving overall 16.7% infection frequency among the sampled mosquito species (Table 1). The Wolbachia infection frequency of Ae. aegypti screened was 3.35% of the wild population (17/507) and Ae. aegypti specimens collected from three different locations including Anuradhapura (4/107, 3.74%, collection time Oct 2014), Colombo (8/150, 5.33%, collection time June 2022), and Gampaha (5/150, 3.33%, collection time June 2022) recorded Wolbachia prevalence while a single infected specimen was not found in the Trincomalee sampling location (0/100, 0%, collection time June 2023) (Supplementary Table S1). For the rest of the Wolbachia-positive species examined, the infection rates were always 100% with 1–10 samples examined per species.
Mosquito species verification by CO I and CO II sequencing
The result of cytochrome oxidase subunit 1 (CO I) and CO II sequencing for Wolbachia-positive Aedes aegypti species (BC161207-011_contig_14) revealed that these species, which was morphologically identified, belongs to Ae. aegypti (Fig. 1). The control specimens of identification based on morphological characters carried out in this study for Ae. aegypti were confirmed as they clustered with corresponding species in the phylogenetic analysis.
Phylogenetic tree for confirmation of the Aedes aegypti sample; molecular identification was performed by PCR followed by sequencing using CO I primers for Aedes aegypti mosquito samples. The results of phylogenetic analysis of the same samples for species confirmation (BC161207-011_contig_14) was displayed.
Genotyping of the Wolbachia strains
A clear single band was obtained for the DNA of 13 mosquito species with five different MLST primers (Table 2). Figure 2 described the variation of DNA band size for Mansonia indiana sample amplified with five different MLST primers. However, sequences obtained for the Culex tritaeniorhynchus sample were failed to submit to the Gene Bank database due to multiple peaks obtained. Ten species showed absence of nucleotide polymorphisms in the wsp sequences, suggesting that a single strain was infecting these mosquito species. The two remaining species gave multiple peaks in the chromatogram for some of the primer PCR sequences denoting possible infection of multiple strains of Wolbachia (results summarized in Tables 1 and 2).
Analysis of the sequences
Polymorphism analysis for the 16S rRNA gene revealed that a total of 55 sites analyzed with 31 invariable (monomorphic) sites and 25 polymorphic (segregating) sites. The number of positions with gaps was four and the number of positions with missing data was recorded as zero. However, the 16S sequence attributed to Wolbachia from Ae. aegypti had highest nucleotide identity (100%) to the Wolbachia 16S from Ae. aegypti [GenBank: MF999263]. This identity was better than that of Wolbachia 16S sequences from the sister species within the genus Aedes namely Ae. albopictus (96%) and Ae. pseudoalbopictus (92%).
Allelic profile form for different Wolbachia strains infecting mosquitoes
Allelic profiles of 12 different Wolbachia strains infecting different mosquito species were given in Table 2. Most of the alleles were exactly matched with the available alleles in the Wolbachia MLST database while the rest were partially matched (Table 2). All sequences obtained for ftsZ MLST gene perfectly matched to the existing alleles in the MLST database. However, only the Ae. albopictus MLST gene profile was exactly matched with the Ae. albopictus allelic profile already present in the Wolbachia MLST database (ST-2). Based on a comparison to the Wolbachia MLST database (http://pubmlst.org/wolbachia), 14 alleles of gatB, coxA, hcpA and fbpA were determined to be novel. The combination of alleles for all five genes in Ae. aegypti Wolbachia was unique and constituted a novel strain type. Most of the Wolbachia strain shared two or three alleles with Wolbachia from closely related species or genus.
Wolbachia strain characterization based on the amino acid motifs of the hypervariable regions (HVRs) of the wsp sequence
Due to multiple peaks or low-quality sequences obtained in sequencing, five samples failed to sequence with wsp primers. Allelic profile form of rest of the samples were shown in Table 3. Accordingly, only Ae. albopictus and Cx. pipiens wsp and MLST alleles perfectly matched with the existing alleles in the database (http://pubmlst.org/wolbachia) and they were from the same mosquito host species. Other alleles partially matched with Wolbachia strains in the same mosquito host species or different arthropod species. Accordingly, a total of 43 new alleles were submitted to the database for allele number assignment which includes at least one allele in all the genes that were completed for both wsp and MLST genes.
Phylogenetic inferences for individual genes of 16S rRNA, wsp and MLST
Based on the phylogenetic relationship between sequences in each gene, phylogenetic trees were generated separately for 16S rRNA (Supplementary Fig. 1A), wsp (Supplementary Fig. 1B) and concatenated MLST sequences (Supplementary Fig. 1C). Supplementary Fig. 1-A showed the phylogenetic tree drawn from the sequence data together with reference sequences obtained from the gene bank database for the gene 16S rRNA. The optimal tree with the sum of branch length is 0.045. Evolutionary distance between Wolbachia strains infected Ae. albopictus and Ae. aegypti was 0.011 with 0.005 base substitutions per site according to the above phylogeny. The 16S sequence attributed to Wolbachia from Ae. aegypti had highest nucleotide identity (100%) to the Wolbachia 16S from Ae. aegypti [GenBank: MF999263]. This identity was higher than that between the Wolbachia 16S sequences from the sister species within the genus Aedes namely Ae. albopictus (96%) and Ae. pseudoalbopictus (92%).
The optimal tree with the sum of branch length was 1.413 shown in Supplementary Fig. 1-B for the phylogeny based on the wsp gene. According to the estimation of evolutionary distance between Wolbachia strains present in the mosquito host species based on the wsp gene sequence, there was no genetic distance between Wolbachia strains infecting Ae. aegypti and Ae. albopictus mosquito host species. Similarly, there was no genetic distance between Wolbachia strains infecting Cx. gelidus and Mn. indiana mosquito hosts. The highest evolutionary distance was obtained for the Wolbachia strains present in Ae. kesseli with both Ae. albopictus and Ae. aegypti mosquito hosts and it was indicated as 1.322.
Phylogenetic analysis of the Wolbachia strains of seven mosquito species, based on complete MLST gene set was given in the Supplementary Fig. 1-C. Concatenated reference sequences were also incorporated and aligned with available sequences as described earlier. Ae. aegypti, Cx. gelidus, Mn. uniformis Wolbachia strains cluster together while the four remaining strains (derived from Cx. quinquefaciatus, Ar. subalbatus, Ae. flavus and Ae. albopictus) clustered in a separate clade. Phylogenies using wsp and the concatenation of the five MLST genes showed some discrepancy in respect to the position of Wolbachia strains present in certain mosquito species hosts with 16S rRNA phylogeny. Based on the 16S rRNA and wsp phylogeny, Ae. aegypti Wolbachia was closely related to Wolbachia from Ae. albopictus (Supplementary Fig. 1- A and B) while the MLST phylogeny placed Ae. aegypti Wolbachia more closely related to Wolbachia from Mn. uniformis (Supplementary Fig. 1-C).
Phylogenetic inferences for individual MLST genes
Since there were inconsistencies regarding the different datasets, not only concatenated, MLST genes were further analyzed individually. Phylogenetic tree was constructed for each aligned multiple gene sequences obtained for each of the MLST genes of the Wolbachia strain present in mosquitoes as assessed by sequencing of corresponding PCR products of each individual species. Phylogenetic trees were given under Supplementary Fig. 2-A—gatB, 2-B – coxA, 2-C – hcpA, 2-D – ftsZ and 2-E – fbpA. Numbers at the nodes of each phylogenetic tree indicated bootstrap values and reference sequences included host strains from PubMLST.
As shown in the Supplementary Fig. 2-A, the gatB gene-based phylogeny analysis of Wolbachia strains present in mosquito species showed 0.00 genetic variation between the test sequences and also with the reference sequences other than one of the Wolbachia B strain presents in Ae. albopictus from China (PubMLST allele 1760). Wolbachia strains present in filarial nematode (super group C) were clustered separately as expected.
According to the coxA gene-based phylogeny analysis of Wolbachia strains present in mosquito species (Supplementary Fig. 2-B), it was clear that this gene sequence was very similar or identical between Wolbachia strains infecting the species in one genus. Therefore, there was no or minimal genetic variation between Wolbachia strains infecting different host species. The hcpA gene-based phylogeny analysis of Wolbachia strains present in the mosquito hosts in Sri Lanka revealed that Wolbachia strains infecting Mn. indiana species had the hcpA gene sequence similar to the reference sequences of Wolbachia strains infecting Cx. quinquefasciatus (1808, 498) and Cx. pipiens (28) (Supplementary Fig. 2-C). Similarly, Wolbachia strains infecting Mn. uniformis, Cx. fuscocephala and Cx. pipiens had identical hcpA gene sequences. In addition, ftsZ gene sequences of Wolbachia strains infecting different mosquito species of the same genus were very similar (Supplementary Fig. 2-D); however, there were exceptions like in the case of the Wolbachia strains infecting Cx. fuscocephala and Ae. pseudoalbopictus from Sri Lanka. Similarly with other MLST genes, the fbpA gene-based phylogeny analysis of Wolbachia strains present in mosquitoes from Sri Lanka showed very close genetic structure within the same host genus (Supplementary Fig. 2-E).
All available concatenated sequences of MLST genes and wsp gene were again aligned and resulted concatenated sequences were aligned with reference sequences attributed in the same way. The resulting phylogenetic tree was given in Fig. 3. Out of the available complete MLST gene profile of eight Wolbachia strains in different mosquito hosts, only six strains had wsp gene sequences. Therefore, this final analysis step involved only seven Wolbachia isolates from different mosquito hosts, i.e., Ae. aegypti, Ae. albopictus, Cx. gelidus, Cx. pipiens, Cx. quinquefasciatus, Mn. indiana and Mn. uniformis (Table 2). According to the phylogeny of these concatenated sequences within group, the mean distance for genus Aedes was 0.428 and that for genus Culex was 0.844. The mean distance between groups were as follows: genus Aedes and Culex – 0.811; genus Aedes and Mansonia – 0.523 and genus Culex and Mansonia – 0.573. The mean diversity of the entire population was 0.706 while the highest genetic diversity was present between Cx. pipiens and Ae. aegypti. As revealed from the overall Wolbachia genotyping work (Fig. 3), Ae. aegypti, Cx. quinquefasciatus, Cx. gelidus and Mn. uniformis mosquito host were infected with Wolbachia B strain while Cx. pipiens infected with wPip strain. Further findings proved that Ae. albopictus was infected with both Wolbachia strains A (wAlbA) and B (wPip) supergroups. Wolbachia strains present in filarial nematode (Brugia malayi) were out rooted separately as expected.
Wolbachia density in different arboviral mosquito hosts
Results of the Wolbachia infection dynamics in terms of Ct value of the wsp qPCR per insect were examined using quantitative PCR technique. Results indicated that the population of Wolbachia density varied between arboviral hosts and as well as depending on the host tissues. The Wolbachia infection density was highest in Ae. albopictus adult females with an average Ct value of 29.26 per insect, and it was not statistically significant between individual female Ae. albopictus specimens (p = 0.071). The lowest Wolbachia density was recorded for Ae. aegypti female mosquitos giving an average Ct value of 36.15, and the Ct value varied between 34.41 and 37.88 (limit of detection was 37.8). As the Wolbachia density was very low, future attempt is necessary to investigate for possible autochthonous Wolbachia infection in Ae. aegypti. In addition, the Wolbachia density was significantly different between individual female specimens (p = 0.001). Wolbachia density showed a gender difference, i.e., females had a higher Wolbachia density than males and it was also significantly different between both arboviral vectors (Ae. aegypti vs Ae. albopictus) (p = 0.001).
Discussion
The presence of Wolbachia in mosquito species and the ability to trans-infect into the native Wolbachia-uninfected species and new host/strain combinations to induce reproductive and other interesting phenotypes has rendered this symbiont a promising tool to control mosquito vectors24. Therefore, both findings related to Wolbachia infection frequencies and genetic diversity were important for the success in Wolbachia-based population suppression strategies. The diversity of Wolbachia infection in mosquito taxa was reported in Southeast Asia25,26, Europe27, Africa28 and North America29. Several studies have unveiled the occurrence of natural Wolbachia infection in various mosquito genera, including Mansonia, Aedes, Armigeres, and Culex. While Wolbachia infection in Ae. albopictus has been frequently documented, there has been limited emphasis on the natural infection in Ae. aegypti mosquitoes30. Interestingly, despite the prevailing absence of natural Wolbachia infection within Ae. aegypti populations in most studies25,28,31, a recent investigation conducted by Balaji and colleagues32 using molecular techniques has demonstrated the presence of Wolbachia in Ae. aegypti mosquitoes collected from Coimbatore, India. Employing PCR amplification with Wolbachia-specific primers targeting 16S rRNA, wsp, and ftsZ genes, this study conclusively identified Wolbachia supergroup B in the Ae. aegypti populations through phylogenetic analysis.
Notably, Wolbachia natural infection has been scarcely recorded in Anopheles and Ae. aegypti mosquitoes, with a few studies conducted in Malaysia26,33, Thailand34,35, Myanmar36, Philippines37,38, Panama39, New Mexico and Florida, USA40, and various regions in Africa41,42,43,44,45. A study by Wong and colleagues in Malaysia26 reported Wolbachia infection in both Anopheles mosquitoes (An. balabacensis, An. introlatus, An. macarthuri, An. latens, An. maculatus, An. barbirostris, An. hyrcanus, and An. sinensis amplified from Wolbachia specific 16S rRNA primer) and Ae. aegypti (amplified from wsp primer).
In Sri Lanka besides from our study, there were two other studies related to frequency and distribution of Wolbachia within wild mosquito populations46,47. Nugapola and colleagues reported that they had screened a total of 330 individual mosquitoes belonging to 22 species and 7 genera, out of which 87 mosquitoes (26.36%), belonging to four species (i.e. Ae. albopictus, Cx. quinquefasciatus, Ar. subalbatus and Mn. uniformis), were reported as positive for Wolbachia natural infections as detected by wsp gene primers46. Aedes aegypti was negative for Wolbachia infection (n = 40) in which 2 samples collected from Battaramulla located around 9 km far from our Colombo study site that we observed 8 Wolbachia-positive Ae. aegypti samples among 150 samples. Another study, Tharsan et al. (2023), determined the Wolbachia infection in Aedes albopictus in Jaffna peninsula and found it widely infected with the wAlbA and wAlbB strains using wsp gene47. The gene sequence in Jaffna Ae. albopictus was identical to a corresponding sequence from South India but different from that in mainland Sri Lanka. Our study screened greater number of mosquitoes (n = 775) including 78 mosquito species collected from various parts of the country and revealed that 13 species were positive for Wolbachia infection. In addition, specific information of Wolbachia infection within mosquitoes in Sri Lanka was not available from the genotyping studies conducted in relation to developed MLST system. Therefore, this study focused to investigate the Wolbachia infections present in Sri Lankan mosquito species and genotyping the strains by several methods including Wolbachia specific 16S rRNA primers, wsp gene based method and MLST scheme developed by Baldo and colleagues48 for a universal genotyping tool for Wolbachia which indexed variations in five conserved genes (ftsZ, gatB, coxA, hcpA, and fbpA).
Thus, 775 individual specimens from 78 mosquito species in Sri Lanka were screened for Wolbachia prior to initiation of Wolbachia-based Aedes mosquito population suppression strategy in Sri Lanka. According to PCR screening from both 16S rRNA and wsp primers out of 78 species tested, 13 were positive, giving ~ 17% frequency of the prevalence of Wolbachia within Sri Lankan genetic background which included the genera of Mansonia, Aedes, Armigeres and Culex. This incidence estimate was compatible with all previously published estimates across arthropods. For an example a similar study conducted in a Thailand revealed that out of 89 mosquito species, the presence of Wolbachia was 28%25, consisting of the genera Aedes, Culex, Armigeres, Coquillettidia, Hodgesia, Mansonia, Tripteroides and Uranotaenia. A complete MLST profile was obtained only for 8 mosquito species and it was used for the construction of final complete phylogenetic evaluation tree. The Wolbachia 16S sequence from different mosquito species had an average nucleotide identity of 96%. We used maximum likelihood to fit a beta distribution to these data to estimate the between-species distribution of prevalence. Accordingly, the 16S phylogenetic tree resolved supergroups A to B and confirmed the identity of the sequence amplified from each mosquito that being infected with Wolbachia strains (Supplementary Fig. 1A). These results were further verified with both wsp and MLST concatenated phylogenetic trees. As observed from the sequencing results, wsp sequences were not identical between subspecies and had ambiguous bases. This was an indication of having multiple Wolbachia strains within some mosquito species. BLAST homology searches of the GenBank database confirmed strain identification as wPip, the type strain associated with Cx. pipiens. Sequence resulted from this study was identical to the existing GenBank wsp sequences from California Cx. pipiens complex mosquitoes25.
In general, arthropod Wolbachia can be divided into a few main clades such as, A and B48,49, and subdivided into further strain groupings49. Most of these strain groups were represented in the mosquitoes and they did not show similarity with mosquito phylogenetic lineages—for example the wPip subgroup occurs in several Aedes and Culex species and in an Armigeres25,49. On the other hand, superinfections of two or more Wolbachia strains within individuals also occurred, such as in Ae. albopictus where two strains from the A- and the B-clades co-existed, labelled wAlbA and wAlbB, respectively47,50. However, comparisons made between host and Wolbachia evolutionary trees strongly suggested that transfers between phylogenetically distant mosquito groups had occurred naturally49,51,52.
According to the maximum likelihood analysis, it revealed two major branches in the phylogenetic trees based on Wolbachia MLST sequences separately for each gene (Supplement Fig. 2 A-E) and collectively for all MLST genes (Fig. 1C) and wsp gene (Fig. 1B). These two characteristic branches clustered Wolbachia sequences from the studied insect host populations into two main supergroups (Fig. 1C). The first branch, which were nearly 70% of populations, harbored strains belonging to the supergroup B. The second branch included Wolbachia-infected populations, which suggested that approximately 30% were infected from an alternative source by strains belonging to supergroup A (e.g., wAlbA or wRi). Additionally, this network brought more information than traditional phylogenetic tree as it showed also multiple connections among examined Wolbachia haplotypes (MLST strains) which could correspond to the recombination events. According to previous findings there could be a coincidental false negative sample due to Wolbachia tissue tropism. Nevertheless, our findings provided an estimate of the prevalence of Wolbachia within 78 mosquito species in Sri Lanka; and this was the first report of Wolbachia infection in such many mosquito species in Sri Lanka. However, consistent with previous studies done in some other countries, none of the Anopheles species were infected with Wolbachia27,28.
Still there are no proper documentation to prove the presence of naturally infected Wolbachia in the main vector of dengue (Ae. aegypti) and malaria (Anopheles spp.) disease transmission30,31. However, research from India recently reported Wolbachia infection in Ae. aegypti mosquito populations in the country32. Though there were no data on its infection frequencies and sampling population data, some of the genotyping data (16S rRNA) was deposited on the GenBank under the accession number MF999263. According to literature, there was no Wolbachia-harboring Ae. aegypti genomic information except that from India (Wolbachia MLST database allele number 1762)32.
In contrast to the above finding, we also found 3.35% sample frequencies of Wolbachia infection among Ae. aegypti mosquitoes collected from Gampaha District, Sri Lanka (n = 507) and were able to amplify all genes with available primers. Therefore, complete genotypic information of the 16S rRNA, wsp and MLST genes were deposited on the gene bank database under the accession number MH447384. Accordingly, the evolutionary distance between Wolbachia strains of Ae. albopictus and Ae. aegypti was 0.011 with 0.0052 base substitutions per site based on the phylogeny of the 16S rRNA gene. At the same time, it had the highest nucleotide identity (100%) with the Wolbachia 16S reference sequence of Ae. aegypti deposited from India [GenBank: MF999263]. This identity was better than that of Wolbachia 16S sequences from the sister species within the genus Aedes namely Ae. albopictus (96%) and Ae. pseudoalbopictus (92%). The complete allelic profile was also submitted to the Wolbachia MLST database.
However, autochthonous Wolbachia infection in Ae. aegypti mosquitoes always create some doubt about the data due to relatively low infection frequency and inconsistency of data30. Therefore, adopting several detection tools simultaneously facilitate the confirmation of the results of Ae. aegypti autochthonous infection with Wolbachia. Conversely, an argument could also be made for the possible Wolbachia leakage from the wild Ae. albopictus to wild Ae. aegypti mosquitoes due to back-crossing and formation of sibling species. Another possibility was the sample contamination with Wolbachia infected Ae. aegypti mosquitoes due to the release program conducted a few years back under the World Mosquito Program (WMP) in Colombo District in Sri Lanka. Even though the first argument could be accepted at certain extent since interspecific cross-mating between these two species has been documented, though until now viable offspring was not observed53,54,55. There may be a rare chance that the hybrid offspring may then mate with one of the parent species or back-cross with other hybrids, leading to further genetic and possible Wolbachia exchange between the two species. For the second argument, WMP released the Wolbachia infected mosquitoes in the Colombo Municipal Council-District 1 and Nugegoda in the years 2020–202111. We detected the positive Ae. aegypti specimens in Anuradhapura (4/107, 3.74%, collection time Oct 2014), Colombo (8/150, 5.33%, collection time June 2022), and Gampaha (5/150, 3.33%, collection time June 2022). The collection in Anuradhapura was before the WMP released time and it was ~ 210 km far from Colombo and Nugegoda. Our Colombo and Gampaha collection sites were around 27.9 km far from the WMP released sites in CMCD1 and Nugegoda. The second argument could be doubtful because Aedes aegypti mosquitoes were known to have a relatively limited flight range, typically between 100 and 200 m during their lifetime. However, under certain conditions, they have been observed to travel up to 400 m or even farther. All these arguments and data suggested the necessity for more in-depth evaluation of wild Ae. aegypti mosquito population for possible autochthonous Wolbachia infection in the future.
The study conducted by the Nugapola and colleagues46 reported that the total of 330 individual mosquitoes, belonging to 22 species and 7 genera collected from 7 provinces in Sri Lanka, were screened for the presence of Wolbachia by PCR using wsp and groE primers. They found only 87 mosquitoes (26.36%) harbored Wolbachia which belonged to four different mosquito species namely Ae. albopictus, Cx. quinquefasciatus, Mn. uniformis and Ar. subalbatus. The results were comparable to our study. However, they have indicated that the infection frequency of Ae. albopictus mosquito varied between provinces while having 100% infection rate for the mosquitoes collected from the Central (n = 33) and Sabaragamuwa (n = 10) provinces followed by Southern (34.6%; 9 out of 26) and Northwestern provinces (31.0%; 9 out of 29), and only one Ae. albopictus was positive for Wolbachia from the Western province samples (3.4%; 1 out of 29)46. In our study, irrespective of the mosquito collection site, we observed 100% infection for all Wolbachia-positive mosquito species. According to the same study findings, Cx. gelidus collected from the Kandy District was not infected with Wolbachia. However, in our study, specimens collected from Colombo, Gampaha and Badulla Districts gave positive results with 100% sample infection frequency (n = 9). The wsp gene sequences of the Wolbachia strains present in Ae. albopictus and Mn. uniformis mosquito hosts clustered with the same gene locus of KY523666 and KY523674 respectively. However, Wolbachia strain from the wsp gene sequence of Cx. quinquefasciatus (KY523673) host was phylogenetically distinct from our results (Supplementary Fig. 1-B). Furthermore, our findings of Ae. albopictus superinfection with both Wolbachia strains belonging to A and B supergroups were in accordance with their findings of Wolbachia group-specific wsp primer PCR assays46. However, the GenBank submitted Ae. albopictus wsp sequences were reported to vary in another study conducted in different regions of Sri Lanka47. They had analyzed Ae. albopictus in Jaffna by using wsp primers and extensively discovered that this mosquito species harbored wAlbA and wAlbB strains of Wolbachia within Ae. albopictus population in the study location of Jaffna peninsula, Sri Lanka. They had reported that the partial gene sequence of the wAlbB wsp in Jaffna's Ae. albopictus matched precisely with a corresponding sequence from South India, yet it exhibited dissimilarities compared to the sequence found in mainland Sri Lanka. We found that this was an interesting finding which could lead to further investigation. In conclusion, the sample size, screening method, proper species identification, and geographic origin might be the reasons for different profiles of the mosquito species reported in these two and our studies.
Conclusion
Infection frequencies and strain types of Wolbachia in mosquito species found in Sri Lankan genetic background was not significantly different from those observed in Asia or Europe, irrespective of the evidence and the presence of having Wolbachia in Aedes aegypti mosquitoes in Sri Lanka where it was not previously recorded from any countries other than India. Infection frequencies of Wolbachia were very low among Ae. aegypti mosquito populations. However, as naturally occurring vector-endosymbiont association, implying coadaptation, may have proved more stable than the artificial infection currently being used for vector control. Therefore, the evidence of natural Wolbachia infections in Ae. aegypti population in Sri Lanka is an extremely rare event which may have a potential to be used in the vector control programs. Furthermore, Wolbachia density as indicated by the qPCR-based methods, created new opportunities not only to determine how bacterial abundance within the arboviral mosquito populations varied but also to give the specific considerations of the specimen selection for Wolbachia screening.
Materials and methods
Collection of mosquitoes
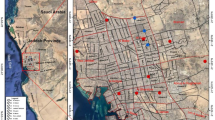
A total of 775 mosquito specimens were collected from western, southern, northern and central part of Sri Lanka including 16 different districts; namely Ampara (7.2917°N, 81.6726°E), Anuradhapura (8.5599°N, 80.4887°E), Badulla (6.9924°N, 81.0550°E), Colombo (6.9271°N, 79.8612°E), Galle (6.0320°N, 80.2170°E), Gampaha (6.999°N, 79.8916°E), Hambanthota (6.1237°N, 81.1034°E), Jaffna (9.6615°N, 80.0255°E), Kagalle (7.2518°N, 80.3466°E), Kandy (7.2906°N, 80.6337°E), Killinochchi (9.39487N, 80.40894E), Kurunegala (7.4840°N, 80.3666°E), Mannar (8.9769°N, 79.9022°E), Matara (5.9493°N, 80.5353°E), Nuwara Eliya (6.9785°N, 80.7133°E) and Tricomalee (8.5921°N, 81.1968°E), Vavuniya (8.7514°N, 80.4987°E) (Fig. 4). Indoor resting mosquitoes were collected using hand nets and mouth aspirators. Field mosquito collections were done using backpack aspirators, light traps, cattle baited traps, and human landing collections. After each collection step, samples were transported to the main laboratories in Sri Lanka. The animal use protocol in this study was approved by the Ethics Review Committees, Faculty of Medicine, University of Kelaniya (FWA00013225, Ref.No.P/25/03/2014); Faculty of Applied Sciences, Rajarata University (Ref: ERC/04/021); and the Animal Care and Use Committee (SCMU-ACUC), Faculty of Science, Mahidol University (Protocol No. MUSC66-031-661).
Distribution of mosquito collected sampling location in Sri Lanka; 17 locations representing the three different climatic zones (2010 – 2023). The map was created by using Qgis version 3.22 (https://download.qgis.org/qgisdata/QGIS-Website/live/html/en/site/forusers/download.html).
Morphological identification of insects
Mosquitoes were sorted into morphospecies using a dissecting microscope. A “voucher” for a set of mosquitoes were taken and they were identified to species level using a set of on-line identification keys, published taxonomic keys and books56,57,58. Briefly, morphological characters present on the main body parts of adult female mosquitoes were used for the identification.
Due to the practical and cost constraint, mosquito specimen screening was limited only to female mosquitoes. The selection of female mosquito was primarily due to high Wolbachia density resulted for immunological staining of female mosquito tissues57. Female mosquitoes belonged to 7 genera, i.e., Anopheles (24), Toxorhynchites (2), Tripteroides (3), Mansonia (5), Aedes (16), Armigeres (7) and Culex (21) were grouped separately, and each group were divided into sub-groups according to the species. Among the 775 individual specimens collected and analyzed, 78 distinct species were identified while five specimens failed to assign to genus/species level (Supplementary Table S1). Following morphological identification, samples were stored in a freezer (-20°C) until DNA extraction.
DNA extraction and screening of Wolbachia infection by Wolbachia-specific PCR assays
Genomic DNA was extracted from individual female mosquitoes using DNeasy Blood & Tissue Kit (Qiagen, The Netherlands) according to the protocol described by the manufacturer. To avoid the sample contamination always sample processing included negative extraction control from the known Wolbachia negative mosquito DNA sample isolated from the antibiotic treated 10% sugar fed Ae. aegypti stock caged mosquitoes maintained at the main laboratory facility in the Sri Lankan institution. Further for every PCR reaction included the known negative samples to verify the absence of contamination during the PCR master mix preparation step. PCR amplification was performed by using several different primer sets described in the Supplementary Table 2. The composition of PCR master mix and thermal profile for each primer sets were described in the Supplementary Tables 3 and 4 respectively. All PCR amplification experiments included positive and negative controls. The positive control was a Wolbachia double-infected Ae. albopictus Thailand strain sample while the negative control consisted of water as the template. Positive mosquito species for possible Wolbachia prevalence by both PCR assays with 16S rRNA and wsp primers were noted. The DNA samples of all above positive PCR products were subjected to PCR with MLST primers to verify the Wolbachia strain. The MLST scheme developed by Baldo and colleagues48 which was used as a universal genotyping tool for Wolbachia which indexed variation at five conserved genes (ftsZ, gatB, coxA, hcpA, and fbpA) of Wolbachia. The PCR primers which robustly amplified loci of strains belonging to supergroups A and B and potentially amplified loci of strains belonging to other supergroups were used. A supplemental typing system developed by the same group48, based on the use of the hypervariable regions (HVRs) of the Wolbachia surface protein (wsp), was also used as an additional marker for strain typing. Importantly, wsp typing could be complement the MLST information as it was analogous to antigen protein typing used for pathogenic bacteria59. Each PCR amplification process underwent three replicates to validate the results obtained. A fourth screening was performed for selected individual samples that had conflicting results based on the above three prior replicates. Therefore, the criteria set in reporting the certainty for Wolbachia infection was based on at least two successful amplifications of the molecular markers. Due to the absence of certain morphological identification keys for some specimens, such specific samples were PCR amplified and sequenced with Cytochrome Oxidase I (CO I) and CO II primers (Supplementary Table S2). For confirmation and validation of the sequencing results, two DNA samples from Ae. albopictus and Ar. subalbatus were also amplified with CO I and CO II primers and sequenced.
Sequencing, sequence alignments and assemblies
The PCR products were purified using either Montage PCR centrifugal filter devices (Millipore, USA) or QIAquick 96 PCR Purification kit (Qiagen, The Netherlands) and bi-directionally sequenced. Amplified PCR products from each molecular marker were sent for sequencing to Eurofins, Operon – Japan. An internal fragment of each PCR product was specifically selected for MLST. Forward and reverse sequences from each PCR product were aligned and visually inspected using both SeqManII by DNAStar and BioEdit DNA Sequence Analysis Software version 7.0.9 (Ibis BioSciences, USA). The contig sequence obtained from aligning both forward and reverse sequences were used for BLAST search of the nucleotide reference sequences on PubMed. Then contig was align with reference sequences and phylogenetic tree was constructed for each contig separately to verify the mosquito species with CO I and CO II PCR products and other primer PCR products for Wolbachia strain identification. In this circumstance, consensus sequences obtained from each individual for each gene were aligned and compared; and all sequence differences between Wolbachia strains were checked to confirm whether they had unambiguous peaks. As bacteria from each mosquito species had the same sequences, a consensus sequence for each gene per mosquito host species was obtained. All consensus sequences were trimmed to the appropriate length for database query. Finally, a BLAST search was performed for each sequence in the Wolbachia MLST database (http://pubmlst.org/wolbachia). Where a sequence had an exact match in the database, it was assigned the designated allele number. The complete MLST profiles were submitted to the Wolbachia MLST database and have been assigned the ID numbers (http://pubmlst.org/wolbachia). Similarly, the Wolbachia-positive 16S rRNA gene PCR products were directly sequenced, and the resulted sequences were compared with the available data in the GenBank database (www.ncbi.nlm.nih.gov) using BLAST search. The above sequences were also deposited in the GenBank under assigned accession numbers provided by the GenBank.
Phylogenetic analysis
All Wolbachia gene sequences generated in this study were manually edited with SeqManII by DNAStar (Version 11.1) and aligned using MUSCLE and ClustalW, as implemented in Geneious 5.3.4, and adjusted by eye. Phylogenetic analyses were performed using Bayesian Inference (BI) and Maximum-Likelihood (ML) estimation for a concatenated data set of the protein-coding genes (gatB, fbpA, hcpA, ftsZ and coxA) and for wsp and 16S rRNA separately. For the Bayesian inference of phylogeny, PAUP version 4.0b10 was used to select the optimal evolution model by critically evaluating the selected parameters using the Akaike Information Criterion. Finally, wsp, Wolbachia specific 16S rRNA and MLST gene forward and reverse sequences were used to reconstruct the contig file for each gene and for each species. Then all contig files were used to do BLAST nucleotide search for the reference sequences and based on the analysis and BLAST search results, the phylogenetic tree was constructed.
Algorithm used for phylogenetic reconstruction was PHASE. The evolutionary history was inferred using the Maximum-Likelihood (ML) method. The optimal tree with the sum of branch length was obtained for all primer pairs individually and for the respective concatenated sequences. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were always maintained more than 75 bootstrap values. The evolutionary distance between Wolbachia strains infecting in each mosquito species were measured based on the base substitutions per site.
Real-time quantitative PCR for Wolbachia density in mosquito
To estimate Wolbachia densities, a real-time quantitative PCR assay based on a single-copy gene wsp encoding a surface protein of Wolbachia was used to determine Wolbachia density in the arboviral hosts including Ae. aegypti and Ae. albopictus. Primers were specifically designed to detect the Wolbachia strain and amplified 332-bp to 513-bp regions of the wsp gene (Supplementary Table S2). The amplification reaction was monitored using a set of fluorescent probes specific to the PCR product. PCR was performed under the following conditions: 10 min at 55 °C, 1 min at 95 °C, followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s, followed by 95 °C for 1 min, 55 °C for 30 s and 95 °C for 30 s. The PCR in a 10 μL reaction system was well-optimized with 5 μL of qPCR dye, the 0.25 μL from 10 μmol/L concentration of each primer (F/R), 1 μL of template DNA, and 3.5 μL of DNase/RNase-free water. Wolbachia density was compared according to Ct values against the threshold value of 0.2.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Roehrdanz, R., Bourchier, R., Cortilet, A., Olson, D. & Sears, S. Phylogeny and genetic diversity of flea beetles (Aphthona sp.) Introduced to North America as biological control agents for leafy spurge. Ann. Entomol. Soc. Am. 104, 966–975. https://doi.org/10.1603/an10145 (2011).
Floate, K., Lysyk, T. & Gibson, G. Haematobia irritans L, horn fly, Musca domestica L, house fly, and Stomoxys calcitrans (L), stable fly (Diptera: Muscidae). in Biological Control Programmes in Canada 2001–. https://doi.org/10.1079/9781780642574.0182 (2013).
Fox, T. et al. Wolbachia-carrying Aedes mosquitoes for preventing dengue infection (Protocol). Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD015636 (2023).
Zhang, D., Lees, R. S., Xi, Z., Gilles, J. R. L. & Bourtzis, K. Combining the sterile insect technique with Wolbachia-based approaches: II- a safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLOS ONE 10, e0135194. https://doi.org/10.1371/journal.pone.0135194 (2015).
Xi, Z., Khoo, C. C. H. & Dobson, S. L. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. R. Soc. B Biol. Sci. 273, 1317–1322. https://doi.org/10.1098/rspb.2005.3405 (2006).
Fofana, A. et al. The strategy of paratransgenesis for the control of malaria transmission. Front. Trop. Dis. https://doi.org/10.3389/fitd.2022.867104 (2022).
Aksoy, S., Weiss, B. & Attardo, G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. In Transgenesis and the Management of Vector-Borne Disease (ed. Aksoy, S.) 35–48 (Springer, New York, 2008).
O’Connor, L. et al. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLOS Neglect. Trop. Dis. 6, e1797. https://doi.org/10.1371/journal.pntd.0001797 (2012).
Moretti, R. & Calvitti, M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta). Med. Veter. Entomol. 27, 377–386. https://doi.org/10.1111/j.1365-2915.2012.01061.x (2013).
Atyame, C. M. et al. Wolbachia-based population control strategy targeting Culex quinquefasciatus mosquitoes proves efficient under semi-field conditions. PLOS ONE 10, e0119288. https://doi.org/10.1371/journal.pone.0119288 (2015).
World Mosquito Program Ltd. Sri Lanka, https://www.worldmosquitoprogram.org/en/global-progress/sri-lanka (2023).
Ranathunge, T. et al. Development of the sterile insect technique to control the dengue vector Aedes aegypti (Linnaeus) in Sri Lanka. PLoS One 17, e0265244. https://doi.org/10.1371/journal.pone.0265244 (2022).
Dutra, H. L. C. et al. From Lab to Field: The influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLOS Neglect. Trop. Dis. 9, e0003689. https://doi.org/10.1371/journal.pntd.0003689 (2015).
Hoffmann, A. A. et al. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLOS Neglect. Trop. Dis. 8, e3115. https://doi.org/10.1371/journal.pntd.0003115 (2014).
Nguyen, T. H. et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors 8, 563. https://doi.org/10.1186/s13071-015-1174-x (2015).
Kittayapong, P. et al. Combined sterile insect technique and incompatible insect technique: the first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Neglecte. Trop. Dis. 13, e0007771–e0007771. https://doi.org/10.1371/journal.pntd.0007771 (2019).
Werren, J. H. Biology of Wolbachia. Ann. Rev. Entomol. 42, 587–609. https://doi.org/10.1146/annurev.ento.42.1.587 (1997).
Aldridge, R. L., Gibson, S. & Linthicum, K. J. Aedes aegypti controls Ae. aegypti: SIT and IIT—an overview. J. Am. Mosq. Control Assoc. 40, 32–49. https://doi.org/10.2987/23-7154 (2024).
Russell, J. A. et al. Specialization and geographic isolation among Wolbachia symbionts from ants and lycaenid butterflies. Evolution 63, 624–640. https://doi.org/10.1111/j.1558-5646.2008.00579.x (2009).
Hoffmann, A. A., Ross, P. A. & Rašić, G. Wolbachia strains for disease control: ecological and evolutionary considerations. Evol. Appl. 8, 751–768. https://doi.org/10.1111/eva.12286 (2015).
Correa, C. C. & Ballard, J. W. O. Wolbachia associations with insects: winning or losing against a master manipulator. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2015.00153 (2016).
Mohanty, I., Rath, A. & Hazra, R. K. Wolbachia: biological control strategy against arboviral diseases in Genetically Modified and other Innovative Vector Control Technologies: Eco-bio-social Considerations for Safe Application (ed. Brij Kishore Tyagi) 215–245 (Springer Singapore, 2021). https://doi.org/10.1007/978-981-16-2964-8_11
Bian, G., Zhou, G., Lu, P. & Xi, Z. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLOS Neglect. Trop. Dis. 7, e2250. https://doi.org/10.1371/journal.pntd.0002250 (2013).
Kamtchum-Tatuene, J., Makepeace, B. L., Benjamin, L., Baylis, M. & Solomon, T. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr. Opin. Infect. Dis. 30, 108–116. https://doi.org/10.1097/qco.0000000000000342 (2017).
Kittayapong, P., Baisley, K. J., Baimai, V. & O’Neill, S. L. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37, 340–345. https://doi.org/10.1093/jmedent/37.3.340 (2000).
Wong, M. L. et al. Natural Wolbachia infection in field-collected Anopheles and other mosquito species from Malaysia. Parasites & Vectors 13, 414. https://doi.org/10.1186/s13071-020-04277-x (2020).
Ricci, I., Cancrini, G., Gabrielli, S., D’amelio, S. & Favia, G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: culicidae): large polymerase chain reaction survey and new identifications. J. Med. Entomol. 39, 562–567. https://doi.org/10.1603/0022-2585-39.4.562 (2002).
da Moura, A. J. F. et al. Screening of natural Wolbachia infection in mosquitoes (Diptera: Culicidae) from the Cape Verde islands. Parasites & Vectors 16, 142. https://doi.org/10.1186/s13071-023-05745-w (2023).
Rasgon, J. L. & Scott, T. W. An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J. Med. Entomol. 41, 255–257. https://doi.org/10.1603/0022-2585-41.2.255 (2004).
Ross, P. A. et al. An elusive endosymbiont: Does Wolbachia occur naturally in Aedes aegypti?. Ecol. Evol. 10, 1581–1591. https://doi.org/10.1002/ece3.6012 (2020).
Gloria-Soria, A., Chiodo, T. G. & Powell, J. R. Lack of evidence for natural Wolbachia infections in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 55, 1354–1356. https://doi.org/10.1093/jme/tjy084 (2018).
Balaji, S., Jayachandran, S. & Prabagaran, S. R. Evidence for the natural occurrence of Wolbachia in Aedes aegypti mosquitoes. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fnz055 (2019).
Teo, C. H. J., Lim, P. K. C., Voon, K. & Mak, J. W. Detection of dengue viruses and Wolbachia in Aedes aegypti and Aedes albopictus larvae from four urban localities in Kuala Lumpur Malaysia. Trop. Biomed. 34, 583–597 (2017).
Thongsripong, P. et al. Mosquito vector-associated microbiota: metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol. 8, 1352–1368. https://doi.org/10.1002/ece3.3676 (2018).
Tongkrajang, N., Ruenchit, P., Tananchai, C., Chareonviriyaphap, T. & Kulkeaw, K. Molecular identification of native Wolbachia pipientis in Anopheles minimus in a low-malaria transmission area of Umphang Valley along the Thailand-Myanmar border. Parasites Vectors 13, 579. https://doi.org/10.1186/s13071-020-04459-7 (2020).
Sawasdichai, S. et al. Detection of diverse Wolbachia 16S rRNA sequences at low titers from malaria vectors in Kayin state, Myanmar [version 4; peer review: 2 approved, 1 approved with reservations]. Wellcome Open Research 4 (2019). https://doi.org/10.12688/wellcomeopenres.15005.4
Carvajal, T. M., Hashimoto, K., Harnandika, R. K., Amalin, D. M. & Watanabe, K. Detection of Wolbachia in field-collected Aedes aegypti mosquitoes in metropolitan Manila. Philippines. Parasites & Vectors 12, 361. https://doi.org/10.1186/s13071-019-3629-y (2019).
Reyes, J. I. L., Suzuki, T., Suzuki, Y. & Watanabe, K. Detection and quantification of natural Wolbachia in Aedes aegypti in metropolitan Manila, Philippines using locally designed primers. Front. Cell. Infect. Microbiol. https://doi.org/10.3389/fcimb.2024.1360438 (2024).
Bennett, K. L. et al. Dynamics and diversity of bacteria associated with the disease vectors Aedes aegypti and Aedes albopictus. Sci. Rep. 9, 12160. https://doi.org/10.1038/s41598-019-48414-8 (2019).
Kulkarni, A. et al. Wolbachia pipientis occurs in Aedes aegypti populations in New Mexico and Florida, USA. Ecol. Evol. 9, 6148–6156. https://doi.org/10.1002/ece3.5198 (2019).
Ayala, D. et al. Natural Wolbachia infections are common in the major malaria vectors in Central Africa. Evol. Appl. 12(1583), 1594. https://doi.org/10.1111/eva.12804 (2019).
Mouillaud, T. et al. Limited association between Wolbachia and Plasmodium falciparum infections in natural populations of the major malaria mosquito Anopheles moucheti. Evol. Appl. 16, 1999–2006. https://doi.org/10.1111/eva.13619 (2023).
Baldini, F. et al. First report of natural Wolbachia infection in the malaria mosquito Anopheles arabiensis in Tanzania. Parasites & Vectors 11, 635. https://doi.org/10.1186/s13071-018-3249-y (2018).
Gomes, F. M. et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proc. Nat. Acad. Sci. 114, 12566–12571. https://doi.org/10.1073/pnas.1716181114 (2017).
Niang, E. H. A. et al. First report of natural Wolbachia infection in wild Anopheles funestus population in Senegal. Malaria J. 17, 408. https://doi.org/10.1186/s12936-018-2559-z (2018).
Nugapola, N. W. N. P., De Silva, W. A. P. P. & Karunaratne, S. H. P. P. Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka. Parasites & Vectors 10, 230. https://doi.org/10.1186/s13071-017-2174-9 (2017).
Tharsan, A. et al. Wolbachia infection is widespread in brackish and fresh water Aedes albopictus (Diptera: Culicidae) in the coastal Jaffna peninsula of northern Sri Lanka. J. Vector Borne Dis. 60(2), 172–178 (2023).
Baldo, L. et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098–7110. https://doi.org/10.1128/aem.00731-06 (2006).
Zhou, W., Rousset, F. & O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265, 509–515. https://doi.org/10.1098/rspb.1998.0324 (1998).
Ruang-Areerate, T., Kittyapong, P., Baimai, V. & O’Neill, S. L. Molecular phylogeny of Wolbachia endosymbionts in Southeast Asian mosquitoes (Diptera: Culicidae) based on wsp gene sequences. J. Med. Entomol. 40, 1–5. https://doi.org/10.1603/0022-2585-40.1.1 (2003).
Ruang-Areerate, T. & Kittayapong, P. Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc. Natl. Acad. Sci. U S A 103, 12534–12539. https://doi.org/10.1073/pnas.0508879103 (2006).
Werren, J. H., Windsor, D. & Guo, L. R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. London Ser. B Biol. Sci. 262, 197–204. https://doi.org/10.1098/rspb.1995.0196 (1995).
Zhou, J. et al. Interspecific mating bias may drive Aedes albopictus displacement of Aedes aegypti during its range expansion. PNAS Nexus 1, pgac041. https://doi.org/10.1093/pnasnexus/pgac041 (2022).
Bargielowski, I. E. et al. Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in nature. Infect. Genet. Evol. 36, 456–461. https://doi.org/10.1016/j.meegid.2015.08.016 (2015).
Marcela, P., Hassan, A. A., Hamdan, A., Dieng, H. & Kumara, T. K. interspecific cross-mating between Aedes aegypti and Aedes albopictus laboratory strains: implication of population density on mating behaviors. J. Am. Mosq. Control Assoc. 31, 313–320 (2015).
Gunathilaka, N. Illustrated key to the adult female Anopheles (Diptera: Culicidae) mosquitoes of Sri Lanka. Appl. Entomol. Zool. 52, 69–77. https://doi.org/10.1007/s13355-016-0455-y (2017).
Gunathilaka, N., Fernando, T., Hapugoda, M., Abeyewickreme, W. & Wickremasinghe, R. Revised morphological identification key to the larval anopheline (Diptera: Culicidae) of Sri Lanka. Asian Pac. J. Trop. Biomed. 4, 222–227. https://doi.org/10.12980/apjtb.4.2014c941 (2014).
Rattanarithikul, R. & Panthusiri, P. Illustrated keys to the medically important mosquitos of Thailand. Southeast Asian J. Tropical Med. Public Health 25(Suppl 1), 1–66 (1994).
Moreira, L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell 139, 1268–1278. https://doi.org/10.1016/j.cell.2009.11.042 (2009).
Acknowledgements
The authors would like to thank R.S. Dassanayake and P.A.D.H.N. Gunathilaka for supporting sample collection, A. Avgoustinos and K. Bourtzis for supervising data analysis, Zhiyong Xi, P. Wongtrakoongate and T. Ruang-Areerate for giving useful suggestions, and Suwannapa Ninphanomchai for her helpful administration. This study received financial support from Mahidol University, NRC/TO14/04, IAEA/RAS5047, IAEA/RAS5066, WHO/TDR/HQTDR1409931, OWSD Early Career Fellowship Award (2022), and WHO/TDR/Crowdfunding Grant 2019.
Author information
Authors and Affiliations
Contributions
P.K. and N.D.A.D.W. conceived and designed the study. P.K., P.T., Y.I.N.S.G., W.A. and T.G.A.N.C. supervised the experiments. N.D.A.D.W. administered and conducted the experiments. N.D.A.D.W. analyzed data. N.D.A.D.W., P.K. and P.T. wrote the original manuscript. N.D.A.D.W., Y.I.N.S.G., W.A., T.G.A.N.C., P.T. and P.K. read, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wijegunawardana, N.D.A.D., Gunawardene, Y.I.N.S., Abeyewickreme, W. et al. Diversity of Wolbachia infections in Sri Lankan mosquitoes with a new record of Wolbachia Supergroup B infecting Aedes aegypti vector populations. Sci Rep 14, 11966 (2024). https://doi.org/10.1038/s41598-024-62476-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62476-3
- Springer Nature Limited