Abstract
Silver nanoparticles (AgNPs) have gained much attention due to their unique physical, and chemical properties. Integration of phytochemicals in nanoformulation might have higher applicability in healthcare. Current work demonstrates the synthesis of green AgNPs with O. gratissimum (gr-AgNPs) O. tenuiflorum (te-AgNPs) and O. americanum (am-AgNPs) followed by an evaluation of their antimicrobial and anticancer properties. SEM analysis revealed spherical-shaped particles with average particle sizes of 69.0 ± 5 nm for te-AgNPs, 46.9 ± 9 nm for gr-AgNPs, and 58.5 ± 18.7 nm for am-AgNPs with a polydispersity index below 0.4. The synthesized am-AgNPs effectively inhibited Klebsiella pneumonia, Escherichia coli, Staphylococcus aureus, Aspergillus niger, and Candida albicans with 23 ± 1.58 mm, 20 ± 1.68 mm, 22 ± 1.80 mm, 26 ± 1.85 mm, and 22 ± 1.40 nm of zone of inhibition respectively. Synthesized AgNPs also induced apoptotic cell death in MCF-7 in concentration-dependent manner. IC50 values for am-AgNPs, te-AgNPs, and gr-AgNPs were 14.78 ± 0.89 µg, 18.04 ± 0.63 and 15.41 ± 0.37 µg respectively which suggested that am-AgNPs were the most effective against cancer. At higher dose size (20 µg) AgNPs were equally effective to commercial standard Doxorubicin (DOX). In comparison to te-AgNPs and gr-AgNPs, am-AgNPs have higher in vitro anticancer and antimicrobial effects. The work reported Ocimum americanum for its anticancer properties with chemical profile (GCMS) and compared it with earlier reported species. The activity against microbial pathogens and selected cancer cells clearly depicted that these species have distinct variations in activity. The results have also emphasized on higher potential of biogenic silver nanoparticles in healthcare but before formulation of commercial products, detailed analysis is required with human and animal models.
Similar content being viewed by others
Introduction
Antibiotics and antimicrobial drugs have become inseparable parts of healthcare used for managing infectious disorders and preventing infection during difficult surgeries. However, overexploitation of antibiotics resulted in the evolution of drug resistance strains of pathogens1 that directly affect hospitalization phase and mortality rates2,3. Similar observations have been recorded in the cases of cancer chemotherapy as drug resistance cases have increased due to its frequent application. It also results in the unchecked development of transformed cells and dynamic genomic alterations that give rise to malignant traits in normal cells4. Besides drug resistance, cancer therapy also faces issues with delayed diagnosis, non-specific systemic distribution, insufficient or high drug dosages, poor drug delivery, and difficulty in tracking therapeutic outcomes5.
The present cancer therapy challenges can be addressed through nanotechnology and nanoformulations6,7. Nanomaterials have shown peculiar natures and characteristics such as optical, magnetic, chemical, and mechanical properties, that allow multifaceted applications in food, healthcare, and agriculture8,9. Metal-based particles like gold (AuNPs) and silver (AgNPs) have drawn considerable interest in infectious diseases and post-surgical infections due to inherent bioactivities and lower possibility of toxicity to normal cells10,11. Due to their unique stability, varied geometries, and simplicity of AgNPs to obstruct bacterial cell growth, AgNPs have extensive usage in the construction of multi-resistant drugs12, speed up the healing process13, ointment balms14, anticancer agents15, and dental fillers16.
Conventional NPs synthesis methods involve the utilization of toxic and dangerous materials and pose a high risk of poisoning17. Green NPs synthesis by using plant extract and natural reducing materials has offered a cost-effective, eco-friendly approach with a high level of reproducibility18,19. Tulsi (holy basil), one of the most imperative herbal plants from the Lamiaceae family is widely used as food and a dominant ingredient of healthcare formulations20. It is native to the Indian subcontinent and has extensively been utilized in Ayurveda and is frequently referred to as an "Elixir of Life"21. The medicinal properties of tulsi led to its use in treating epilepsy22,23, asthma24, cough25, anemia26, parasite infections27,28, neuralgia29, and inflammation30. Tulsi leaf extracts are recommended in the Indian Materia Medica for the treatment of pyrexia, rheumatism, and bronchitis31 while the tea infusion has been used to treat hepatic and gastrointestinal diseases, the leaf juice has been utilized topically to cure earaches, malaria, snakebites, and mosquito bites32. In the current work, AgNPs were synthesized with aqueous leaf extracts of O. gratissimum, O. tenuiflorum, and O. americanum and compared for antimicrobial and in vitro anticancer activities.
Results
The biogenic nanoparticles i.e. te-AgNPs, am-AgNPs, and gr-AgNPs were prepared from AgNO3 as a precursor using extract of different species of Ocimum. The basic concept behind AgNPs synthesis is the use of plant extract as a reducing and stabilizing agent33,34. During the nanoparticle synthesis process, initially, the emergence of a dark brown hue in aqueous solutions of (te), (gr), and (am) signifies the formation of te-AgNPs, am-AgNPs, and gr-AgNPs, respectively35. During the interaction, some of the biomolecules from plant extract involved in the reduction of Ag+ ions into Ag0 reduced or metallic form of silver followed by their stabilization by biomolecules present in the same extract to yield silver nanoparticles. Bioactive compounds present in the extracts of Ocimum species such as alkaloids, saponins, flavonoids, phenols, tannins, and terpenoids are actively involved in the reduction and stabilization of nanoparticle synthesis35. Previous literature also supported the fact that phytochemicals can act as reducing agents as well as capping agents. Phenolics and flavonoids are known to act as reducing agents while xanthones and phloroglucinols act as capping agents in nanoparticle synthesis36. The confirmation of silver nanoparticle synthesis was done through UV–visible spectroscopy. The surface plasmon resonance (SPR) peaks of te-AgNPs, gr-AgNPs, and gr-AgNPs were observed at 426.10 nm (Fig. 1a), 434.70 nm (Fig. 1b), and 444.60 nm (Fig. 1c), respectively. The SPR peaks for each of the silver nanoparticles were in accordance with the previous literature. The correlation of SPR band of synthesized silver nanoparticles has been established in section "UV–visible spectroscopic analysis".
Characterization of synthesized AgNPs
UV–visible spectroscopic analysis
UV–visible spectrophotometer serves as an important tool to determine if the plant extract can reduce silver salt to NPs. Following the blending of the extract and silver nitrate solution, UV absorption spectra were measured.
The UV–visible spectrum analysis of synthesized AgNPs demonstrated the maximum absorbance peak at 426.10 nm for te-AgNPs from O. tenuiflorum (Fig. 1a), 434.70 nm for gr-AgNPs from O. gratissimum (Fig. 1b), and 444.60 nm for am-AgNPs from O. americanum (Fig. 1c), respectively, indicating the occurrence of surface plasmon resonance (SPR). The similar peak shifting and occurrence of SPR was also reported in earlier literature with silver nanoparticles37,38. Li et al. (2023) showed that AgNPs exhibited local SPR due to scattering of radiated light on particle surface38. In another work, Benjamin et al. (2006) proved that SPR in AgNPs and peak position depend upon the shape of the particle. Sharp peaks around 400–450 nm can be observed in the case of spherical nanoparticles while cubical, octahedron, and tetrahedron-shaped particles have multiple peaks of different intensities due to multiple dipole symmetries39.
SEM analysis
SEM analysis proved that the synthesized AgNPs are rocky surfaced spherical morphology was found in te-AgNPs from O. tenuiflorum (Fig. 2a) and flake surfaced spherical shape was found in gr-AgNPs from O. gratissimum (Fig. 2b) whereas spherical shape was observed in am-AgNPs from O. americanum (Fig. 2C). The morphology and molecule size of formulated AgNPs were studied by utilizing SEM investigation. The SEM micrographs of AgNPs recommended the amalgamation of mono-scattered AgNPs of high thickness. The micrographs additionally uncovered consistency in the size and shape of the orchestrated AgNPs.
DLS analysis
The DLS study revealed the size distribution of fabricated te-AgNPs, gr-AgNPs, and am-AgNPs. The average diameter of te-AgNPs was 69.0 ± 5.4 nm (Fig. 3a), 46.9 ± 9.0 nm for gr-AgNPs (Fig. 3b), and 58.5 ± 18.7 nm from am-AgNPs (Fig. 3c). The findings of the DLS study of te-AgNPs showed the zeta average diameter of 69.0 nm with PI value of 0.356. Whereas gr-AgNPs have a zeta average diameter of 46.9 nm with a PI value of 0.207 and am-AgNPs have a zeta average diameter of 58.5 nm with a PI value of 0.3870 were observed.
FT-IR analysis of AgNPs from Ocimum species
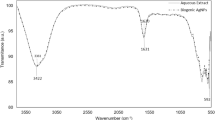
FT-IR assessments were performed to detect the functional groups that present on the formulated te-AgNPs, gr-AgNPs, and am-AgNPs. The peaks obtained for fabricated te-AgNPs, gr-AgNPs, and am-AgNPs from different Ocimum species were revealed in Fig. 4. Almost the same with slight variations at absorbed wavelengths and percentage transmittance to elucidate the functional groups and the metabolites that have reduced silver ions. It was apparent that strong peaks obtained at 3311 cm-1 and 3342 cm-1 resemble O–H stretching. The peak at 2947 cm-1 and 2077 cm-1 resembles carboxylic acid O–H Stretch. The peaks at 1641 cm-1 and 1637 cm-1 resemble N–H bend primary amines. The bands observed in 1163 cm-1 and 1170 cm-1 resemble C–N stretching alcohols. The FT-IR study revealed that the carbonyl group from proteins and amino acid residues has a strong ability to bend metal, suggesting that the proteins may be made of synthetic metal nanoparticles. As shown in Fig. FTIR spectrum A band at 1200–1800 cm−1 corresponded to the bending vibrations of the amide I and amide II bands of protein40.
The phytochemicals attached to the particle's surface may have served as capping agents to prevent agglomeration and stabilize the medium41,42. This implies that the creation and stabilization of te-AgNPs, gr-AgNPs, and am-AgNPs in the aqueous media may both be accomplished by biological agents. The solid assimilation groups at 3468.5 cm−1 and 3417.56 cm−1 compare to O–H extending vibration likewise, we additionally noticed solid pinnacles showing up at 1634.25 cm−1 and 1616.84 cm−1 in the IR spectra which are allocated as carbonyl stretch of amide-I bond and of –N–H stretch vibrations of amide-II bond demonstrating the presence of proteins. The trademark groups showed up at 2924.72 cm−1 and 2853.53 cm−1 compared to C–H for alkenes. Bands at 1384.53 cm−1 and 1354 cm−1 credited to C–H lopsided extending of alkanes. Bands at 1124.83 cm−1 and 1107.8 cm−1 are because of the C–OH extending of carboxylic corrosive. Additionally, tops saw at 780.76 cm−1 and 804.63 cm−1 were allotted to C–H extending of fragrant gathering, and the top at 521.22 cm−1 addresses the O–H bowing of phenol bunch.
The FTIR spectrum revealed the presence of carboxylic acid, and hydroxyl group dominantly along with C–N and N–H which are associated with primary as well as secondary metabolites. The extract usually contains enzymes and proteins like Superoxide dismutase (SOD), Catalase, Glutathione peroxidase (GPx), Glutathione reductase (GR) and Glutathione S Transferase43. In Ocimum, octa-aminopropyl may be present, potentially as a synthetic compound or a modification introduced to a protein. Its role could involve enhancing solubility, and stability, or facilitating interactions with other molecules44. Besides, –COOH and –OH functional groups are associated with flavanols, phenolics, and tannins. Previous literature has identified rosmarinic acid, chlorogenic acid, caffeic acid, aesculin, quercetin, luteolin, and apigenin from Ocimum sanctum that might contribute to carboxylic and OH groups45.
Antimicrobial activity of synthesized AgNPs from Ocimum species
The antimicrobial assessments of the fabricated te-AgNPs, gr-AgNPs, and am-AgNPs showed the maximum inhibition zone (23 ± 1.58 mm) against K. pneumoniae whereas normal aqueous leaf extracts show (20 ± 1.15 mm) inhibition zone from am-AgNPs (Fig. 5). The S. aureus and E. coli showed 22 ± 1.80 mm and 20 ± 1.68 mm, respectively followed by (19 ± 1.50 mm) B. subtilis (Table 1). Antifungal properties of am-AgNPs synthesized from the O. americanum exhibited a maximum inhibition zone (26 ± 1.85 mm) against A. niger and 22 ± 1.40 mm against C. albicans. The te-AgNPs and gr-AgNPs of O. tenuiflorum and O. gratissimum respectively show the inhibition zones of 16 ± 1.2 mm against S. aureus. The gr-AgNPs showed 16 ± 1.12 mm against S. aureus, 14 ± 0.67 mm against K. pneumoniae, 15 ± 1.03 mm against E. coli, and 11 ± 0.54 mm against B. subtilis. Meanwhile, the antifungal results revealed 15 ± 0.80 mm against A. niger and 13 ± 1.05 mm against C. albicans. Data are demonstrated as mean ± SD of triplicates. The suitability of antimicrobial analysis, conducted with one-way ANOVA revealed the F-value and Fcrit of 60.04 and 2.25 respectively which suggested that the null hypothesis can be rejected and obtained results were statistically significant.
Cytotoxic effect of AgNPs from the O. tenuiflorum, O. gratissimum, and O. americanum leaf extracts on MCF-7 cells by (a). MTT assay; (b). XTT assay; and (c). SRB assay, (IC50 te-AgNPS: 18.04 ± 0.63 µg; gr-AgNPs: 15.41 ± 0.37 µg and am-AgNPs: 14.78 ± 0.89 µg). MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; XTT: 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl) -2H-tetrazolium-5-carboxanilide; SRB: Sulforhodamine B.
In vitro anticancer activity
The influence of fabricated te-AgNPs, gr-AgNPs, and am-AgNPs from the Ocimum species has been investigated against the MCF-7 cells by four different methods including MTT assay, Sulforhodamine-B assay, XTT assay, and Clonogenic assay. Figure 5 shows the in-vitro cytotoxicity of synthesized te-AgNPs, gr-AgNPs, and am-AgNPs against MCF-7 cells via different methods. The results from the MTT assay indicate that aqueous extract-based nanoparticles (te-AgNPs, gr-AgNPs, and am-AgNPs) substantially diminished the viability of MCF-7 cells in dose dose-dependent manner. In comparison to te-AgNPs, gr-AgNPs, and am-AgNPs showed more pronounced cytotoxic activity and substantially inhibited the viability of MCF-7 cells (Fig. 5a). Similar patterns were also observed in other cytotoxic assays as well. The XTT assay (Fig. 5b), SRB assay (Fig. 5c), and Clonogenic assay (Fig. 6a–b) also showed dose-dependent cytotoxicity in MCF-7 cells. The IC50 value for te-AgNPs was 18.04 ± 0.63 µg, for gr-AgNPs, 15.41 ± 0.37 µg and for am-AgNPs, it was 14.78 ± 0.89 µg. Therefore, it was clear that am-AgNPs demonstrated more cytotoxicity in comparison to te-AgNPs as well as gr-AgNPs on MCF-7 cells. The apoptosis in cell demise is essentially portrayed by morphological changes like shrinkage of cells, chromatin build-up, core discontinuity, blebbing, and development of apoptotic bodies. Staining with AO and PI staining was used for the determination of live and dead cells. The control cells showed green fluorescence, whereas the synthesized te-AgNPs, gr-AgNPs, and am-AgNPs treated MCF-7 cells demonstrated an increased yellow and orange fluorescence, which indicates the occurrence of apoptotic cells. The DOX treatment also revealed increased apoptosis in the MCF-7 cells. The findings of AO/PI staining proved that the am-AgNPs treatment induced more apoptotic cell death in MCF-7 cells than the gr-AgNPs and te-AgNPs (Fig. 6c).
Statistical analysis
Antimicrobial and anticancer analysis was assessed with ANOVA analysis using Microsoft Excel with the ‘Data analysis’ tool pack (Table 2). ANOVA analysis of the antimicrobial test revealed a P-value of 0.0016 (for rows) and 2E-20 (for table) which suggested the analysis was significantly suitable. A similar analysis was also conducted against MCF-7 cell lines via MTT, XTT, and SRB assay. ANOVA analysis was conducted with individual tests. The P-values for rows and columns were 6.5E-10 and 0.025 for MTT assay, 1.07E-09 and 0.02 for XTT assay, and 3.07E-10, 0.02 for SRB assay. In addition, the IC50 value was calculated for individual tests followed by the average IC50 calculation. Average IC50 values were 18.04 ± 0.63 (for te-AgNPs), 14.77 ± 0.89 (am-AgNPs), and 15.42 ± 0.37 (gr-AgNPs). To find the best AgNPs against cancer cells were determined by two-way ANOVA analysis between three groups. The P values for the IC50 were 0.037 and 0.000771 which not only identified the significance of the model but also suggested that am-AgNPs have significantly higher anticancer potential among the group.
Chemical profiling of extract by GC–MS
Ocimum americanum has shown the maximum efficiency in cancer inhibition among all three extract-based nanoparticles hence chemical profiling was done via GC–MS for the identification of compounds. The scanning was done for upto 40–33 min and with the molecular weight range of 50–500 da (Fig. 7). Total 45 peaks were identified from the extract out of which only 12 peaks have area of more than 1%. Octadecadienoic acid (7.05) and its derivative (27.99%), oleic acid (23.82%), and hexadecenoic acid (13.53%) have occupied more than 50% area on the peaks (Table 3). These compounds are responsible for the bioactivity of the extract against microorganisms as well as cancer cells. 9,12-Octadecadienoic acid derivatives are one of the most prominent oil/polyunsaturated fatty acids in plants and have bioactive potential46,47. Oleic acid is a monounsaturated fatty acid that has a direct role in cancer management. It has shown anti-proliferative activity that aids in cancer inhibition. Besides, it also suppresses inflammation by modulating the production of inflammatory mediators and VEGF effector pathway48. Abietic, palustric, and eicosanoic acids were also detected in extract and have also been reported for their applications in pharmaceuticals.
Discussion
In contrast to bulk materials, NPs have distinct physical, chemical, and biological characteristics, including crystal structures, and specific targeting and controlled release features. Drug delivery, aesthetics, tissue engineering, theranostics, and agriculture are just a few of the industries that are fast-growing NP applications49. AgNPs are gaining more and more attention for their numerous uses as antimicrobial, antioxidant, and anticancer, as well as for improving vaccine immunogenicity50. Nanoparticles, particularly AgNPs have been widely utilized as antimicrobial sources in the medical field. AgNPs are more effective against microbes than bulk silver due to their colossal specific surface area to surface atom ratio51. As a result of their biological activities, AgNPs are employed in the battle against multidrug-resistant bacteria. Researchers looked at the fabrication of AgNPs for effective cancer therapy, inquiry, and diagnostics52. To increase AgNPs' sensitivity and effectiveness against different tumor cells, they can be coupled to or loaded with different drugs, polysaccharides, and nanostructures53. The development of new antimicrobial drugs has been encouraged by the emergence of multidrug-resistant pathogens. AgNPs have powerful antimicrobial properties against several pathogens.
In addition, AgNPs have substantial anticancer properties against several cancers including lung, hepatocellular, breast, and colorectal in both in vitro and in vivo settings54. Due to their capacity for oxidation and reduction, biomolecules like polyphenol and its derivatives were used to reduce Ag+ ions into AgNPs55. It was highlighted that plant metabolites have exceptional efficiency in the bio-reduction of Ag+ ions and the stability of AgNPs56. AgNPs from plants contain phytochemical substances that may reduce silver ions; however, this process is currently unknown. In this study, we witnessed that the te-AgNPs, gr-AgNPs, and am-AgNPs fabricated from the aqueous leaf extracts of Ocimum leaf extracts were found effective against the clinical pathogens and breast cancer MCF-7 cells.
AgNPs generally have an antimicrobial effect, although the exact mechanism by which they do so is unknown. However, it has been proposed that the mechanism by which they are antibacterial is the binding of negative charge on the bacterial cell membrane to positive charge Ag+ ions, which may cause the bacterial cell to die. According to this, AgNPs can cause damage to membranes, which can either impede bacterial growth or cause bacteria to die57. Similar to this, AgNPs can saturate and cling to fungal hyphae and generate insoluble compounds that bind to lipids and enzymes, rupturing the membrane and resulting in cell lysis58.
AgNPs have been shown to have antibacterial action through a number of methods, including direct bacterial cell membrane destruction. Increased membrane permeability and DNA damage are brought on by the discharge of Ag+ ions, which also generate reactive oxygen species59. AgNPs' cytotoxicity and antibacterial activity, as well as their capacity for penetrating cells, are all greatly predisposed by their size and surface chemistry, as has been demonstrated. As a result, choosing the right stabilizing reducing agents can increase the nanoparticles' antibacterial activity, which can therefore result in a reduction in the therapeutic dose60. Additionally, the functionalization of AgNPs with natural organic compounds known to have antimicrobial activity may enhance such effects, which are referred to as capping agents. By preventing AgNPs aggregation, these capping agents may also enhance the AgNPs' colloidal stability and have a substantial impact on how they interact with other in vivo components61.
Our results indicated that Gram-negative bacteria were more vulnerable than Gram-positive species62. This is because of the thin peptidoglycan layer and additional lipopolysaccharide outer membrane present in the Gram-negative strain, which suggests the existence of a periplasmic membrane layer. The NPs entry and discharged ions into the cell might be facilitated by this arrangement. The thick coating of peptidoglycan seen in the cell membranes of Gram-positive bacteria, however, contains covalently bonded teichoic and teichuronic acids and may serve as a shield against the AgNPs63. In this study, our findings demonstrated that the fabricated te-AgNPs, gr-AgNPs, and am-AgNPs efficiently decreased the growth of different pathogenic bacteria and fungi, which is witnessed by the higher inhibitory zones around the AgNPs-loaded wells. The am-AgNPs treatment more effectively inhibited the growth of the pathogens than the te-AgNPs and gr-AgNPs. The subsequent production of extracellular ROS disrupts the cell's natural antioxidant defense, leading to a higher cell wall injury or cell necrosis via impairing ATP synthesis and DNA replication64. Two potential mechanisms of toxicity include inducing the production of ROS, which results in DNA injury and apoptosis. The Ag+ ions in the cytosol interfere with natural metabolic and cell cycle systems, they are primarily responsible for cell death65.
Additionally, several studies have shown that the NPs' surface morphology significantly affects how active they are; NPs' small size and high surface area facilitate cell penetration. It appears that NPs with a small diameter and a high dosage of Ag+ ions can enter the intracellular space of bacteria66. It was highlighted that cells administered with AgNPs can produce higher ROS than cells administered with Ag+ ions alone, leading to the development of oxidative stress. Ag+, however, inhibits the site and raises ROS, which can damage DNA without obviously disrupting the membrane, signifying a complex toxicity strategy for inhibiting the growth of tumor cells67. Ocimum species exhibited cytotoxic activity on the MCF-7 cell line68. Cell death expanded with expanding groupings of AgNPs and hindrance of half-cell passing requires a little portion of AgNPs. It has been accounted for that the AgNPs treatment causes harm to the mitochondrial film and delivers cytochrome c, which is a basic angle showing apoptosis in cells animated by cytoplasm69. Here, our outcomes demonstrated that the formulated te-AgNPs, gr-AgNPs, and am-AgNPs effectively inhibited growth and induced apoptosis in the breast cancer MCF-7 cells. Apoptosis and growth retardation incidence were observed in am-AgNPs treated MCF-7 cells in all cytotoxicity tests revealing the potent invitro anticancer activity of am-AgNPs higher than the te-AgNPs and gr-AgNPs. Hence, it was clear that the am-AgNPs from the O. americanum species are effective in inhibiting the pathogenic bacterial and fungal growth and induction of apoptotic cell death in the MCF-7 cells.
Materials and methods
Collection of plant materials
The fresh plant materials of O. tenuiflorum, O. gratissimum, and O. americanum were collected from the Kulumani (Latitude: 10.80915; Longitude: 78.59011), Kallikudi (Latitude: 10.9772; Longitude: 78.9515), and Palkalaiperur villages of Tiruchirappalli district in the month of April to May. During the collection period, the temperature, and humidity of 36 ± 5 °C and 60 ± 5% respectively. The collected plant materials were taxonomically identified by Dr. S. Soosairaj, Department of Botany, St. Joseph’s College (Autonomous) Tiruchirappalli. National guidelines and legislation were strictly followed for the sample collection of plant sample. The voucher specimens of the collected plant materials were deposited at the Rapinet Herbarium Centre (Herbarium Accession numbers—2794, 2793, and 2792, respectively).
Preparation of Ocimum leaf extract
To make leaf extract, the leaves were gathered in a beaker and twice rinsed with deionized water to discard the dust that remained. 100 ml of double-distilled water and 10 g of properly cleaned dried leaves were crushed together in a mortar and pestle70. The aqueous extract was pulverized before being added to a 250 ml beaker and heated there for 10 min at 80 °C. After cooling to 37 °C, the extract was filtered and utilized to synthesize AgNPs.
Green synthesis of AgNPs
The AgNPs synthesis is a one-step procedure. The mixture was heated at 80 °C for 15 min after being blended with 90 ml of AgNO3 and 10 ml of leaf extracts. The solution color reformed from pale yellow to dark brown due to the development of AgNPs. The reaction mixture was centrifuged at 15,000 rpm for 20 min at 4 °C to separate the generated AgNPs (te-AgNPs for O. tenuiflorum, gr-AgNPs for O. gratissimum, and am-AgNPs for O. americanum). The AgNPs pellet was collected and washed with deionized water to remove phytochemical and silver ions residues and then AgNPs were lyophilized and stored in a cool, and dry environment till further use71.
Characterization of synthesized AgNPs
To recognize the development of AgNPs in the suspension, the UV–visible spectroscopic analysis was performed using Perkin Elmer equipment. A wavelength between 200 and 1000 nm was utilized to investigate the sample. The size and shape of AgNPs prepared from AgNO3 using Ocimum extract were confirmed by scanning through a Scanning electron Microscope (SEM; Carl Zeiss Ultra 55 FESEM). Besides, the particle size and distribution were confirmed by the DLS-Zeta sizer (Micromeritics).
The AgNPs were prepared with plant extract hence nanoparticles ay accompanied by biomolecules. The presence of biomolecules was assessed by FT-IR (Perkin Elmer) analysis by which available functional groups can be identified. For analysis, the sample powder was mixed with the KBr disc technique, and the spectrum was recorded at 4000–500 cm−1.
Antimicrobial assay
The clinical pathogens were acquired and collected from the Department of Microbiology, Bharathidasan University, Tiruchirappalli. The synthesized AgNPs were dissolved in sterile DMSO and used as a stock (1 mg/mL) for antimicrobial testing. The antimicrobial potential of AgNPs was evaluated by the well diffusion technique71. Different concentrations of AgNPs (40, 50, and 60 µg/disk) were prepared with dried AgNPs in Milli Q water (1 mg/mL) and applied to the microbiological strains of S. pneumoniae, E. coli, K. pneumoniae, S. aureus, A. niger, and C. albicans. Amoxicillin and ketoconazole (1 mg/mL) were used as a positive control against bacteria and fungi respectively. The inhibition zones were recorded following a 24 h treatment period.
In vitro anticancer assays
MTT(3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay
The DMEM enhanced with 10% FBS was utilized to maintain the MCF-7 cells, which were then kept at 37 °C in an incubator with 5% CO2. The growing cells were trypsinized and used for further experiments after it had reached 80% confluency. Cytotoxicity analysis of AgNPs was conducted using MTT assay72 by confirming the viability of MCF-7 cells followed by AgNPs administration. In order to test their ability to adhere, the cells were loaded at a population of 5 × 103 cells per well in a 96-well plate that had been filled with DMEM. The growth medium of the cells was then refilled and administered with different dosages of AgNPs (2.5, 5, 7.5, 10, 20, and 30 μg) for 24 h at 37 °C. Following the treatment phase, 20 μl of MTT and 100 μl of DMEM were mixed in each well and kept there for 4 h at 37 °C. To liquefy the generated formazan stone, 100 μl of DMSO was later mixed into the wells. By determining the absorbance at 570 nm, the cell viability was assessed. The apoptosis in MCF-7 cells after treatment with AgNPs was determined by acridine orange/ethidium bromide (AO/EB) staining73. In brief, 5 × 103 MCF-7 cells were loaded into each well of a 24-well plate, and the cells were then maintained for 24 h at 37 °C. Following that, cells were supplemented with AgNPs at doses of 10 and 20 μg and 2 μg of doxorubicin (DOX) as a standard drug, and they were then maintained for 24 h at 37 °C. The cells were then stained for 5 min using 100 μg/ml of AO/EB stain in a 1:1 ratio after the treatment period. Using a fluorescent microscope, the apoptosis in treated cells was evaluated.
(2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)–2H-tetrazolium-5-carboxanilide (XTT assay)
XTT assay relies on the activity of mitochondrial dehydrogenase enzyme from the viable cells. The active enzyme can act on the tetrazolium ring and soluble formazan will be formed as product74. In comparison to other tests, the XTT assay is highly sensitive and accurate however it uses a highly toxic XTT reagent75. In order to test the viability, 5 × 103 cells per well were loaded in a 96-well plate with DMEM. Different dosages of AgNPs (2.5, 5, 7.5, 10, 20, and 30 μg) were added to separate wells and incubated at 24 h at 37 °C. Post-incubation, 100 µL of prepared XTT reagent was added and incubated at 37 °C for 4 h. Absorbance was recorded at 450 nm and activity was assessed.
SRB (Sulforhodamine B) assay
Sulforhodamine B assay is one of the most reliable methods to assess drug-induced cytotoxicity with high accuracy and affordability. SRB is an amino xanthene dye with two sulfonic groups that can bind with amino residues of cellular proteins76,77. The estimation can be done by following the method described by Vichai and Kritikara76. MCF-7 cells (5 × 103 cells) were added to each well of 96-well plate and fixed with 25 μL of 10% (wt/vol) TCA solution and incubated for up to an hour at 4 °C. After fixing, cells were washed slowly and dried with paper towels. 100 µL of SRB dye solution (0.057% wt/vol) was added and mixed to each well and incubated for 30 min. Unbounded dye was removed by washing with 1% (V/V) acetic acid. The protein-bound dye was dissolved in 200 µL of a 10 mM Tris base solution (pH 10.5) and quantified at 510 nm.
Clonogenic assay
The Clonogenic assay relies on the fact that each cell can grow into a larger colony by division. The assay was earlier designed to test the efficiency of radiation on cancer cells but later it was adopted for evaluating the cytotoxic effect of drugs on cancer cell78,79,80. The assay was performed by following the protocol of Munshi et al.78. MCF-7 cells (5 × 103 cells per well) were added to each well as well plate with complete media and incubated at 37 °C for 24 h. The cells were treated with different dosages of AgNPs (2.5, 5, 7.5, 10, 20, and 30 μg) for 24 h at 37 °C. The medium was replaced and incubated for a week to attain the ideal colony size and density. Cells were gently washed with 1X PBS and fixed with 500 µL of 100% methanol at − 20 °C for 15–20 min. Remove methanol by aspiration and wash the cells with 1X PBS 3–4 times. The cells were stained with 500 μL of Coomassie blue and incubated for 15–30 min. Unbounded stain was removed with 1X PBS and colonies were counted.
Statistical analysis
The fitness of the analysis related to antimicrobial and anticancer activity was determined by two Analysis of Variance (ANOVA) analyses. The analysis was conducted with Microsoft Excel using a data analysis tool pack. The model fitness was determined with a p-value which must be < 0.05 to consider a model significant.
Chemical profiling of extract with GC–MS
The phytochemical constituents in Ocimum leaf extract were analyzed and identified using GC–MS (Perkin Elmer Clarus 500, Shelton, CT, USA) following the method described81. For analysis, Helium gas was used as mobile/carrier and operated at a flow rate of 1.20 mL/min. The temperature of the injection port and oven were kept at 250 °C and 280 °C. The mass spectrum analysis was conducted with ion source and interface temperature of 200 °C and 250 °C respectively. The spectrum was recorded between 4–40.33 min with full scan mode considering the range of 50–500 Daltons. The compound peaks were identified with the standard libraries from NIST (The National Institute of Standards and Technology) and Wiley compound libraries.
Conclusion
The synthesized te-AgNPs, gr-AgNPs, and am-AgNPs from the O. tenuiflorum, O. gratissimum, and O. americanum, respectively by the green route method effectively decreased the growth of the pathogenic strains. Furthermore, the synthesized te-AgNPs, gr-AgNPs, and am-AgNPs substantially inhibited cell growth and triggered apoptotic cell death in the MCF-7 cells. The am-AgNPs treatment revealed more antimicrobial and potent in vitro anticancer activity on MCF-7 cells than the te-AgNPs and gr-AgNPs treatments. As far as is known, the plant material employed in the green synthesis of nanoparticles was first used. It is a common weed. It is noteworthy that Ocimum americanum can produce finer AgNPs than the other species in this genus. The approach was original and economical. To support its additional therapeutic use, we strongly recommend additional studies in the future.
Data availability
The data used to support the findings of this study are included within the article. Further information can be made available upon request from corresponding authors.
References
Abalkhil, T. A., Alharbi, S. A., Salmen, S. H. & Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 31, 411–417 (2017).
Escárcega-González, C. E. et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int J Nanomedicine 13, 2349–2363 (2018).
Hoffman, S. B. Mechanisms of antibiotic resistance. Comp. Continu. Edu. Practicing Veterinarian 23, 464–472 (2001).
Mansoori, B., Mohammadi, A., Davudian, S., Shirjang, S. & Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 7, 339 (2017).
Nile, A., Nile, S. H., Shin, J., Park, G. & Oh, J.-W. Quercetin-3-glucoside extracted from apple pomace induces cell cycle arrest and apoptosis by increasing intracellular ROS levels. Int. J. Mol. Sci. 22, 10749 (2021).
Roy, S. et al. Recent nanobiotechnological advancements in lignocellulosic biomass valorization: A review. J. Environ. Manag. 297, 113422 (2021).
Siddiqi, K. S., Husen, A. & Rao, R. A. K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechn. 16, 14 (2018).
Gogoi, B., Kumar, R., Upadhyay, J. & Borah, D. Facile biogenic synthesis of silver nanoparticles (AgNPs) by Citrus grandis (L.) Osbeck fruit extract with excellent antimicrobial potential against plant pathogens. SN Appl. Sci. 2, 1723 (2020).
Ajitha, B., Ashok Kumar Reddy, Y. & Sreedhara Reddy, P. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C 49, 373–381 (2015).
Ahmad, N. et al. Green Fabrication of Silver Nanoparticles using Euphorbia serpens Kunth Aqueous Extract, Their Characterization, and Investigation of Its In Vitro Antioxidative, Antimicrobial, Insecticidal, and Cytotoxic Activities. BioMed. Res. Int. 2022, e5562849 (2022).
Haleem, A., Javaid, M., Singh, R. P., Rab, S. & Suman, R. Applications of nanotechnology in medical field: A brief review. Global Health J. 7, 70–77 (2023).
Ojemaye, M. O., Okoh, S. O. & Okoh, A. I. Silver nanoparticles (AgNPs) facilitated by plant parts of Crataegus ambigua Becker AK extracts and their antibacterial, antioxidant and antimalarial activities. Green Chem. Lett. Rev. 14, 51–61 (2021).
Iftikhar, M. et al. Green synthesis of silver nanoparticles using Grewia optiva leaf aqueous extract and isolated compounds as reducing agent and their biological activities. J. Nanomater. 2020, e8949674 (2020).
Mehwish, H. M. et al. Therapeutic potential of Moringa oleifera seed polysaccharide embedded silver nanoparticles in wound healing. Int. J. Biol. Macromol. 184, 144–158 (2021).
Kaur, J. & Tikoo, K. Evaluating cell specific cytotoxicity of differentially charged silver nanoparticles. Food Chem. Toxicol. 51, 1–14 (2013).
Kim, J. S. et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 3, 95–101 (2007).
Khan, I., Saeed, K. & Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12, 908–931 (2019).
Hano, C. & Abbasi, B. H. Plant-based green synthesis of nanoparticles: Production characterization and applications. Biomolecules 12, 31 (2022).
Ansar, S. et al. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep. 10, 18564 (2020).
Calderón Bravo, H., Vera Céspedes, N., Zura-Bravo, L. & Muñoz, L. A. Basil seeds as a novel food, source of nutrients and functional ingredients with beneficial properties: A review. Foods 10, 1467 (2021).
Bhamra, S., Heinrich, M., Howard, C., Johnson, M. & Slater, A. DNA authentication of tulsi (Ocimum tenuiflorum) using the nuclear ribosomal internal transcribed spacer (ITS) and the chloroplast intergenic spacer trnH-psbA. Planta Med. https://doi.org/10.1055/s-0035-1565644 (2015).
Agarwal, C., Sharma, N. L. & Gaurav, S. S. Anti epileptic activity of ocimum species: A brief review. Int. J. Appl. Sci. Biotechn. 1, 180–183 (2013).
Sarangi, S. C., Pattnaik, S. S., Katyal, J., Kaleekal, T. & Dinda, A. K. An interaction study of Ocimum sanctum L and levetiracetam in pentylenetetrazole kindling model of epilepsy. J Ethnopharmacol 249, 112389 (2020).
Bezerra, J. J. L., Pinheiro, A. A. V. & de Barreto, E. O. Medicinal plants used in the treatment of asthma in different regions of Brazil: A comprehensive review of ethnomedicinal evidence, preclinical pharmacology and clinical trials. Phytomed. Plus 2, 100376 (2022).
Dharsono, H. D. A., Putri, S. A., Kurnia, D., Dudi, D. & Satari, M. H. Ocimum species: A review on chemical constituents and antibacterial activity. Molecules 27, 6350 (2022).
Akara, E. U. et al. Ocimum gratissimum leaf extract ameliorates phenylhydrazine-induced anaemia and toxicity in Wistar rats. Drug Metab. Pers. Ther. https://doi.org/10.1515/dmdi-2020-0185 (2021).
Kanu, C. S., Aloke, C., Elom, I. N. & Eleazu, O. C. Effects of co-treatment of Plasmodium berghei-infected mice with aqueous extract of Ocimum gratissimum leaves and primaquine on glucose-6-phosphate dehydrogenase activity, hematological, and antioxidant parameters. J. Ayurveda Integr. Med. 13, 100656 (2022).
Luanda, A., Ripanda, A., Sahini, M. G. & Makangara, J. J. Ethnomedicinal uses, phytochemistry and pharmacological study of Ocimum americanum L: A review. Phytomed. Plus 3, 100433 (2023).
Paula-Freire, L. I. G., Molska, G. R., Andersen, M. L. & de Carlini, E. L. A. Ocimum gratissimum essential oil and its isolated compounds (Eugenol and Myrcene) reduce neuropathic pain in mice. Planta Med. 82, 211–216 (2016).
Eftekhar, N., Moghimi, A., Mohammadian Roshan, N., Saadat, S. & Boskabady, M. H. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Compl. Altern. Med. 19, 349 (2019).
Jamshidi, N. & Cohen, M. M. The clinical efficacy and safety of tulsi in humans: A systematic review of the literature. Evid. Based Compl. Alternat. Med. 2017, 9217567 (2017).
Dadysett, H. J. On the various domestic remedies, with their effects, used by the people of india for certain diseases of the ear. The Lancet 154, 781–782 (1899).
Singh, J. et al. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechn. 16, 84 (2018).
Adeyemi, J. O., Oriola, A. O., Onwudiwe, D. C. & Oyedeji, A. O. Plant extracts mediated metal-based nanoparticles: synthesis and biological applications. Biomolecules 12, 627 (2022).
Roy, P., Das, B., Mohanty, A. & Mohapatra, S. Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Appl. Nanosci. 7, 843–850 (2017).
Pradeep, M., Kruszka, D., Kachlicki, P., Mondal, D. & Franklin, G. Uncovering the phytochemical basis and the mechanism of plant extract-mediated eco-friendly synthesis of silver nanoparticles using ultra-performance liquid chromatography coupled with a photodiode array and high-resolution mass spectrometry. ACS Sust. Chem. Eng. 10, 562–571 (2022).
Kaur, H., Kaur, H. & Sharma, A. Study of SPR peak shifting of silver nanoparticles with change in surrounding medium. Mater. Today Proc. 37, 3574–3576 (2021).
Li, X., Yang, W., Deng, J. & Lin, Y. Surface plasmon resonance effects of silver nanoparticles in graphene-based dye-sensitized solar cells. Front. Mater. 10, 1137771 (2023).
Wiley, B. J. et al. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 110, 15666–15675 (2006).
Gole, A. et al. Pepsin−gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir 17, 1674–1679 (2001).
Javed, R., Sajjad, A., Naz, S., Sajjad, H. & Ao, Q. Significance of capping agents of colloidal nanoparticles from the perspective of drug and gene delivery, bioimaging, and biosensing: An insight. Int. J. Mol. Sci. 23, 10521 (2022).
Sidhu, A. K., Verma, N. & Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 3, 801620 (2022).
Srivastava, A. et al. Potential of hydroethanolic leaf extract of Ocimum sanctum in ameliorating redox status and lung injury in COPD: an in vivo and in silico study. Sci. Rep. 13, 1131 (2023).
Gohari, G. et al. Characterization of Octa-aminopropyl polyhedral oligomeric silsesquioxanes (OA-POSS) nanoparticles and their effect on sweet basil (Ocimum basilicum L.) response to salinity stress. Plant Physiol. Biochem. 196, 89–102 (2023).
Chaudhary, A., Sharma, S., Mittal, A., Gupta, S. & Dua, A. Phytochemical and antioxidant profiling of Ocimum sanctum. J. Food Sci. Technol. 57, 3852–3863 (2020).
Lan, W., Lin, S., Li, X., Zhang, Q. & Qin, W. Chemical composition of the leaf and stem essential oil of Adenophorae Radix. 1820, 030001 (2017).
Zheng, Y.-F., Liu, X.-M., Zhang, Q., Lai, F. & Ma, L. Constituents of the essential oil and fatty acid from rare and endangered plant magnolia kwangsiensis figlar & noot. J. Essential Oil Bearing Plants 22, 141–150 (2019).
Farag, M. A. & Gad, M. Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 20, 48 (2022).
Batool, A., Menaa, F., Uzair, B., Khan, B. A. & Menaa, B. Progress and prospects in translating nanobiotechnology in medical theranostics. Curr. Nanosci. 16, 685–707 (2020).
Mohanta, Y. K. et al. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front. Microbiol. 11, 1143 (2020).
Salem, S. S. et al. Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials 10, 1–20 (2020).
Păduraru, D. N. et al. Recent developments in metallic nanomaterials for cancer therapy diagnosing and imaging applications. Pharmaceutics 14, 435 (2022).
Capanema, N. S. V. et al. Hybrid hydrogel composed of carboxymethylcellulose-silver nanoparticles–doxorubicin for anticancer and antibacterial therapies against melanoma skin cancer cells. ACS Appl. Nano Mater. 2, 7393–7408 (2019).
Pei, J., Fu, B., Jiang, L. & Sun, T. Biosynthesis, characterization, and anticancer effect of plant-mediated silver nanoparticles using Coptis chinensis. Int J Nanomed. 14, 1969–1978 (2019).
Shaikh, W. A., Chakraborty, S., Owens, G. & Islam, R. U. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): The wonder particle of the past decade. Appl. Nanosci. 11, 2625–2660 (2021).
Albukhari, S. M., Ismail, M., Akhtar, K. & Danish, E. Y. Catalytic reduction of nitrophenols and dyes using silver nanoparticles @ cellulose polymer paper for the resolution of waste water treatment challenges. Coll. Surfaces A Physicochem. Eng. Aspects 577, 548–561 (2019).
Venkatesan, J., Kim, S. K. & Shim, M. S. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae ecklonia cava. Nanomaterials 6, 235 (2016).
Hamedi, S., Ghaseminezhad, M., Shokrollahzadeh, S. & Shojaosadati, S. A. Controlled biosynthesis of silver nanoparticles using nitrate reductase enzyme induction of filamentous fungus and their antibacterial evaluation. Artif. Cells Nanomed. Biotechnol. 45, 1588–1596 (2017).
Azócar, M. I. et al. A systematic study of antibacterial silver nanoparticles: Efficiency, enhanced permeability, and cytotoxic effects. J. Nanopart Res. 16, 2465 (2014).
Bruna, T., Maldonado-Bravo, F., Jara, P. & Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 22, 7202 (2021).
Hamelian, M., Zangeneh, M. M., Amisama, A., Varmira, K. & Veisi, H. Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl. Organomet. Chem. 32, 1–8 (2018).
Feng, Q. L. et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater Res. 52, 662–668 (2000).
Garde, S., Chodisetti, P. K. & Reddy, M. Peptidoglycan: Structure, synthesis, and regulation. EcoSal. Plus https://doi.org/10.1128/ecosalplus.ESP-0010-2020 (2021).
Snezhkina, A. V. et al. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell Longev. 2019, 6175804 (2019).
Mikhailova, E. O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater 11, 84 (2020).
More, P. R. et al. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 11, 369 (2023).
Gomes, H. I. O., Martins, C. S. M. & Prior, J. A. V. Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials (Basel) 11, 964 (2021).
Torres, R. G. et al. Ocimum basilicum but not Ocimum gratissimum present cytotoxic effects on human breast cancer cell line MCF-7, inducing apoptosis and triggering mTOR/Akt/p70S6K pathway. J. Bioenerg. Biomembr. 50, 93–105 (2018).
Takáč, P. et al. The role of silver nanoparticles in the diagnosis and treatment of cancer: Are there any perspectives for the future?. Life 13, 466 (2023).
Gautam, D. et al. Green synthesis of silver nanoparticles using Ocimum sanctum Linn. and its antibacterial activity against multidrug resistant Acinetobacter baumannii. Peer J. 11, e15590 (2023).
Rautela, A., Rani, J. & Das Debnath, M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 10, 5 (2019).
Ghasemi, M., Turnbull, T., Sebastian, S. & Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 22, 12827 (2021).
Liu, K., Liu, P., Liu, R. & Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 21, 15–20 (2015).
Batool, S. et al. Addressing artifacts of colorimetric anticancer assays for plant-based drug development. Med. Oncol. 39, 198 (2022).
Uzunoglu, S. et al. Comparison of XTT and Alamar blue assays in the assessment of the viability of various human cancer cell lines by AT-101 (−/− gossypol). Toxicol. Mechan. Methods 20, 482–486 (2010).
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1, 1112–1116 (2006).
Skehan, P. et al. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J. Nat. Cancer Inst. 82, 1107–1112 (1990).
Munshi, A., Hobbs, M. & Meyn, R. E. Clonogenic Cell Survival Assay. In Chemosensitivity In Vitro Assays Vol. 1 (ed. Blumenthal, R. D.) 21–28 (Humana Press, Totowa, 2005). https://doi.org/10.1385/1-59259-869-2:021.
Franken, N. A. P., Rodermond, H. M., Stap, J., Haveman, J. & van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 1, 2315–2319 (2006).
Kumari, P. & Gupta, A. Chapter 3 - Assays to assess the proliferative behavior of cancer cells. In Protocol Handbook for Cancer Biology (eds Misra, G. & Rajawat, J.) 23–41 (Academic Press, Cambridge, 2021). https://doi.org/10.1016/B978-0-323-90006-5.00002-1.
Veeramuthu, K. et al. Chemical profiling and biological activity of psydrax dicoccos gaertn. Molecules 28, 7101 (2023).
Author information
Authors and Affiliations
Contributions
Asha Monica Alex: data collection and original data analysis, Senthilkumar Subburaman: Conceptualization, data presentation, funding acquisition and project leader; Shikha Chauhan: methodology, data collection and original data analysis, reviewing and editing; Vishal Ahuja: Conceptualization, methodology, data collection and original data analysis, data presentation, writing, reviewing and editing. Maryam Abbasi Tarighat; Original Data Analysis, writing, editing. Gholamreza Abdi; Methodology, reviewing and editing, methodology, data analysis. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alex, A.M., Subburaman, S., Chauhan, S. et al. Green synthesis of silver nanoparticle prepared with Ocimum species and assessment of anticancer potential. Sci Rep 14, 11707 (2024). https://doi.org/10.1038/s41598-024-61946-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61946-y
- Springer Nature Limited