Abstract
Fish are poikilothermic vertebrates and their physiological activities are affected by water temperature. In recent years, extreme weather has occurred frequently, and temperature changes have adversely affected the growth of farmed fish. To explore the changes in gill tissue structure caused by changing the water temperature and the relationship between the intestinal microbiota and the Leiocassis longirostris host adaptation mechanism, gill tissue sections and intestinal microbial 16S rRNA amplicon sequencing were conducted under different temperature stress (low temperature 4 °C, normal temperature 26 °C and high temperature 32 °C). The results showed that heat stress and cold stress caused injury and swelling, terminal congestion, cell vacuolation, and necrosis of the gill tissue of L. longirostris. For intestinal microbiota, the abundances of Pseudomonadota and Bacillota increased at the cold stress, while the abundances of Fusobacteriota and Bacteroidota increased at the heat stress. The number of opportunistic bacteria, mainly Aeromonas and Acinetobacter, was the highest under cold stress. In addition, the richness of the intestinal microbiota decreased significantly at heat and cold stresses, while evenness increased. Prediction of intestinal microbiota function showed that most common functions, such as metabolism of cofactors and vitamins, energy metabolism and replication and repair, were decreased significantly at heat stress and cold stress, and phylogenetic relationship analysis revealed significant differences among the groups. In conclusion, the change of temperature altered the gill tissue structure, and affected the structure and homeostasis of the intestinal microbiota, thus affecting the survival time of L. longirostris, and cold stress had a greater effect than heat stress.
Similar content being viewed by others
Introduction
Leiocassis longirostris a freshwater fish in the family Bagridae. It is widely distributed in the Yangtze, Liao, and Huaihe Rivers of China. L. longirostris is a sought-after commercially important species1. However, overfishing, environmental pollution, construction of water conservancy projects, and habitat degradation and fragmentation of wild L. longirostris populations have contributed to sharp declines. Thus, artificial culture was initiated to alleviate the increasing demand for L. longirostris2.
The gastrointestinal tract is an important immune organ in fish and plays a very important role in the resistance to pathogens. This important function of the gut is largely the responsibility of the intestinal microbiota. Intestinal microorganisms form a large microbial population, and according to the state of the host organism, they are always in a dynamic change and mutually-beneficial symbiosis with the host3. Research on the function of the intestinal microbiota has become increasingly in-depth and specific. As an “extra organ” of fish, the intestinal microbiota directly or indirectly assists the body in various physiological and metabolic activities4. One of the most important functions is the metabolism of substances5. In addition, intestinal microbiota act as a natural biological barrier for the host. The microbial flora covers the surface of the intestinal mucosa, effectively reducing damage to intestinal epithelial cells caused by sudden external stimulation. When pathogenic bacteria enter the intestine with food, the intestinal microbiota is the first barrier encountered. Some microorganisms directly eliminate some pathogens, and the intestinal microbiota stimulates the intestinal mucosa to secrete bactericidal substances to maintain normal immune function6.
The differences in the composition of fish intestinal microbiota communities are caused by differences in feeding habits among fish7,8,9. However, changes in environmental temperature also have a significant impact on the composition of fish intestinal microbiota. Environmental temperature is an important abiotic variable, which affects the adaptation of the fish to the environment10. Changes in ambient temperature affect the abundance of the host intestinal microbiota and the concentrations of metabolites11. The number of intestinal microbiotas in red hybrid tilapia at 18 °C was higher than that at 26 °C12. The study results of Yoshimizu et al. showed that the community structure of the intestinal microbiota of fish varied to a certain extent in different seasons. The number of intestinal microbiotas was the lowest in winter and the highest in summer13. In addition, ambient temperature induces changes in the intestinal microbiota and affects host adaptation, including resistance to intestinal colonization, host energy and nutrient assimilation, and host immunity10. Animals living under extreme temperature conditions may have specific microbiota in their intestine, and these temperature-induced microbiota play an integral role in maintaining host health, growth, and development14.
The histological changes in the gills are widely used as a health status indicator in fish. The large surface area of the gills is in contact with the water, and histological and subcellular changes effectively reflect the degree of body injury15. Heat stress causes gill damage in Salmo salar and Paralichthys olivaceus, such as swelling of the lamella, high peeling, and rupturing of the epithelium16,17. Under the stress of high temperature and low temperature in Oryzias latipes, the gill morphology would change, different degrees of oxidative stress would be generated, and a large number of ROS would be released, thus inducing the apoptosis of gill tissue18. Although L. longirostris has a wide temperature adaptability range, it often experiences temperature stress during production. Therefore, studying the histological changes in gills and intestinal microbiota of L. longirostris under various temperature stress levels is significant in guiding production, and can provide a theoretical basis and preventive measures for production and breeding practices.
Materials and methods
Fish of the study
The experimental animals were L. longirostris bred by the Fisheries Research Institute of Sichuan Academy of Agricultural Sciences. L. longirostris at 3 months of age, similar body length and weight (body length, 10.45 ± 0.26 cm; body weight, 18.32 ± 1.11 g), normal swimming behavior and no physical injury was selected as the experimental fish. Before the formal experiment, L. longirostris were acclimated for 2 weeks with water temperature (26 ± 0.5) °C, dissolved oxygen ≥ 5 mg·L−1, 24 h aeration, 14 h light/10 h dark cycle, and fed twice a day (9:00 am and 5:00 pm). The daily feeding amount is 3% of the body mass of the fish, and the water is changed every 3 days.
Temperature stress experiment
L. longirostris can survive at 0–38 °C, but the optimum growth temperature is 25–28 °C19. Therefore, the experiment consisted of 3 groups, cold stress group (4 °C), heat stress group (32 °C) and normal temperature (26 °C) control group. In the control group, the cold and heat stress groups were subjected to gradient cooling or heating at a rate of 1 °C/h starting from 26 °C. Each group comprised of six replicates with 10 fish in each, and placed in incubators with constant temperature. Samples were collected after 12 h and 24 h of stress. In this experiment, H12, H24, C12, C24 and CK represent 12 and 24 h at 32 °C, 12 and 24 h at 4 °C and 24 h at 26 °C, respectively (Fig. 1).
Section of gill tissue
One L. longirostris was selected from each parallel of the three groups after 24 h of stress. After anesthetizing the fish in MS-222 solution, the gill tissue was dissected immediately and fixed overnight in a 4% volume fraction polyformaldehyde solution. After dehydration with gradient ethanol, the gill tissue was treated with xylene for 1 min and then immersed in wax for 3 h. The gill tissue was sliced with a Leica microtome at a thickness of 5 μm, and the slices were maintained overnight in a 37 °C dryer. The sections were taken for hematoxylin–eosin (H&E) staining20. Three stained gill plates were used from each group for analysis. At least five fields of view were observed for each section under an optical microscope (TS-200B, SDPTOP, Ningbo, China), and the most representative field of view was selected as the final result (Fig. 1).
Gut microbiome
After 12 h and 24 h under the different temperature stressors, six tails (two/parallel) were randomly selected from each group. After anesthesia, the spine was incised and the fish was euthanized in a sterile environment. The intestine was dissected and separated in normal saline. Prior to anatomical examinations, all fish specimens were administered a thorough anesthesia protocol in accordance with local standard practices. This procedure involved immersing the fish in a solution containing 100 mg/L of MS-222 until they exhibited no response upon manual tail manipulation, signifying a state of profound anesthesia. After documenting their weight and length measurements, the fish were euthanized via caudal venipuncture. The outer wall of the intestine was flushed with normal saline three times to remove the intestinal contents. Subsequently, the intestinal contents of two L. longirostris from the same aquarium were pooled into 1 sample, and six samples were used as parallel groups. The 16S rRNA amplicon sequencing was completed by Guangzhou Gidio Biotechnology Co., Ltd. (Guangzhou, China). Total genomic DNA was extracted using the FastDNA®SPIN Kit for Soil (MP Biomedical, Santa Ana, CA, USA). Genomic DNA integrity was determined by agarose gel electrophoresis. The concentration and purity of the genomic DNA were determined by Nanodrop 2000 and Qubit3.0 spectropheatometers. The 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′) primers were used to amplify the V3–V4 hypervariable region of the 16S rRNA gene. The sequencing was performed using an Illumina NovaSeq 6000 sequencer (Fig. 1).
Statistical analysis
To get high quality clean reads, raw reads were further filtered using FASTP21 (version 0.18.0). Paired end clean reads were merged as raw tags using FLASH22 (version 1.2.11) with a minimum overlap of 10 bp and mismatch error rates of 2%. Noisy sequences of raw tags were filtered under specific filtering conditions23 to obtain the high-quality clean tags. The clean tags were clustered into operational taxonomic units (OTUs) of ≥ 97% similarity using and UPARSE24 (version 9.2.64) pipeline. All chimeric tags were removed using and UCHIME algorithm10 and finally obtained effective tags for further analysis. The representative OTU sequences were classified into organisms by a naive Bayesian model using RDP classifier25 (version 2.2) based on SILVA database26 (version 138.1), with the confidence threshold value of 0.8.
The community composition stacked bar plot was visualized in the R project ggplot2 package (version 2.2.1)27. Biomarker features in each group were screened using LEfSe software (version 1.0)28, the randomforest package (version 4.6.12)29, the pROC package (version 1.10.0)30, and the labdsv package (version 2.0-1) in R31. The OTU rarefaction and rank abundance curves were plotted in the R ggplot2 package (version 2.2.1)27. The alpha index comparison between the groups was calculated using Welch’s t-test and the Wilcoxon rank test in the R vegan package (version 2.5.3)32. Sequence alignment was performed using Muscle (version 3.8.31) and the phylogenetic tree was constructed using FastTree (version 2.1). The weighted and unweighted unifrac distance matrix was generated with the GuniFrac package (version 1.0) in R. Sequence alignment was performed using Muscle (version 3.8.31) and the phylogenetic tree was constructed using FastTree (version 2.1). The weighted and unweighted unifrac distance matrix was generated using the GuniFrac package (version 1.0) in R33,34,35. The KEGG pathway analysis of the OTUs was inferred using PICRUSt (version 2.1.4)36. The microbiome phenotypes of the bacteria were classified using BugBase36,37,38.
Results
Changes in L. longirostris gill structure at different temperatures
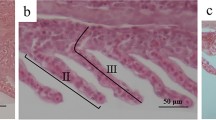
L. longirostris under cold stress remained at the bottom of the tank, moved slowly, and consumed less food. Under heat stress, L. longirostris swam frequently, their gills turned red, food intake decreased significantly, and no deaths were reported. The results of tissue sections showed that the gill filaments from the control fish had a normal structure, uniform color, regular arrangement of the layered structure, and normal morphology of the gill slices and epidermal cells (Fig. 2A). The structure of the gills changes under cold- and heat-stress. Under heat stress, the branchial lamella exhibited extensive edema, with highly exfoliated and ruptured epithelial cells, terminal hyperemia, cell vacuolation and necrosis, and some fusion of the branchial lamellae (Fig. 2B). In addition to swelling of epithelial cells and lamellar deformation, basal cell proliferation also occurred under cold stress (Fig. 2C). These results indicate that both high and low-temperature stressors cause structural changes in L. longirostris gills, which may affect survival.
Microstructure of L. longirostris gills at different temperatures. (A) represent the control group. (B) represent the heat stress group. (C) represent the cold stress group. Note: Red arrow point to swelling of epithelial cells; blue arrow point to lamellar deformation; black arrow point to basal cell hyperplasia. Scale bar: 100 μm.
Sequencing characteristics of the L. longirostris intestinal microbiota
We obtained 3,851,442 raw tags from the 30 fish samples with overlap assembly, and 3,837,327 clean tags after quality control. The remaining 3,543,079 effective tags were obtained after removing the chimeric tags detected in the clustering analogy pairs. The number of effective tags in all samples accounted for more than 85.0% of the number of original raw pair-end reads (Table 1). The rank abundance curves of all samples were obtained based on the OTU abundance of each sample and its ranking. Figure 3 shows the richness and evenness of the species composition in each sample.
Dilution curves for the Sob index of the 16S rRNA gene MiSeq sequences from the different temperature samples. Different colored lines represent different samples. The horizontal and vertical coordinates respectively represent the number of tags extracted and the number of tags extracted corresponding to the calculation of the diversity index value. CK control group, C12 12-h cold stress group, C24 24-h cold stress group, H12 12-h heat stress group, H24 24-h heat stress group.
Differences in L. longirostris intestinal microbiota composition at different temperatures
The top 10 dominant bacteria phyla in the intestinal microbiota of the 30 samples accounted for more than 99.93% of all sequence reads. Pseudomonadota dominated the intestinal microbiota from the treatment groups, all of which were more than 42.14%. The contents of Pseudomonadota and Bacillota increased significantly after the cold stress, while Bacteroidota content decreased significantly. In addition, Bacteroidota content increased significantly after the heat stress, while Pseudomonadota content decreased (Fig. 4A). The bacterial composition of the heat-stressed and CK groups was similar based on a bacterial community analysis at the genus level, including Cetobacterium and Plesiomonas, followed by Edwardsiella. Cetobacterium and Plesiomonas contents decreased significantly in the intestinal microbiota community after cold stress, while Aeromonas, Acinetobacter, Bacillus, and Psychrobacter contents increased significantly (Fig. 4B).
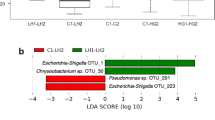
Changes in the intestinal microbiota composition after different temperature stressors. (A) Distribution of intestinal bacterial phyla in the experimental treatment and control groups. (B) Distribution of intestinal bacteria genera in the treatment and control groups. (C) Indicator analysis between the treatment groups. The size of the bubble represents the IndVal value of the species in the corresponding group, and the color of the bubble indicates group information. The larger the value, the more likely the species is an indicator species of that group.
According to the index analysis of the abundance and frequency of species in the samples among the cold-stressed groups, the heat-stressed groups and the CK group, the index values of Aeromonas in C24, Staphylococcus in C12, Plesiomonas in CK, Cetobacterium in H12 and Paraclostridium in H24 were the highest, indicating that these five genera may be C24, C12, CK, H12 and H24 indicator species, respectively (Fig. 4C).
Diversity analysis of the L. longirostris intestinal microbiota under different temperatures
Alpha diversity refers to the diversity within a particular environment or ecosystem. This index was mainly used to reflect the richness and evenness of species. Compared with the control group, the Chao1 diversity index of the L. longirostris intestinal microbiota showed no difference in C12 and H12 groups, but decreased in C24 and H24 groups (Fig. 5A). These results indicate that 24-h heat and cold stress decreased the richness of the L. longirostris intestinal microbiota. No significant difference in the Shannon diversity index was observed in the C24, C12 and H12 groups, but it increased significantly in the H24 group, indicating that the evenness of the intestinal microbiota improved significantly under 24-h heat stress (Fig. 5A). These results indicate that 24-h heat and cold stress reduced the richness of the L. longirostris intestinal microbiota.
Diversity of bacteria in the gut of the treatment groups. (A) Box chart of the inter-group differences in the Chao 1 and Shannon indices. (B) Principal Component Analysis (PCA) based on OUT abundance information of species. (C,D) Phylogenetic relationship among the genera of horizontal species. The phylogenetic tree was constructed with representative sequences of the genera of horizontal species. The colors of the branches and fan-shaped branches represent the corresponding gates, and the stacking histogram outside the fan ring represents the abundance of the genus in the samples.
The closer the samples were to each other, the more similar their microbiome structures were. Principal Component Analysis (PCA) based on the OTU abundance information of species found that the samples under different temperature stress were divided into the same group, in which the distance between the heat stress group and the control group was closer, while the distance between the cold stress group and the control group was farther, indicating that the intestinal microbiota of the heat stress group and the control group were more similar (Fig. 5B). In addition, according to the bacterial evolutionary tree analysis of C24 and H24 groups with large changes, the number of bacteria in Acinetobacter, Citrobacter, Aeromonas, Providencia, and Vagococcus in the C24 group was significantly higher than that in the CK group, while the number of bacteria in Cetobacterium, Erwinia, and Turicibacter was significantly lower than that in the CK group (Fig. 5C). The numbers of Providencia, Citrobacter, Vagococcus, and Kurthia were significantly higher in the H24 group than those in the C group, and the numbers of Synechococcus, Lactobacillus, and Bacillus were significantly lower than those in the CK group (Fig. 5D).
Prediction of intestinal microbiota function of L. longirostris under different temperature stressors
Based on the PICRUSt functional prediction, the KEGG functional abundance of the sample is obtained. According to the KEGG database functional prediction analysis, all the intestinal microbiota samples in the CK, C24 and H24 groups are mainly involved in the metabolism of cofactors and vitamins, carbohydrate metabolism, amino acid metabolism, metabolism of terpenoids and polyketides, metabolism of other amino acids, energy metabolism and lipid metabolism (Fig. 6A). The KEGG database shows that there were differences in the major metabolic functions between the different treatment groups. Overall, the metabolism of cofactors and vitamins, carbohydrate metabolism, metabolism of terpenoids and polyketides, energy metabolism, replication and repair and glycan biosynthesis and metabolism were significantly lower in the C24 group than in the CK group (p < 0.05). The functions of replication and repair and glycan biosynthesis and metabolism under the H24 group were significantly lower than those of the CK group (p < 0.05) (Fig. 6B). The analysis of the bacterial interaction showed that Pseudomonadota was the most important bacteria in the L. longirostris intestinal microbiota and was related to most of the other bacteria (Fig. 6C).
Functional prediction of the intestinal microbiota. (A) Histogram of the functional abundance of each sample. The ordinate represents the percentage of relative abundance (%). (B) Tukey Honestly Significant Difference (HSD) rank sum test of functional significance difference between different groups. The ten functions predicted to have the highest abundance were compared for differences between the groups, with an asterisk indicating significance. (C) Network diagram of the microbial community structure. The circles represent the bacteria, the lines represent the relationships between different bacteria, with red representing positive correlations and blue representing negative correlations.
Ethical approval
All animal handling procedures were approved by the Animal Care and Use Committee of the Fisheries Research Institute, Sichuan Academy of Agricultural Sciences (Chengdu, China), following the recommendations in the ARRIVE guidelines, under permit number 20210307001–5. At the same time, all methods were carried out by relevant guidelines and regulations.
Discussion
Too high or too low of a water temperature destroys homeostasis and causes a stress response. A continuous stress state destroys the defense system, eventually leading to the death of the fish39. Gills are the most important functional organs of fish, as they are involved in respiration, osmosis, and nitrogen excretion40. Cold stress and hypoxia change the gill morphology of Carassius auratus by decreasing cell proliferation and inducing apoptosis41,42. In addition, a study on gill tissue apoptosis in Danio rerio and Oreochromis niloticus under cold stress of 8 °C for 12 h reported that apoptosis of the gill was the most obvious among the eight organs in which it was detected, suggesting that the gills are sensitive organs to perceive external changes43. In addition to cold stress, heat stress increases peroxide content in mitochondria and intracellular reactive oxygen species (ROS), leading to oxidative damage44. The gills of pikeperch are sensitive to heat stress and pikeperch actively responds to heat stress through regulating the antioxidant system, and the expression of Hsp genes and genes involved in energy metabolism45. This study observed swelling of gill epithelial cells under the cold- and heat-stress. This change in gill tissue structure can prevent stress factors from penetrating deep tissues and has a protective effect on fish gills. However, it comes at the cost of reduced gas and material exchange46. Furthermore, the fusion of gill plates can directly decrease the respiratory surface area, which reduces the impact of stress factors on the gill interior47. However, this reduction in respiratory surface area may result in issues such as hypoxia and respiratory failure. The degree of apoptosis of gill cells under cold stress and heat stress and the reasons requires further study.
The change in water temperature also significantly affects the composition of the intestinal microbiota of fish. Pseudomonadota, Bacillota, Fusobacteriota, and Bacteroidota dominate the gut microflora of most marine and freshwater fish48,49,50. Our study indicated that in the gut microbiome of L. longirostris, the dominant flora of different temperature treatments were relatively consistent, and included Proteobacteria, Firmicutes, and Fusobacteriota. These three phyla have also been found to be dominant in the intestinal tracts of many other marine and freshwater fish specie48,51. In this study, although the different temperature treatments did not change the dominant bacteria in L. longirostris, they affected their relative abundances. The abundances of Pseudomonadota and Bacillota increased significantly in the cold stress group, while the abundances of Fusobacteriota and Bacteroidota increased significantly in the heat stress group. It has been reported that an increase in the abundance of Pseudomonadota in the host intestine may be caused by pathogenic bacteria52. Aeromonas, Acinetobacter, and Cetobacterium are Pseudomonadota, whereas Aeromonas and Acinetobacter are Gamma-pseudomonadota. Widely distributed in nature, these are typical opportunistic pathogens, which may cause many diseases such as enteritis and septicemia in humans and animals53,54. Bacillota produces short-chain fatty acids in the gut, and a higher abundance of Bacillota helps to obtain more energy from the diet55. Most of the bacteria in Fusobacteriota and Bacteroidota are typical anaerobic bacteria. In general, anaerobic bacteria and the bioprotective film formed by intestinal mucosa are the first barrier against pathogenic bacteria56. We speculate that the relative abundance of opportunistic pathogens in the intestinal tract of L. longirostris in the cold stress group was higher, and the resistance to diseases was relatively weaker when the internal environment was disturbed by environmental stress.
Alpha and beta diversity are important parameters for evaluating the level of organization of the community structure57. The higher the Chao1 index, the richer the community, and the larger the Shannon index, the greater the uniformity of the community. The species richness values of the temperature treatment groups were significantly lower than that of the control group, but the evenness values were slightly higher than that of the control group, indicating that the temperature treatments affected the richness and evenness of the intestinal microbiota. Beta diversity is an algorithm that converts the evolutionary relationship and abundance information of each sample sequence into an inter-sample distance, which directly reflects the community differences between the sample groups57. In this study, the two treatment groups and the control group were divided into three subgroups with statistical differences. Intestinal microbial diversity is closely related to the health of the body, and the decrease of intestinal microbial diversity may induce many diseases58. In addition, evidence shows that the functional composition of the microbial community is closely related to environmental factors, and different environments affect differences in microbial community functions59,60. Bacterial interaction analysis also found that Fusobacteriota and Proteobacteria were associated with most bacteria, which was consistent with the results of previous studies61. The functional prediction results showed that the functional abundance of the intestinal microbiota decreased significantly under the different temperature stressors. The precise causes of this phenomenon require further examination through analysis of the transcriptome and metabolome. In general, the intestinal microbial diversity of L. longirostris decreased, the abundance of pathogenic bacteria was higher, and intestinal functioning decreased in the cold- and heat-stress environments, particularly in the cold stress environment. Therefore, the L. longirostris intestinal microbiota detected in high or low temperature environment is not conducive to growth and resistance to disease, and the appropriate environmental temperature provides a better living environment for aquaculture animals.
Conclusion
In this study, gill tissue sections and 16S rRNA sequencing technology were used to investigate the effects of different temperatures on the gill tissue structure and intestinal microbial homeostasis of L. longirostris. The cold- and heat-stress environments caused damage, swelling, terminal congestion, cellular vacuolation, and necrosis in the gills of L. longirostris. The stress causing ambient temperatures changed the composition of the intestinal microbiota, significantly affected the intestinal microbiota richness, and significantly reduced intestinal microbial function in L. longirostris. Based on these results, a suitable ambient temperature can maintain a rich gut microbiota, which has a stronger ability to resist disease for L. longirostris.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive62 in National Genomics Data Center63, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA011773) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
References
Xiao, M. S., Cui, F., Kang, J. & Ma, Y. H. Analysis on sequence polymorphism of the mitochondrial DNA control region and population genetic diversity of the cultivated and natural Chinese longsnout catfish (Leiocassis longirostris). Acta Hydrobiol. Sin. 37, 10. https://doi.org/10.7541/2013.90 (2013).
Cao, J., Zhang, F. P. & Song, J. Comparative analysis of nutrient composition of muscles of farmed and wild Leiocassis longirostris. Food Sci. 36, 6 (2015) (in Chinese).
Rawls, J. F., Samuel, B. S. & Gordon, J. I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. 101, 6. https://doi.org/10.1073/PNAS.0400706101 (2004).
Li, X. M. et al. Gut microbiota contributes to the growth of fast-growing transgenic common carp (Cyprinus carpio L.). PLoS One 8, 7. https://doi.org/10.1371/journal.pone.0064577 (2013).
Gould, A. L. et al. Microbiome interactions shape host fitness. Proc. Natl. Acad. Sci. 115, 10. https://doi.org/10.1073/pnas.1809349115 (2018).
Kabouridis, P. S. et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85, 7. https://doi.org/10.1016/j.neuron.2014.12.037 (2015).
Liu, H. et al. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci. Rep. 6, 11. https://doi.org/10.1038/srep24340 (2016).
Ni, J. J., Yu, Y. H., Zhang, T. & Gao, L. Comparison of intestinal bacterial communities in grass carp, Ctenopharyngodon idellus, from two different habitats. Chin. J. Oceanol. Limnol. 30, 757. https://doi.org/10.1006/jfbi.1996.0339 (2012).
Wu, S. G. et al. Composition, diversity, and origin of the bacterial community in grass carp intestine. PloS One 7, e30440. https://doi.org/10.1371/journal.pone.0030440 (2012).
Sepulveda, J. & Moeller, A. H. The effects of temperature on animal gut microbiomes. Front. Microbiol. https://doi.org/10.3389/fmicb.2020.00384 (2020).
Osadchiy, V., Martin, C. R. & Mayer, E. A. The gut-brain axis and the microbimoe: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 17, 11. https://doi.org/10.1016/j.cgh.2018.10.002 (2019).
Leamaster, B., Walsh, W. A. & Brock, J. A. Cold stress induced changes in the aerobic heterotrophic gastrointestinal tract bacterial flora of red hybrid tilapia. J. Fish Biol. 50, 11. https://doi.org/10.1006/jfbi.1996.0339 (2005).
Yoshimizu, M., Kimura, T. & Sakai, M. Studies on the intestinal microflora of salmonids, II: Effects of artificial transplanting from fresh water into sea water on the intestinal microflora of feeding and non-feeding fish. Bull. Jpn. Soc. Sci. Fish. 42, 11. https://doi.org/10.2331/suisan.42.863 (1976).
Liu, X., Sha, Y. Z., Dingkao, R. Q., Zhang, W. & Luo, Y. Z. Interactions between rumen microbes, VFAs, and host genes regulate nutrient absorption and epithelial barrier function during cold season nutritional stress in Tibetan sheep. Front. Microbiol. https://doi.org/10.3389/fmicb.2020.593062 (2020).
Sales, C. F., Santos, K. P. E. D., Rizzo, E. & Elizete, R. M. A. Proliferation, survival and cell death in fish gills remodeling: From injury to recovery. Fish Shellfish Immunol. 68, 9. https://doi.org/10.1016/j.fsi.2017.07.001 (2017).
Moltumyr, L., Gismervik, S., Gu, J., Gsnes, S. & Stien, L. H. Does the thermal component of warm water treatment inflict acute lesions on Atlantic salmon (Salmo salar). Aquaculture 532, 12. https://doi.org/10.1016/j.aquaculture.2020.736048 (2021).
Liu, Y. F., Ma, D. Y., Zhao, C. Y., Wang, W. Q. & Li, J. Histological and enzymatic responses of Japanese flounder (Paralichthy olivaceus) and its hybrids (P. olivaceus♀×P. dentatus♂) to chronic heat stress. Fish Physiol. Biochem. 40, 11. https://doi.org/10.1007/s10695-013-9903-6 (2014).
Hu, L. H., Wang, Y., Wang, H. M. & Chen, L. B. Effects of different temperature stress on gill apoptosis of medaka Oryzias latipes. J. Dalian Ocean Univ. 36, 8. https://doi.org/10.16535/j.cnki.dlhyxb.2021-053 (2021) (in Chinese).
Chen, P. Artificial high-yield culture technique of Leiocassis longirostris. Mod. Agric. Sci. Technol. 317–319 (2009) (in Chinese).
Lu, Y. F. et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 50, 7. https://doi.org/10.1021/acs.est.6b00183 (2016).
Chen, S. F., Zhou, Y. Q., Chen, Y. R. & Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. BioRxiv https://doi.org/10.1101/274100 (2018).
Magoc, T. & Salzberg, S. L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 7. https://doi.org/10.1093/bioinformatics/btr507 (2011).
Bokulich, A. N. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 3. https://doi.org/10.1038/nmeth.2276 (2013).
Edgar, R. C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 3. https://doi.org/10.1038/nmeth.2604 (2013).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 7. https://doi.org/10.1128/AEM.00062-07 (2007).
Pruesse, E. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 9. https://doi.org/10.1093/nar/gkm864 (2015).
Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 3, 6 (2011).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. https://doi.org/10.1186/gb-2011-12-6-r60 (2011).
Liaw, A. & Wiener, M. Classification and regression by randomForest. RNews 2, 5 (2002).
Robin, X. et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. https://doi.org/10.1186/1471-2105-12-77 (2011).
Roberts, D. W. & Roberts, M. D. W. Package ‘labdsv’. Ordination and Multivariate (2016).
Oksanen, J., Blanchet, F. G. & Kindt, R. Vegan: Community ecology package. R package version 1.17-4. Acesso em 23. http://cran.r-project.org (2010).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 6. https://doi.org/10.2460/ajvr.69.1.82 (2004).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS One https://doi.org/10.1371/journal.pone.0009490 (2010).
Lozupone, C. & Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 9. https://doi.org/10.1128/AEM.71.12.8228-8235.2005 (2005).
Langille, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 8. https://doi.org/10.1038/nbt.2676 (2013).
Aßhauer, K. P., Wemheuer, R. & Daniel, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31, 3. https://doi.org/10.1093/bioinformatics/btv287 (2015).
Ward, T. et al. BugBase predicts organism level microbiome phenotypes. BioRxiv https://doi.org/10.1101/133462 (2017).
Bonga, S. E. W. The stress response in fish. Physiol. Rev. 77, 2. https://doi.org/10.1152/physrev.1997.77.3.591 (1997).
Evans, D. H., Piermarini, P. M. & Choe, K. P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 85, 81. https://doi.org/10.1152/physrev.00050.2003 (2005).
Sollid, J., De, A. & Gundersen, K. Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J. Exp. Biol. 206, 7. https://doi.org/10.1242/jeb.00594 (2003).
Nilsson, G. E. Commentary: Gill remodeling in fish—A new fashion or an ancient secret?. J. Exp. Biol. 210, 107. https://doi.org/10.1242/jeb.000281 (2007).
Hu, P. et al. Transcriptome comparison reveals a genetic network regulating the lower temperature limit in fish. Sci. Rep. https://doi.org/10.1038/srep28952 (2016).
Kikusato, M., Yoshida, H., Furukawa, K. & Toyomizu, M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 52, 6. https://doi.org/10.1016/j.jtherbio.2015.04.005 (2015).
Chen, Y. N. et al. Effects of heat stress on histopathology, antioxidant enzymes, and transcriptomic profiles in gills of pikeperch Sander lucioperca. Aquaculture 534, 736277. https://doi.org/10.1016/j.aquaculture.2020.736277 (2021).
Camargo, M. M. P. & Martinez, C. B. R. Histopathology of gills, kidney and liver of a neotropical fish caged in an urban stream. Neotrop. Ichthyol. 5, 327–336. https://doi.org/10.1590/S1679-62252007000300013 (2007).
Mohamed, F. Histopathological studies on some organs of Oreochromis niloticus, Tilapia zillii and Synodontis schall from El-Salam Canal Egypt, Egypt. Egypt. J. Aquat. Biol. 7, 99–138. https://doi.org/10.21608/EJABF.2003.1770 (2003).
Llewellyn, M. S., Boutin, S., Hoseinifar, S. H. & Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 5, 17. https://doi.org/10.3389/fmicb.2014.00207 (2014).
Wu, S. G. et al. Microbial diversity of intestinal contents and mucus in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 303, 1–7. https://doi.org/10.1016/j.aquaculture.2009.12.025 (2010).
Zhu, H. J. et al. Physiological and gut microbiome changes associated with low dietary protein level in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) determined by 16S rRNA sequence analysis. Microbiol. Open https://doi.org/10.1002/mbo3.1000 (2020).
Yan, S., Zheng, B. G., Guo, F. L. & Li, M. F. Gut microbiota of red swamp crayfish Procambarus clarkii in integrated crayfish-rice cultivation model. AMB Express https://doi.org/10.1186/s13568-019-0944-9 (2020).
Nelson, A. M. et al. Disruption of the human gut microbiota following norovirus infection. Plos One 7, e48224. https://doi.org/10.1371/journal.pone.0048224 (2012).
Shotts, E. B. & Rimler, R. Medium for the isolation of Aeromonas hydrophila. Appl. Microbiol. 26, 4. https://doi.org/10.1128/am.26.4.550-553.1973 (1973).
Zhai, P. P., Wu, Y. Q. & Lu, J. Progress of study on Acinetobacter classification. Electron. J. Emerg. Infect. Dis. 26, 9. https://doi.org/10.19871/j.cnki.xfcrbzz.2020.01.012 (2020) (in Chinese).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Grodon, J. I. Microbial ecology: Human gut microbes associated with obesity. Nature 444, 2. https://doi.org/10.1038/4441022a (2006).
Wen, J. & Sun, X. F. Research progress on intestinal microecological regulation of aquatic animals. Feed Res. 3, 1 (2009) (in Chinese).
Willis, K. J. & Whittaker, R. J. Ecology Species diversity–scale matters. Science 295, 4. https://doi.org/10.1126/science.1067335 (2002).
Fergus, S. Probiotics in perspective. Gastroenterology 139, 5. https://doi.org/10.1053/j.gastro.2010.10.025 (2010).
Gibbons, S. M. Microbial community ecology: Function over phylogeny. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-016-0032 (2017).
Louca, S., Parfrey, L. W. & Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 353, 6. https://doi.org/10.1126/science.aaf4507 (2016).
Yukgehnaish, K., Kumar, P., Sivachandran, P., Marimuthu, K. & Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 12, 1903–1927. https://doi.org/10.1111/raq.12416 (2020).
Chen, T. T., Chen, X., Zhang, S. S., Zhu, J. W. & Zhao, W. M. The genome sequence archive family: Toward explosive data growth and diverse data types. Genomics Proteomics Bioinform. https://doi.org/10.1101/2021.06.29.449849 (2021).
Memberspartners, C. N. Database resources of the national genomics data center, China national center for bioinformation in 2022. Nucleic Acids Res. 50, 12. https://doi.org/10.1093/nar/gkab951 (2022).
Funding
This research was supported by The National Key Research and Development Program of China (2022YFD2400903), The China Agriculture Research System of MOF and MARA (CARS-46), Technology Program of Sichuan Academy of Agricultural Sciences (1 + 9KJGG004), and The Sichuan Freshwater Fish Innovation Team of the National Modern Agricultural Industrial Technology System.
Author information
Authors and Affiliations
Contributions
Z.Z.M., and L.Q. conceived and designed research. Z.Z.M., Z.H., K.H.Y., M.C.Y., W.X.Y., and Z.J. conducted experiments. Z.Z.M., Z.L., H.Z.P., D.Y.L., and L.Q. analyzed data. Z.Z.M., and L.Q. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Z., Zhao, H., Wang, X. et al. Effects of different temperatures on Leiocassis longirostris gill structure and intestinal microbial composition. Sci Rep 14, 7150 (2024). https://doi.org/10.1038/s41598-024-57731-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57731-6
- Springer Nature Limited