Abstract
Metabolic disorder has been found to be an important factor in the pathogenesis and progression of sepsis. However, the causation of such an association between serum metabolites and sepsis has not been established. We conducted a two-sample Mendelian randomization (MR) study. A genome-wide association study of 486 human serum metabolites was used as the exposure, whereas sepsis and sepsis mortality within 28 days were set as the outcomes. In MR analysis, 6 serum metabolites were identified to be associated with an increased risk of sepsis, and 6 serum metabolites were found to be related to a reduced risk of sepsis. Furthermore, there were 9 metabolites positively associated with sepsis-related mortality, and 8 metabolites were negatively correlated with sepsis mortality. In addition, “glycolysis/gluconeogenesis” (p = 0.001), and “pyruvate metabolism” (p = 0.042) two metabolic pathways were associated with the incidence of sepsis. This MR study suggested that serum metabolites played significant roles in the pathogenesis of sepsis, which may provide helpful biomarkers for early disease diagnosis, therapeutic interventions, and prognostic assessments for sepsis.
Similar content being viewed by others
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host immune response triggered by infection1. In a report about sepsis worldwide, the incidence of hospital-treated adult sepsis was 189 cases per 100,000 persons, which is likely higher in low-income and middle-income countries owing to the high infection rate and backward medical environment2. Another research based on analyzing death records to calculate mortality related to sepsis and estimated the global incidence of sepsis was 677.5 cases per 100,000 persons worldwide3. In fact, sepsis incidence and mortality varied substantially across regions, and the global incidence of sepsis is likely to be higher than estimated. The number of sepsis diagnoses continues to rise year by year despite advancements in critical care and antimicrobial therapy, making it a leading cause of mortality among ICU patients4. Therefore, finding accurate and effective sepsis biomarkers is of great significance to patient treatment and prognosis. According to previous research, C-reactive protein (CRP)5, procalcitonin (PCT)6, pancreatic stone protein (PSP)7, interleukin 6 (IL-6)8, high-mobility group box 1 (HMGB1)9, soluble triggering receptor expressed on myeloid cell-1 (sTREM-1)10 are commonly used for early identification and severity assessment of sepsis, and serum amyloid A protein (SAA)11, HMGB1, adrenomedullin (ADM)12, programmed death-1 (PD-1)13, endothelial cell specific molecule-1 (ESM-1)14, plasminogen activator inhibitor 1 (PAI-1)15, lncRNA CASC216 are important predictors of sepsis-related death.
In recent years, there has been an increasing body of evidence supporting the notion that metabolic reprogramming is a crucial condition contributing to immune dysregulation in sepsis17. Cellular metabolism within the body is highly heterogeneous, dynamic, and plastic at different stages of sepsis. Raymond et al. have revealed robust and reproducible metabolic differences in host responses to sepsis despite significant inter-individual metabolic heterogeneity between septic and non-infected SIRS patients and between sepsis survivors and non-survivors18. The study identified 63 metabolites that differed between sepsis patients and non-infected SIRS patients, with lower concentrations of succinate, citrate, glycerol, glycerol-3-phosphate, phosphate, 21 amino acids and their catabolites, 12 glycerophospholipids, and glycerophosphoethanolamines, as well as 6 acylcarnitines in the plasma of sepsis patients. The differences were more pronounced between sepsis survivors (within 28 days) and non-survivors, with significant increases in 17 amino acid breakdown products, 16 acylcarnitines, 11 nucleotide breakdown products, 5 glycolytic and citric acid cycle components (citrate, succinate, pyruvate, dihydroxyacetone, and phosphate), and 4 free fatty acids in the non-survivor group. This study, in line with several other investigations, collectively underscores the significant role of metabolites in the pathogenesis and prognosis of sepsis19,20. Nevertheless, our understanding of the causal relationships between specific serum metabolites and the occurrence and mortality of sepsis remains highly limited.
Research on the association between blood metabolites and the occurrence and prognosis of sepsis primarily relies on observational studies and basic experiments. However, observational studies are susceptible to confounding factors21, and the results of basic experiments are challenging to validate in natural populations due to expensive prices, time-consuming, complex ethical issues, and so on22. To overcome these limitations, we employed a Mendelian Randomization (MR) research approach, an application of instrumental variables (IVs) analysis, that aims to test a causal hypothesis in non-experimental data. In an MR analysis, genetic variation, commonly single nucleotide polymorphism (SNP), is used as an IV for assuming risk factors. The principle of MR is based on Mendel’s second law of independent segregation of genetic alleles when DNA is transmitted from parents to offspring at gamete formation, and utilizes the random allocation characteristics of genotypes to phenotypes in nature for causal inference, therefore overcoming the limitations of residual confounding and reverse causation in conventional observational studies23,24. MR, as a genetic epidemiological method, has become a widely used approach to explore the potential causal relationships between a modifiable exposure and a clinically relevant outcome25. Accumulating evidence has proven the reliability of MR. For instance, Thorkildsen et al.26 have demonstrated insomnia is potentially causally associated with the risk of sepsis through MR analysis. In addition, You et al.27 have confirmed the specific intestinal flora that had a causal relationship with the risk and prognosis of sepsis at the level of gene prediction.
Some MR studies have been performed to explore the relationship between exposure and sepsis. However, the main focus was single exposures or common exposure factors, such as polyunsaturated fatty acids28, vitamins29, body mass index30, and iron status31. Few studies have focused on blood metabolites and sepsis and mortality. In this study, we performed a two-sample MR approach to assess the causal relationship between 486 human blood metabolites and sepsis occurrence and mortality risk to provide a deeper understanding of the pathogenesis of sepsis.
Materials and methods
Study design
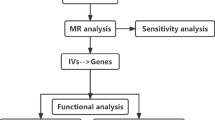
We assessed the causal relationship between human serum metabolites and sepsis using an MR design with two distinct samples. MR studies rely on three fundamental assumptions: (1) the genetic instruments used must be directly associated with the exposure (serum metabolites), (2) these instruments should have no impact on the outcome (sepsis and sepsis mortality within 28 days) and should be independent of known and unknown confounders, and (3) the effect of the instruments on the outcome (sepsis and sepsis mortality within 28 days) is entirely mediated through the exposure (serum metabolites).To prevent sample overlap, we obtained genetic information for metabolites and sepsis separately from independent GWAS datasets. An overview of this study is presented in Fig. 1.
Overview of this Mendelian randomization (MR) analysis. Assumption 1: The genetic instruments are directly associated with the exposure. Assumption 2: Genetic instruments have no impact on the outcome and are independent of known and unknown confounders. Assumption 3: Genetic instruments are unrelated to the outcome and affect the outcome entirely through exposure.
GWAS data and cohort characteristics
The serum metabolite GWAS data were obtained from the study by Shin et al.32 which is the most comprehensive analysis of human metabolites to date, and its complete summary statistics are publicly available through the Metabolomics GWAS server (http://metabolomics.helmholtz-muenchen.de/gwas/). Plasma or serum samples from 7824 adult individuals from two European population studies (the Twins UK cohort and KORA F4 cohort) were collected and analyzed for metabolites using liquid chromatography and gas chromatography coupled to tandem mass spectrometry. A total of 486 metabolites were measured by the MS (Metabolon) platform and GWAS analysis was performed on the HapMap2-based imputed genotype dataset. After data processing, 2.1 million single nucleotide polymorphisms (SNPs) for 486 metabolites were identified, including 309 known and 177 unknown metabolites, respectively. According to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, 309 known metabolites can be divided into eight broad metabolite groups: amino acids, carbohydrates, cofactors and vitamins, energy, lipids, nucleosides, peptides, and xenobiotic metabolism. Meanwhile, the chemical properties of another 177 unknown metabolites have yet to be conclusively determined.
The GWAS data for sepsis and 28-day mortality after sepsis were sourced from the UK Biobank (http://www.nealelab.is/uk-biobank/). These datasets encompassed a combined total of 486,484 European adult volunteers, with 11,643 sepsis patients and 474,841 controls (IEU GWAS ID: ieu-b-4980), as well as 1896 sepsis patients who succumbed within 28 days and 484,588 controls (IEU GWAS ID: ieu-b-5086). Sepsis cases were identified by the International Classification of Diseases (ICD) 10th revision codes A02, A39, A40, and A41, in line with definitions used in recent literature33.
Selection of SNPs
SNPs selected as IVs should satisfy three MR assumptions: relevance, independence, and exclusion restriction. The selection of SNPs in this study was based on the following criteria: (1) SNPs significantly associated with risk factors at the genome-wide level (p < 1 × 10−5); (2) SNPs were mutually independent (linkage disequilibrium with r2 < 0.01 within a 500 kb range). The statistical power of SNPs depends on their explanatory power for the phenotype (R2), which can be assessed through the strength of the correlation between SNPs and the phenotype. R2 refers to the proportion of variance in the exposure explained by the genetic variant. It gives an indication of the strength of the genetic instrument, with higher values suggesting a stronger instrument. Its formula is as follows34. \(R2=\frac{2\times EAF\times \left(1-EAF\right)\times beta2}{2\times EAF\times \left(1-EAF\right)\times beta2+2\times EAF\times \left(1-EAF\right)\times N\times se(beta)2}\) In the formula, EAF stands for Effect Allele Frequency, that is, the frequency with which the effect allele appears in the population. beta is the effect size of the SNP on the metabolite and is a measure of the strength and direction of the association between the SNP and the metabolite. se(beta) is the standard error of the effect size and it represents the precision of the estimated effect size. N is the sample size. The above indicators collectively determine the IV’s strength and relevance. The F-statistic can reflect the strength of the correlation between SNPs and phenotypes, and its formula is as follows35. \(F=\frac{N-K-1}{K}\times \frac{R2}{1-R2}\) Where N represents the sample size, K represents the number of SNPs. The magnitude of the F-statistic decreases with an increase in the number of SNPs, while it increases with an increase in the sample size and the explanatory power of SNPs on the exposure. In order to remove the weak instruments, we calculated the F statistics for each IV and excluded those with an F-statistic lower than 1036.
MR and sensitivity analysis
Considering that the random-effects inverse variance weighting (IVW) can provide the most accurate assessment, assuming that all SNPs are valid instruments, we employed the IVW method as the primary analysis to evaluate the causal relationship between serum metabolites and sepsis and sepsis mortality within 28 days with p < 0.05.
To obtain more reliable results, we applied weighted median (WM), MR-Egger, and MR-PRESSO, the other three additional methods, as complementary analyses to further assess metabolites with significant estimates (IVW derived p < 0.05). The WM method provides a reliable estimate that the compelling IV accounts for more than 50% of the weight37, and the MR-Egger method can supply unbiased estimations under the strength of IVs independent of direct effect38. Moreover, MR-PRESSO can check and rectify horizontal pleiotropic outliers, thus providing correct assessments39.
Sensitivity analysis is crucial because it examines horizontal pleiotropy and heterogeneity, which may severely violate MR estimates. Horizontal pleiotropy can be observed when the IVs affect the outcome via pathways other than the exposure of interest. Therefore, we conducted several methods, including the Cochran Q test, MR-Egger intercept test, and MR-PRESSO, to detect and correct for heterogeneity and pleiotropy. The Cochran’s Q test identified heterogeneity among used SNPs estimates, with p < 0.05 indicating potential heterogeneity. We employed the MR-Egger intercept test to evaluate the possibility of horizontal pleiotropy, with p < 0.05 indicating possible pleiotropic effects. Furthermore, this study used the MR-PRESSO global test to evaluate the presence of horizontal pleiotropy, and p < 0.05 suggested the presence of horizontal pleiotropy.
In summary, we rigorously screened serum metabolites with potential causal effects on sepsis and sepsis mortality within 28 days via multiple criteria: (1) The p-value of the IVW method was significant (p < 0.05). (2) There is no heterogeneity and horizontal pleiotropy in MR results. (3) The directions of the four MR methods are consistent.
Reverse MR analysis
To evaluate the causal relationship between serum metabolites and sepsis and sepsis mortality within 28 days, we also performed reverse MR analysis on serum metabolites causally related to sepsis and sepsis mortality within 28 days in the forward MR analysis. The methods and settings used are consistent with forward MR.
Metabolic pathway analysis
Based on the analysis of known metabolite pathways and referencing the KEGG database, we conducted a metabolic pathway analysis using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) to investigate the causal associations of metabolites identified through MR analysis with sepsis incidence and sepsis-related 28-day mortality40,41. This study only analyzed metabolites that passed the suggested association threshold by the IVW method (p < 0.05). Pathways were considered significantly enriched if p < 0.05 and the impact score > 0.1.
Ethics approval and consent to participate
This study contains human participants collected from several studies conducted by previous researches. All participants gave informed consent in all corresponding original studies. Our study is based on the large-scale GWAS dataset rather than individual-level data. Hence, ethical approval is not applicable.
Results
The selection of instrumental variables
For each metabolite, we extracted genetic variants as IVs to test their causal relationship with the outcomes. SNP and their F-values, R2, for all IVs used in the forward MR, are provided in Supplementary Tables 1 and 2. A total of 9077 SNPs for Sepsis and 8651 SNPs for sepsis mortality within 28 days were finally included in the analysis. The number of SNPs for each metabolite ranged from 7 to 37. The minimum F statistic of these IVs was 17.41, indicating that all IVs were sufficiently compelling for the MR analysis of the 486 metabolites.
Causal effects of serum metabolites on sepsis and sepsis mortality within 28 days
Sepsis
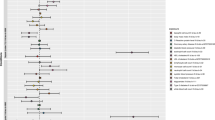
Using the above serum metabolites as IVs, we estimated causal associations between the 486 serum metabolites and sepsis, and a total of 14 metabolites were identified to have causal associations with sepsis (IVW method, p < 0.05) (Fig. 2). Combined with complementary analysis methods (WM, MR-Egger, and MR-PRESSO), we removed two metabolites (epiandrosterone sulfate and X-14304-leucylalanine) because of inconsistent with the direction of IVW, and 12 metabolites were finally obtained that were causally related to sepsis. These metabolites include 9 known metabolites with established structures and functions and 3 unknown metabolites. The known metabolites are derived from metabolic pathways such as carbohydrate, lipid, peptide, and xenobiotics. Among these, glucose (OR 1.95, 95% CI 1.17–3.26, p = 0.011), androsterone sulfate (OR 1.14, 95% CI 1.03–1.26, p = 0.009), propionylcarnitine (OR 1.50, 95% CI 1.03–2.18, p = 0.034), salicylate (OR 1.04, 95% CI 1.00–1.08, p = 0.031), metoprolol acid metabolite (OR 1.02, 95% CI 1.00–1.04, p = 0.017) and X-11787 (OR 1.72, 95% CI 1.06–2.79, p = 0.028) were positively associated with sepsis. While, heptanoate (7:0) (OR 0.50, 95% CI 0.31–0.79, p = 0.003), 1-oleoylglycerophosphoethanolamine (OR 0.52, 95% CI 0.31–0.87, p = 0.012), X-14205-alpha-glutamyl tyrosine (OR 0.76, 95% CI 0.61–0.95, p = 0.016), saccharin (OR 0.84, 95% CI 0.74–0.94, p = 0.004), X-12063 (OR 0.84, 95% CI 0.72–0.98, p = 0.027) and X-13435 (OR 0.71, 95% CI 0.51–0.99, p = 0.044) were negatively associated with sepsis (Fig. 3).
Causal association heatmap of serum metabolites with sepsis and 28-day mortality in sepsis by IVW analysis. Rows in the figure represent different serum metabolites, while columns depict two outcomes: sepsis and 28-day mortality in sepsis. Different metabolites are presented in distinct colors, with pink and blue indicating positive and negative factors, respectively. Deeper colors on the heatmap signify higher significance.
Sepsis mortality within 28 days
This study identified 21 causal relationships between the serum metabolites and sepsis mortality within 28 days (IVW method, p < 0.05) (Fig. 2). By utilizing WM, MR-Egger, and MR-PRESSO analysis, three candidate metabolites, including acetylcarnitine, 1-palmitoylglycerophosphoinositol, and N1-methyladenosine inconsistent with the direction of IVW, were excluded, and 18 metabolites were finally identified as having a causal relationship with 28-day death in sepsis. These metabolites include 13 known metabolites with established structures and functions and 5 unknown metabolites. The known metabolites are derived from various metabolic pathways such as amino acid, lipid, peptide, and xenobiotics. Genetically predicted 10 serum metabolites were associated with a increased risk of sepsis mortality in 28 days, including 4-acetamidobutanoate (OR 3.83, 95% CI 1.15–5.76, p = 0.029), 3-(3-hydroxyphenyl)propionate (OR 1.70, 95% CI 1.02–2.84, p = 0.043), glycocholate (OR 1.74, 95% CI 1.34–2.27, p = 4E-05), taurochenodeoxycholate (OR 1.72, 95% CI 1.19–2.49, p = 0.004), 1-stearoylglycerophosphocholine (OR 3.50, 95% CI 1.47–5.36, p = 0.005), X-13183-stearamide (OR 1.87, 95% CI 1.12–3.10, p = 0.016), aspartylphenylalanine (OR 3.17, 95% CI 1.05–5.56, p = 0.041), gamma-glutamylphenylalanine (OR 3.94, 95% CI 1.17–5.27, p = 0.027), gamma-glutamylglutamate (OR 2.03, 95% CI 1.24–3.33, p = 0.005), cotinine (OR 1.17, 95% CI 1.02–1.33, p = 0.021). Conversely, methionine (OR 0.6, 95% CI 0.3–0.91, p = 0.004), cysteine–glutathione disulfide (OR 0.51, 95% CI 0.33–0.78, p = 0.002), 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF) (OR 0.48, 95% CI 0.35–0.65, p = 2.1E-06), X-11529 (OR 0.76, 95% CI 0.59–0.98, p = 0.033), X-11538 (OR 0.60, 95% CI 0.42–0.86, p = 0.006), X-11550 (OR 0.61, 95% CI 0.41–1.00, p = 0.05), X-12465 (OR 0.46, 95% CI 0.29–0.72, p = 0.001), X-13549 (OR 0.84, 95% CI 0.75–0.92, p = 0.04) were related with a decreased risk of 28-day death in sepsis (Fig. 4).
Since the p-value threshold is artificially prescribed, it only represents a low false positive result. It cannot guarantee an accurate result, no matter how small the p-value is. In addition, p < 0.05 is a relatively loose threshold, and we need to implement multiple tests to eliminate false positives by modifying the p-value threshold. The formula for Bonferroni correction is p*(1/486), where p is the original threshold, and 486 is the total number of tests. After Bonferroni correction, only glycocholate and CMPF passed this criterion; all other metabolites were nominally significant.
Sensitivity analysis
Although the IVW method is highly effective in inferring causal relationships between exposures and disease outcomes, it is well known to be susceptible to weak instrument bias. Therefore, we further carried out sensitivity and pleiotropy analyses to evaluate the robustness of the causal relationship. As shown in Table 1, results from Cochran’s Q test suggested that no apparent heterogeneity was found in the selected SNPs (p > 0.05) except for 3-(3-hydroxyphenyl)propionate (p = 0.041), which serves as a positive factor for mortality within 28 days in sepsis. Moreover, both the MR-PRESSO test and MR-Egger interception test did not detect any signs of horizontal pleiotropy (p > 0.05). These findings indicate that the levels of heterogeneity and pleiotropy are not pronounced, supporting the robustness of the estimated causal relationships between these serum metabolites and the occurrence and mortality of sepsis.
Reverse causation between metabolites and sepsis-related phenotypes
To obtain robust MR evidence, we performed a reverse causal effect analysis on the sepsis-related phenotypes on the identified candidate metabolites. In the IVW model, none of the sepsis and sepsis mortality within 28 days had opposite effects on these candidate metabolites, further supported by the other three MR models. Reverse MR is detailed in Supplementary Tables 3 and 4.
Metabolic pathway analysis
The results of metabolic pathway analysis (Table 2) reveal three metabolic pathways associated with the risk of sepsis, namely Glycolysis/Gluconeogenesis (p < 0.001), and Pyruvate metabolism (p = 0.042). Additionally, we did not observed any pathway is involved in sepsis mortality within 28 days.
Discussion
In this study, we firstly explored causal relationships between 486 human blood metabolites and risk of sepsis and mortality within 28 days by utilizing the MR causal effects model. We identified 12 blood metabolites that were causally related to sepsis and these metabolites may influence sepsis development through pathways such as glycolysis/gluconeogenesis, and pyruvate metabolism. Among the 12 blood metabolites, in addition to unknown metabolites and reported glucose42 and propionylcarnitine43 as risk factors for sepsis, we also discovered that elevated levels of salicylate, metoprolol acid metabolite, and androsterone sulfate were associated with an increased risk of sepsis. While, elevated levels of heptanoate (7:0), 1-oleoylglycerophosphoeth-anolamine, alpha-glutamyltyrosine, and saccharin could reduce the risk of sepsis. Meanwhile, we found 17 blood metabolites that were causally associated with 28-day mortality in sepsis. In addition to the previously reported associations between taurochenodeoxycholate44, stearamide45, cotinine46, methionine47, CMPF48 and sepsis mortality, we also discovered that 4-acetamidobutanoate, glycocholate, 1-stearoylglycerophosphocholine, aspartylphenylalanine, gamma-glutamylphenylalanine, gamma-glutamylglutamate and cysteine-glutathione disulfide is causally associated with 28-day mortality in sepsis. These results may provide biomarkers for early diagnosis of sepsis and targets for timely treatment of sepsis.
Through MR research, we establish causal relationships between 9 metabolites and sepsis, of which glucose has been intensively studied in sepsis. Recently, Jiang et al. established a predictive model for 47,185 septic patients in a retrospective observational cohort study, and they identified glucose levels as a significant risk factor for sepsis42. We further added genetic evidence for the role of glucose in sepsis. Neugebauer et al.43 conducted a retrospective analysis of 406 patients and found that the serum concentrations of most acylcarnitines including propionylcarnitine were altered in sepsis compared with systemic inflammatory response syndrome, which is also consistent with our research results. Surprisingly, salicylate and metoprolol acid metabolite were identified as the risk factors for sepsis in our study. Salicylate in the human body mainly comes from the intake of external drugs, especially aspirin. Although multiple studies have shown that aspirin can reduce sepsis progression by inhibiting the release of inflammatory factors and platelet aggregation, a growing number of studies have shown that the use of aspirin as a primary prevention strategy does not reduce the burden of sepsis49. What is more, studies have reported that the use of aspirin in critically ill patients can increase the incidence of sepsis50,51. The mechanism may be that aspirin irreversibly inhibit platelet function, hindering its activation and surface expression of adhesion molecules, thereby forming microvascular thrombus and causing ischemia, leading to tissue damage and multiple organ dysfunction syndrome. This seemingly contradictory result highlights the complexity of sepsis pathogenesis and requires further studies to explain and validate. Marika et al. discovered significant changes in androgen metabolism when analyzing novel potential molecular biomarkers of sepsis through untargeted metabolites. Among them, androsterone sulfate is significantly increased in sepsis patients with complicated febrile neutropenia52, and our study provided further evidence to support the role of androgen metabolism in sepsis. However, further research is needed to explore the mechanism of androsterone sulfate in sepsis.
We also found that elevated levels of 1-oleoylglycerophosphoeth-anolamine, alpha-glutamyltyrosine, heptanoate (7:0), and saccharin exhibited protective roles against sepsis. 1-oleoylglycerophosphoeth-anolamine is an important component of phosphatidylethanolamine (PE). PE is the main phospholipid in mammalian cell membranes and can attenuate inflammatory responses and increase cell survival53. Studies have shown that pretreatment with phosphatidylethanolamine diethylene triamine pentaacetate (PE-DTPA) inhibits LPS-induced TNF-α production in human myeloid cells, leading to NF-κB activation54. Therefore, we hypothesized that 1-oleoylglycerolphosphoethanolamine may affect the development of sepsis by regulating inflammatory responses. Inflammatory mechanisms play a key role in the pathogenesis of sepsis caused by changes in metabolic phenotype. Immune cells activated by inflammation, require large amounts of energy to perform their functions, which increases the body’s demand for glucose and fatty acids. This increased energy demand can alter an individual’s metabolic status, leading to changes in glucose metabolism and lipid metabolism. Cytokines released during inflammation, such as TNF-α, IL-1, and IL-6, not only play a role in the immune response but also directly or indirectly affect metabolic pathways. For example, TNF-α is thought to be involved in the development of insulin resistance, where dysregulation of glucose metabolism may promote the development of sepsis55. Increased production of ROS caused by inflammation can cause cell damage and impairment of energy metabolism. The role of oxidative stress in inflammation links reductions in red blood cell oxygenation and energy production, factors that can influence the development of sepsis. Research also shows that the gut microbiome is closely connected to the host’s metabolic and immune systems. Intestinal barrier damage and bacterial translocation during inflammation can further affect metabolic status, promote the production of inflammatory factors, and aggravate the process of sepsis56. In sepsis, there is a bidirectional relationship between altered metabolic phenotypes and inflammation. Inflammation leads to metabolic abnormalities, and these metabolic abnormalities can in turn exacerbate inflammation, forming a vicious cycle. This cycle may be one of the key drivers in the development of sepsis, triggering systemic inflammatory response and multiorgan failure.
As a tyrosine-containing dipeptide, alpha-glutamyltyrosine has high antioxidant properties and can improve oxidative stress in the pathogenesis of sepsis57. Heptanoate (7:0) is a derivative of heptanoic acid. The exogenous addition of medium-chain fatty acids (including heptanoic acid) could increase mitochondrial respiratory capacity under starvation and inflammation and improve mitochondrial dysfunction in sepsis58. Mitochondrial dysfunction plays a crucial role in the pathogenesis of sepsis. Inflammatory mechanisms and alterations in metabolic pathways contribute significantly to this dysfunction, leading to the development and progression of sepsis. Inflammatory cytokines elevate ROS production, overwhelming mitochondrial antioxidant defenses. Excessive ROS can damage mitochondrial DNA, proteins, and lipids, impairing the electron transport chain and ATP production. Furthermore, the energetic dysfunction extends to alterations in substrate utilization and metabolic reprogramming. Sepsis induces a shift from oxidative phosphorylation to aerobic glycolysis in immune cells upon activation, contributing to lactate release into the blood. This metabolic condition, resembling starvation, is characterized by the breakdown of protein, carbohydrate, and fat reserves, leading to further mitochondrial injury and organ dysfunction. The lipolysis-related rise in free fatty acid concentrations and the impaired fatty acid oxidation in the mitochondria are particularly noteworthy, contributing to a metabolic deficit that is associated with sepsis-related cardiomyopathy and increased hospital mortality59. Therefore, heptanoate (7:0) may play a protective role in sepsis by restoring mitochondrial function and providing energy to the body in inflammatory conditions. Studies have shown that saccharin intake in mice can change the composition of the intestinal microbiome, reduce bacterial load and increase the relative abundance of bacteroidetes, thereby alleviating intestinal inflammatory response and playing a protective role in dextran sodium sulfate-induced experimental colitis, which is also consistent with the results that saccharin can reduce the occurrence of sepsis in our study60.
Our study on metabolic pathway analysis suggests the involvement of glucose metabolism pathways such as glycolysis/gluconeogenesis, and pyruvate metabolism in the occurrence of sepsis in the population, with glycolysis being the most prominent. During the hyperinflammatory phase of sepsis, the expression of genes related to glycolysis in monocytes/macrophages increases; while during the immune tolerance phase, glycolysis levels decrease61. Tannahill et al. found that LPS stimulation of mouse BMDMs could increase the expression of Glut1 and HK3, increase glycolysis, and promote IL-1β expression, while using 2-deoxy-D-glucose (2-DG) to inhibit glycolysis in BMDMs significantly reduced IL-1β production62. In addition, metabolomic and transcriptomic analysis of polymorphonuclear neutrophils isolated from sepsis patients showed that glycolysis and the Warburg effect were significantly altered and downregulation of LDHA mediated by the PI3K/Akt-HIF-1α pathway caused inhibition of glycolysis, leading to neutrophil immunosuppression during sepsis63. During pyruvate metabolism, when pyruvate is transferred to mitochondria, pyruvate dehydrogenase complex (PDHC) oxidizes pyruvate to acetyl-CoA, thereby accelerating aerobic oxidation. Clinical studies have shown that PDHC activity in peripheral blood mononuclear cells of sepsis patients is significantly lower than that of healthy controls, and this reduced activity may affect the prognosis of patients with sepsis64. Targeted activation of PDHC can not only regulate glucose metabolism and inhibit lactate accumulation in septic cells but can also significantly restore TCA metabolite levels to control levels and improve liver function in sepsis65. Our findings further confirm the roles of glycolysis/gluconeogenesis and pyruvate metabolism in the occurrence and development of sepsis.
Given the persistently high mortality rate among sepsis patients, researchers have been investigating the risk factors for sepsis-related mortality. Our study demonstrates that serum metabolites, such as glycocholate and taurochenodeoxycholate, are identified as risk factors for 28-day mortality in sepsis. Taurochenodeoxycholate, formed by combining free primary bile acids in the liver with taurine is also considered closely related to inflammatory responses and the regulation of immune cells66,67. Recently, Long et al.44 conducted a metabolomic analysis of fecal samples from 23 sepsis patients. The data revealed a significant increase in taurine levels during the third week of illness in sepsis patients who ultimately succumbed, compared to the first week. A linear model depicted that higher concentrations of taurine-conjugated bile acids constitute a risk factor for sepsis mortality. Additionally, the blood metabolites of membrane synthesis (1-stearoylglycerophosphocholine) and amino acid metabolism (aspartylphenylalanine, gamma-glutamyl phenylalanine, gamma-glutamyl glutamate) closely correlate with the 28-day mortality risk factors for sepsis. 1-stearoylglycerophosphocholine is synthesized from stearic acid and glycerophosphocholine, both contributing to membrane formation. As the cellular signaling platform, the composition and physicochemical properties of the membrane, including membrane fluidity and potential, can cooperatively regulate immune cell signaling and receptor functions (including T cell receptors and B cell receptors). Both palmitic acid and stearic acid are saturated fatty acids that can reduce membrane fluidity, thereby increasing susceptibility to diseases68,69. In addition, circulatory failure secondary to shock is the most common direct cause of sepsis-related death. Angiotensin-converting enzyme (ACE) is a critical enzyme that converts angiotensin I to angiotensin II, participating in the regulation of cardiovascular functions, including blood pressure70. Therefore, as a product of ACE-catalyzed peptide cleavage, aspartate phenylalanine may be involved in septic shock. Dynamic plasma lipidomic analysis revealed that stearamide was increased significantly in patients with sepsis after cardiopulmonary bypass cardiovascular surgery, accompanied by high mortality45. Plasma cotinine is a nicotine metabolite. Romina et al. have shown that increased neutrophil extracellular traps (NETs) formation may be a trigger for sepsis in smokers and cotinine could effectively induce the formation of NETs, which may be the main reason why cotinine aggravates the death of patients with sepsis46.
Our study also suggests a connection between metabolic-induced liver dysfunction and susceptibility to circulatory failure, resulting in a higher risk of mortality within 28 days in sepsis. Traditionally, abnormal gamma-glutamyl transferase (GGT) has been seen as an indicator of liver dysfunction in critically ill patients, linked to increased mortality and longer hospital stays71. Thomson et al.72 found that among 263 ICU patients without pre-existing liver or biliary diseases, 61% had abnormal liver function upon admission and abnormal GGT significantly increased the risk of death within 30 days of admission. Our results indicate that gamma-glutamyl phenylalanine and gamma-glutamyl glutamate, associated with GGT, constitute high-risk factors for 28-day mortality in sepsis. In addition, studies have shown that plasma 4-acetamidobutanoate levels are elevated in patients with severe cirrhosis, suggesting that 4-acetamidobutanoate is associated with liver dysfunction in humans73. In fact, liver dysfunction plays an important role in sepsis caused by alteration of bile acids metabolism. Liver malfunction significantly alters bile acids metabolism during sepsis, affecting both the liver’s function and its communication with the gut, known as the gut-liver axis. In sepsis, liver dysfunction can lead to increased levels of bile acids, which in turn activate inflammatory and oxidative stress pathways, potentially resulting in cellular damage such as apoptosis, necrosis, fibrosis, and cirrhosis. The farnesoid X receptor (FXR) and G protein-coupled receptors like TGR5 are key mediators in the regulatory actions of bile acids, influencing various metabolic processes including lipid and glucose homeostasis74,75. The interaction between bile acids and the liver, underscores a complex regulatory network that is significantly impacted during sepsis. Understanding this network is crucial for identifying potential therapeutic targets aimed at mitigating liver injury and improving outcomes in septic patients. Therefore, we speculate that the reason for the increased mortality in sepsis caused by glycocholate and taurochenodeoxycholate may be related to liver dysfunction.
In contrast, methionine serves as a protective factor for 28-day mortality in sepsis. Methionine is enzymatically converted to S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH) is the by-product of SAM transmethylation reactions. An observational study showed that SAM and SAH were significantly higher in patients who died from sepsis than in those who survived47. Our results also demonstrate a strong causal relationship between CMPF and reduced sepsis mortality within 28 days. CMPF is a major furan fatty acid metabolite whose role in disease has been controversial. Some studies have shown that CMPF can increase the production of ROS in human kidney cells and induce kidney injury76. However, there was also literature showing that higher CMPF levels were associated with a reduced risk of all-cause death48 and periodontitis77. Consistent with our study, Wei et al. found that (CMPF) is causally related to a reduced risk of sepsis in an MR study.
Beyond the known constituents and functions mentioned above, this study also suggests that 8 unknown metabolites are associated with sepsis occurrence and mortality. Most of these metabolites appear to serve as protective factors in the development and outcome of sepsis. Further identification, analysis, and exploration of the relationship between these unknown metabolites and sepsis may offer new insights into diagnosing and treating sepsis.
Our study employed four causal inference MR models to enhance the reliability of research findings. Among these, the IVW model serves as the primary method in MR research by averaging the effects of multiple genetic variants to improve the robustness of estimates. Each MR method is designed with unique assumptions and advantages. The observed heterogeneity in p values may stem from the different assumptions and mechanisms used by these methods to account for pleiotropic and invalid instruments. Despite these differences, the consistency in the direction of effects across all methods enhances the credibility of our findings. This consistency suggests that our results are robust to the various forms of bias that each method is designed to address. Furthermore, MR-PRESSO, Cochran’s Q statistic, and MR-Egger models were utilized to detect heterogeneity and horizontal pleiotropy among genetic variants. Additionally, bidirectional MR analysis confirmed causal inferences between metabolites and the occurrence of sepsis and 28-day mortality.
While our study offers valuable insights into the potential causal relationships between metabolites and sepsis outcomes, we acknowledge several limitations that are inherent to our research design and methodology. Firstly, since all samples in this study are of European descent, caution should be exercised when extrapolating the results to other populations. Secondly, the SNP selection employed a p-value threshold of 1 × 10−5, resulting in a limited number of selected SNPs. This may only account for partial exposure variations and could impact the statistical power of causal estimation. Thirdly, in view of the complexity and multi-factor etiology of sepsis as a disease, we prioritized establishing a broad understanding of potential causal relationships and did not perform an in-depth analysis of the impact of confounding on the exposure-outcome relationship by subgroups defined by age, gender, or comorbidities. Future studies are needed to build on our findings by incorporating stratified analyses and multivariable adjustments to elaborate how different subgroups (based on age, gender, or underlying health conditions) exhibit different causal relationships between metabolites and sepsis outcomes.
In summary, this MR study provides preliminary evidence of a causal relationship between blood metabolites and the occurrence of sepsis, as well as the risk of death within 28 days. The study results underscore the involvement of cellular glycolysis and energy-related serum metabolite heterogeneity in the susceptibility to sepsis formation. Additionally, metabolites related to liver function impairment demonstrate a causal relationship with sepsis-related mortality within 28 days, highlighting the susceptibility to sepsis occurrence and death based on different metabolite phenotypes.
Data availability
The datasets used during the current study are publicly available. The data for serum metabolite can be found here: http://metabolomics.helmholtz-muenchen.de/gwas/. The GWAS data for sepsis and 28-day mortality after sepsis were sourced from the UK Biobank: http://www.nealelab.is/uk-biobank/.
References
Rhodes, A. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43(3), 304–377 (2017).
Fleischmann-Struzek, C. et al. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 46(8), 1552–1562 (2020).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 395(10219), 200–211 (2020).
Vincent, J. L. Current sepsis therapeutics. Ebiomedicine 86, 104318 (2022).
Tan, J. et al. Procalcitonin kinetics early after severe burn injury and its value in diagnosis of sepsis. Burns 47(8), 1802–1809 (2021).
Li, S. et al. Serum procalcitonin levels distinguish gram-negative bacterial sepsis from gram-positive bacterial and fungal sepsis. J. Res. Med. Sci. 21, 39 (2016).
Klein, H. J. et al. Pancreatic stone protein predicts sepsis in severely burned patients irrespective of trauma severity: A monocentric observational study. Ann. Surg. 274(6), e1179–e1186 (2021).
Ling, H. et al. Evaluation of qSOFA combined with inflammatory mediators for diagnosing sepsis and predicting mortality among emergency department. Clin. Chim. Acta 544, 117352 (2023).
Karakike, E. et al. Late peaks of HMGB1 and sepsis outcome: Evidence for synergy with chronic inflammatory disorders. Shock 52(3), 334–339 (2019).
Qin, Q., Liang, L. & Xia, Y. Diagnostic and prognostic predictive values of circulating sTREM-1 in sepsis: A meta-analysis. Infect. Genet. Evol. 96, 105074 (2021).
Yu, M. H. et al. Prognostic value of the biomarkers serum amyloid A and nitric oxide in patients with sepsis. Int. Immunopharmacol. 62, 287–292 (2018).
Andaluz-Ojeda, D. et al. Superior accuracy of mid-regional proadrenomedullin for mortality prediction in sepsis with varying levels of illness severity. Ann. Intensive Care 7(1), 15 (2017).
Jiang, W. et al. PD-1 in Tregs predicts the survival in sepsis patients using sepsis-3 criteria: A prospective, two-stage study. Int. Immunopharmacol. 89(Pt A), 107175 (2020).
Hsiao, S. Y. et al. Concentration and value of endocan on outcome in adult patients after severe sepsis. Clin. Chim. Acta 483, 275–280 (2018).
Hoshino, K. et al. Usefulness of plasminogen activator inhibitor-1 as a predictive marker of mortality in sepsis. J. Intensive Care 5, 42 (2017).
Wang, R. et al. Potential of circulating lncRNA CASC2 as a biomarker in reflecting the inflammatory cytokines, multi-organ dysfunction, disease severity, and mortality in sepsis patients. J. Clin. Lab. Anal. 36(8), e24569 (2022).
Liu, J. J., Zhou, G. S., Wang, X. T. & Liu, D. W. Metabolic reprogramming consequences of sepsis: adaptations and contradictions. Cell. Mol. Life Sci. 79(8), 456 (2022).
Langley, R. J. et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 5(195), 195ra95-195ra95 (2013).
Sreekumar, A. et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457(7231), 910–914 (2009).
Wishart, D. S. et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 37, D603–D610 (2009).
D’Onofrio, B. M., Sjolander, A., Lahey, B. B., Lichtenstein, P. & Oberg, A. S. Accounting for confounding in observational studies. Annu. Rev. Clin. Psychol. 16, 25–48 (2020).
Mielke, D. & Rohde, V. Randomized controlled trials-a critical re-appraisal. Neurosurg. Rev. 44(4), 2085–2089 (2021).
Larsson, S. C., Butterworth, A. S. & Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 44(47), 4913–4924 (2023).
Larsson, S. C., Burgess, S. & Michaelsson, K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA 318(4), 371–380 (2017).
Li, X. et al. Triglyceride-glucose index and the risk of heart failure: Evidence from two large cohorts and a Mendelian randomization analysis. Cardiovasc. Diabetol. 21(1), 229 (2022).
Thorkildsen, M. S. et al. Association of genetically predicted insomnia with risk of sepsis: A Mendelian randomization study. JAMA Psychiatry 80(10), 1061–1065 (2023).
You, J. Y. et al. Causal associations between gut microbiota and sepsis: A two-sample Mendelian randomization study. Eur. J. Clin. Invest. 53, e14064 (2023).
Lei, P. P. et al. Mendelian randomization analysis reveals causal associations of polyunsaturated fatty acids with sepsis and mortality risk. Infect. Dis. Ther. 12(7), 1797–1808 (2023).
Lou, C. et al. Causal effects of genetically vitamins and sepsis risk: A two-sample Mendelian randomization study. BMC Infect. Dis. 23(1), 766 (2023).
Wang, J. T. et al. Exploring the causality between body mass index and sepsis: A two-sample Mendelian randomization study. Int. J. Public Health 68, 1605548 (2023).
Hamilton, F., Mitchell, R., Ahmed, H., Ghazal, P. & Timpson, N. J. An observational and Mendelian randomisation study on iron status and sepsis. Sci. Rep. 13(1), 2867 (2023).
Shin, S. Y. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46(6), 543–550 (2014).
Zekavat, S. M. et al. Hematopoietic mosaic chromosomal alterations increase the risk for diverse types of infection. Nat. Med. 27(6), 1012–1024 (2021).
Papadimitriou, N. et al. Physical activity and risks of breast and colorectal cancer: A Mendelian randomisation analysis. Nat. Commun. 11(1), 597 (2020).
Burgess, S., Thompson, S. G. & Collaboration, C. C. G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40(3), 755–764 (2011).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37(7), 658–665 (2013).
Bowden, J., Smith, G. D., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40(4), 304–314 (2016).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32(5), 377–389 (2017).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50(5), 693–698 (2018).
Frolkis, A. et al. SMPDB: The small molecule pathway database. Nucleic Acids Res. 38, D480–D487 (2010).
Kanehisa, M., Goto, S., Sato, Y., Furumichi, M. & Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40(D1), D109–D114 (2012).
Jiang, Z. Y. et al. Interpretable machine-learning model for real-time, clustered risk factor analysis of sepsis and septic death in critical care. Comput. Methods Prog. Biomed. 241, 107772 (2023).
Neugebauer, S. et al. Metabolite profiles in sepsis: Developing prognostic tools based on the type of infection. Crit. Care Med. 44(9), 1649–1662 (2016).
Long, X. Y. et al. Global signatures of the microbiome and metabolome during hospitalization of septic patients. Shock 59(5), 716–724 (2023).
Ding, W. Y. et al. Dynamic plasma lipidomic analysis revealed cholesterol ester and amides associated with sepsis development in critically ill patients after cardiovascular surgery with cardiopulmonary bypass. J. Pers. Med. 12(11), 1838 (2022).
Aspera-Werz, R. H. et al. Nicotine and cotinine induce neutrophil extracellular trap formation-potential risk for impaired wound healing in smokers. Antioxidants-Basel 11(12), 2424 (2022).
Wexler, O. et al. Methionine metabolites in patients with sepsis. J. Intensive Care Med. 33(1), 37–47 (2018).
Dai, L. et al. The association between TMAO, CMPF, and clinical outcomes in advanced chronic kidney disease: Results from the European QUALity (EQUAL) study. Am. J. Clin. Nutr. 116(6), 1842–1851 (2022).
Eisen, D. P. et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): A randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir. Med. 9(2), 186–195 (2021).
Al Harbi, S. A., Tamim, H. M., Al-Dorzi, H. M., Sadat, M. & Arabi, Y. M. Association between aspirin therapy and the outcome in critically ill patients: A nested cohort study. BMC Pharmacol. Toxicol. 17, 1–7 (2016).
Hsu, J. et al. Aspirin use and long-term rates of sepsis: A population-based cohort study. PLoS One 13(4), e0194829 (2018).
Lappalainen, M. et al. Novel biomarker candidates for febrile neutropenia in hematological patients using nontargeted metabolomics. Dis. Markers 2018, 6964529 (2018).
Eros, G. et al. Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur. Surg. Res. 42(1), 40–48 (2009).
Lee, C., An, H. J., Kim, J. L., Lee, H. & Paik, S. G. Inhibitory effect of a phosphatidyl ethanolamine derivative on LPS-induced sepsis. Mol. Cells 27(2), 251–255 (2009).
Qu, W., Han, C., Li, M., Zhang, J. & Jiang, Z. Anti-TNF-alpha antibody alleviates insulin resistance in rats with sepsis-induced stress hyperglycemia. J. Endocrinol. Invest. 41(4), 455–463 (2018).
Adelman, M. W. et al. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 24(1), 278 (2020).
Torkova, A., Koroleva, O., Khrameeva, E., Fedorova, T. & Tsentalovich, M. Structure-functional study of tyrosine and methionine dipeptides: An approach to antioxidant activity prediction. Int. J. Mol. Sci. 16(10), 25353–25376 (2015).
Hecker, M. et al. Impact of short- and medium-chain fatty acids on mitochondrial function in severe inflammation. JPEN J. Parenter. Enter. Nutr. 38(5), 587–594 (2014).
Rahmel, T. et al. Mitochondrial dysfunction in sepsis is associated with diminished intramitochondrial TFAM despite its increased cellular expression. Sci. Rep. 10(1), 21029 (2020).
Sunderhauf, A. et al. Saccharin supplementation inhibits bacterial growth and reduces experimental colitis in mice. Nutrients 12(4), 1122 (2020).
Cheng, S. C. et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 17(4), 406–413 (2016).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496(7444), 238–242 (2013).
Pan, T. et al. Immune effects of PI3K/Akt/HIF-1alpha-regulated glycolysis in polymorphonuclear neutrophils during sepsis. Crit. Care 26(1), 29 (2022).
Nuzzo, E. et al. Pyruvate dehydrogenase activity is decreased in the peripheral blood mononuclear cells of patients with sepsis. A prospective observational trial. Ann. Am. Thorac. Soc. 12(11), 1662–1666 (2015).
Mainali, R. et al. Dichloroacetate reverses sepsis-induced hepatic metabolic dysfunction. Elife 10, e64611 (2021).
Xun, Z. et al. Taurocholic acid inhibits the response to interferon-α therapy in patients with HBeAg-positive chronic hepatitis B by impairing CD8 T and NK cell function. Cell. Mol. Immunol. 18(2), 461–471 (2021).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in mice. Nature 487(7405), 104–108 (2012).
Zierer, J. et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging-Us 8(1), 77–94 (2016).
Kamada, T. & Otsuji, S. Lower levels of erythrocyte-membrane fluidity in diabetic-patients: A spin label study. Diabetes 32(7), 585–591 (1983).
Qiu, G. K. et al. Nontargeted metabolomics revealed novel association between serum metabolites and incident acute coronary syndrome: A Mendelian randomization study. J. Am. Heart Assoc. 12(13), e028540 (2023).
Jenniskens, M., Langouche, L., Vanwijngaerden, Y. M., Mesotten, D. & Van den Berghe, G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 42(1), 16–27 (2016).
Thomson, S. J. et al. ‘Liver function tests’ on the intensive care unit: A prospective, observational study. Intensive Care Med. 35(8), 1406–1411 (2009).
Mindikoglu, A. L. et al. Unique metabolomic signature associated with hepatorenal dysfunction and mortality in cirrhosis. Transl. Res. 195, 25–47 (2018).
Kim, T. S. & Choi, D. H. Liver dysfunction in sepsis. Korean J. Gastroenterol. 75(4), 182–187 (2020).
Sun, J. et al. Gut-liver crosstalk in sepsis-induced liver injury. Crit. Care 24(1), 614 (2020).
Miyamoto, Y. et al. A uremic toxin, 3-carboxy-4-methyl-5-propyl-2-furanpropionate induces cell damage to proximal tubular cells the generation of a radical intermediate. Biochem. Pharmacol. 84(9), 1207–1214 (2012).
Ottosson, F. et al. The inverse association between a fish consumption biomarker and gingival inflammation and periodontitis: A population-based study. J. Clin. Periodontol. 49(4), 353–361 (2022).
Acknowledgements
Thanks to all participants and researchers involved in this MR study. The authors also thank the UK Biobank Consortium for publishing the GWAS summary statistics.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 82371370, 82172144, and 82372156) and Excellent Doctoral Fund of Zhongnan Hospital of Wuhan University (ZNYB2023018).
Author information
Authors and Affiliations
Contributions
X.W. and X.S. were responsible for the study conception and study design. G.J. and J.Z. participated in data acquisition and study execution. H.L., Z.L., and M.C. analyzed the data and drafted the manuscript. M.Y. and H.G. contributed to the interpretation and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jing, G., Zuo, J., Liu, Z. et al. Mendelian randomization analysis reveals causal associations of serum metabolites with sepsis and 28-day mortality. Sci Rep 14, 11551 (2024). https://doi.org/10.1038/s41598-024-58160-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58160-1
- Springer Nature Limited