Abstract
The cerebral arteries, specifically the anterior cerebral artery (ACA) and posterior cerebral artery (PCA), work together with the smaller calibre arteries to provide effective communication between the anterior and posterior circuits of the brain via the circle of Willis (CoW). Morphologic variations of the cerebral arteries and the CoW may alter blood flow to the brain, resulting in intracranial vascular disorders associated with stroke, and aneurysms. This study aimed to document the morphology of the cerebral arteries and the CoW in the South African population. Two hundred and thirty-nine computed tomography angiography scans were assessed. Cerebral arteries and CoW normal morphology and variations were classified as complete, absent, or hypoplastic. The ACA A1 was absent in 4.91%, hypoplastic in 30.40%, fenestrated in 1.06%, and typical in 63.6%. The ACA A2 was absent in 0.42%, hypoplastic in 26.28%, and typical in 69.44%. We found triple ACA A2 in 2.98%, azygos in 1.28% and fenestrated in 1.28%. The middle cerebral artery (MCA) was hypoplastic in 7.35% and typical in 92.64%. The PCA was hypoplastic in 28.74% and typical in 71.25%. Knowledge of the configuration of the CoW plays a significant role in guiding therapeutic decision-making in treating various neurovascular pathologies.
Similar content being viewed by others
Introduction
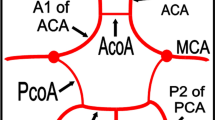
The cerebral arteries and their branches perfuse the cerebral cortex and, most importantly, form the larger arteries of the circle of Willis (CoW). Specifically, the ACA and PCA work together with the smaller calibre arteries (anterior and posterior communicating arteries) to provide effective communication between the anterior and posterior circuits of the brain. The CoW is a vascular network formed at the base of the skull in the interpeduncular fossa1. It is classically described as a symmetrical polygon derived from anastomoses between branches of the internal carotid arteries (ICA) and vertebral arteries (VA)2. The anterior cerebral (ACA), posterior cerebral (PCA), anterior communicating (AComA), and posterior communicating arteries (PComA) constitute the CoW. The internal carotid artery also participates in the construction of the CoW. This configuration allows collateral blood flow and pressure equalization3. The AComA and PComA are important components of the CoW as they act as collateral channels to stabilize blood flow between the two hemispheres1. Adequate collateral circulation across the vessels of CoW will reduce the risk of transient ischemic attack and ischemic stroke. Similarly, a complete CoW has been associated with reduced stroke severity, recurrence, death, and better recovery4.
The presence of variation in the configuration of the cerebral arteries that formed the CoW has been associated with cerebrovascular risk factors and intracranial vascular disorders, such as stroke, aneurysms, and white matter hyperintensities5,6,7. The extent of variation of these arteries is not predictable and may include but is not limited to duplication, absence or aplasia, fenestration, hypoplasia, and accessory vessels1,8,9. Another rare variation is the azygos ACA. Azygos ACA has been linked with developmental disorders such as holoprosencephaly, neuronal migration anomalies, and aneurysm formation10. These morphologic variations may modify the natural, symmetrical geometry of CoW and thus counterbalance the haemodynamics of blood flow, resulting in hypoperfusion in some parts of the brain. Some variations have been identified as risk factors for acute ischemic stroke or related to a neurological disease such as migraine5,10. For instance, changes in the diameter of some arterial segments of the CoW due to hypoplasia have been observed in the case of ischemic cerebral accidents11. In addition to the associated pathologies, variations have surgical implications. It is important to determine the adequacy of the collateral circulation when planning for procedures to treat intracranial vascular pathologies, which may involve ligations or parent vessel occlusion.
In recent times, most intracranial vascular pathologies have been treated using endovascular procedures, which require knowledge of detailed anatomy and the extent of variation. Prevalence of incomplete or atypical CoW has been reported in various population groups using different image modalities4,5,12,13. Previous reports from the South African population used cadaveric samples with a limited sample size14. Due to the multiracial composition of the South African population, it is crucial to consider the racial groups to provide detailed information on the configuration of the cerebral arteries and CoW in this region. Racial/ethnic differences associated with the incidence of vascular variation have been identified to contribute partially to the differences in the incidence of some cerebrovascular diseases and cerebral vascular morbidity1,15. In the present study, we assessed the morphology and described the variations of the cerebral arteries and the CoW in South African patients using multidetector computed tomography angiography (MDCTA). We also investigated the influence of demographic factors such as age, gender, and race. Such information is essential for diagnosis and treatment using endovascular procedures and surgical interventions.
Materials and method
Patient population
This study is a retrospective observational review of 239 MDCTA images of South African patients. The images were obtained from a private hospital in Durban, South Africa, between January 2009 and September 2019. The Biomedical Research Ethics Committee of the University of KwaZulu-Natal approved the study (BREC/00004487/2022) and waived the need for informed consent as this study utilized retrospective chart analyses. All the images were anonymized, with no patient contact or information. All methods were carried out following relevant guidelines and regulations. Exclusion criteria included MDCTA scans that showed no clarity of the cerebral arteries and CoW segments, scans with motion artifacts or poor-quality imaging, and scans performed on foreign patients. The angiographies were from 141 males (59%) and 98 females (41%). The age range of the patients is between 19 and 105 years. The average age of the patients is reported as mean (standard deviation) 65.7 (15.93). Three population groups were included in the present study: Caucasian 139 (58.16%), Indian 66 (27.61%), and Black 34 (14.23%). Race was defined according to the guidelines outlined in the modern systems of racial classification in the Republic of South Africa16.
CTA imaging protocol
The imaging examination was performed on a 64-detector row computed tomography (CT) scanner (Lightspeed CT, GE Healthcare Medical Systems, Milwaukee, WI, USA) with the scanning protocol as follows: 120 kVp, 697 mAs, beam collimation 64 × 0.625 mm, gantry rotation time 0.4 s, section thickness of 0.625 mm, pitch 0.969:1 and reconstruction interval of 0.625 mm. During the procedure, 80 mL of non-ionic iodinated contrast was infused, followed by 40 mL saline, and injected via a double power injector (Medex flowSens, Geubert USA) into the patient’s antecubital vein (4 mL/s)16.
Imaging reconstruction
Postprocessing of three-dimensional images was performed by using a multiplanar reformation (MPR), maximum intensity projection (MIP), multiplanar reconstruction (MPR), and volume rendering (VR) algorithms. The volumetric MDCTA data sets were processed on Advanced Workstation 4.2, (GE Healthcare, Milwaukee, USA)16. The CTAs were performed for diagnostic purposes in the context of various cerebrovascular accidents or diseases. Some of the common clinically diagnosed neurological pathologies include transient ischemic attack (TIA), infarction, unilateral body weakness, aneurysm, and ataxia. While some diseases have been linked with vascular variations, the incidence of variation can also be asymptomatic. Therefore, in some cases, the suspected diseases were not found on CT angiography; thus, some materials in this study were derived from a healthy population. Images were analysed using the Picture Archiving Communication System (PACS Syngo Plaza version VB30D https://doclib.siemens-healthineers.com/home). Each radiological image was evaluated for the following parameters, and variables were recorded:
-
1.
The morphology of the following cerebral vessels: the ACA, MCA, and PCA.
-
2.
The ACA, MCA, and PCA diameters were measured using PACS to determine the distance between two points (Fig. 1). Measurements were taken at the maximum part of the segment (the point where the segment was the widest in diameter). Each measurement was taken three times to reduce error, and the average values were used for the statistical analysis.
To be consistent with previous studies2,17, vessels with diameters less than one millimeter (1 mm) were classified as hypoplastic (Fig. 4). In the present study, CoW with complete arterial segments were defined as complete CoW (Fig. 1). The accuracy and repeatability of the measurements were determined by random sampling of 25 scans, and a second observer took measurements for inter-observer reliability testing.
Statistical analysis
Statistical analysis was conducted using SPSS version 28 (SPSS Inc., Chicago, IL, USA https://www.ibm.com/support/pages/ibm-spss-statistics-server280x), and p-values less than 0.05 were considered statistically significant. The distribution of variables was tested using the Kolmogorov–Smirnov test. Because some of the variables are not normally distributed, while some are normally distributed, both parametric and non-parametric tests were used. The Wilcoxon Signed Rank test was used to compare paired samples (gender). The Kruskal–Wallis test was used to determine statistically significant differences in the dependent variables between the three racial groups, and the Chi-square test was used for categorical variables. Some continuous variables are presented as mean (interquartile range), age was presented as mean ± standard deviation, and the categorical variables are represented by a number (N) and percentage. The interclass coefficient correlation (ICC) was used to examine the reliability of measurements.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The design was approved by the Institutional Review Board/Ethics Committee (Biomedical Research Ethics Committee of the University of KwaZulu-Natal with ethical No: BREC/00004487/2022).
Results
Morphology
A complete CoW was seen in 74.23% and variations in 10.84%. The ACA, MCA, and PCA were assessed by laterality for incidence of variations. According to Cilliers et al., the segments of the ACA were described as ACA A1 (precommunicating) and ACA A2 (postcommunicating)18. The most reported variation is hypoplasia affecting the three cerebral arteries. This is followed by absence, triple, fenestration, and azygos ACA, making the ACA the most variable of the three arteries (Figs. 2, 3, 4). Detailed results of the morphology are summarized in Table 1. Regarding the influence of demographic factors on the incidence of variation in the morphology of the cerebral arteries, the parameters of the segments of the arteries were contrasted against age, sex, and race (Table 2, 3, 4). A significant difference was noted in the incidence of variation across the age group on the right involving only ACA A1 (Table 2). A partial significant difference between genders was registered involving only the ACA (A1 and A2 segments) (Table 3). The incidence of the MCA and PCA hypoplasia showed a significant difference across the racial groups (Table 3).
Diameter
The ICC for intra-observer reliability testing ranged between 86 and 97% for ACA, MCA and PCA. ICC ranged between 84 and 96% for inter-observer reliability testing for ACA, MCA, and PCA.
The average diameter of ACA A1 (left-L and right-R), ACA A2 (L and R), MCA (L and R), and PCA (L and R) were summarized in Table 5. The average diameter of each of the arteries was contrasted against gender (Table 6), race (Table 7), and age (Table 8) to determine if these demographic factors influenced the size of the arteries. Cerebral arteries with pathological vascular changes such as aneurysms were excluded from the study as this may influence the average diameter values during analysis.
Discussion
Arteries of the CoW provide collateral circulation and compensation from arteries of the contralateral side in cases of diseases or injury to any of the arteries. Morphologic variations of the cerebral arteries contributing to the CoW have been associated with an increased risk of vasospasm, subarachnoid haemorrhage, and acute ischemic stroke16. Authors have reported disparity in the prevalence of these variations across different population groups. In a recent meta-analysis, authors have hypothesized that the prevalence of atypical CoW may be as high as 68%2. Also, using human embryo samples, some researchers have reported variation in 85% of the total samples and suggested that variations are congenital and are present from the initiation of CoW formation during embryogenesis19. However, other researchers have reported that the complete or classical text-book described variant of the CoW is the most common in their subjects14,20. Our findings support the latter report as we found complete CoW in 74% of the patients. Variations such as absence, hypoplasia, duplication, fenestration, accessory vessels, and asymmetry of the arteries have been associated with the alteration of blood flow to the brain tissue and thus enhance vascular diseases1,7. For instance, asymmetric CoW configurations have been linked with an increased risk of aneurysm rupture21.
The available evidence in the literature from original and review studies using imaging and cadaveric samples has shown that the PcomA hypoplasia/aplasia is the most frequently reported variation of the CoW2,22,23. This is followed by the AComA hypoplasia23. This situation is responsible for the prevalence of incomplete CoW, as reported by most authors. Despite the non-invasive nature of the CTA and its capacity to display intricate vascular architecture, it has technical limitations in the adequate assessment of arteries with small calibers, such as the communicating arteries. Some authors have hypothesized that CTA may not provide a good image analysis of vessels below 0.6 mm diameter22. Thus, most studies that used CTA have reported a high prevalence of absent/hypoplastic/aplasia or invisible PcomA23,24. The major role of the communicating arteries is to redistribute blood between adjacent arteries of larger caliber22. Therefore, it is sometimes common for the communicating arteries to have smaller diameters, and they have been reported with a high prevalence of hypoplasia/aplasia. This is one of the reasons why a complete CoW is rarely seen on radiographs. Similarly, in the present study, the authors could not investigate each arterial segment of the CoW. The AComA and PComA are not clearly seen in some images and are therefore excluded from the analysis.
The most prevalent variation in the present study is the hypoplasia of the ACA (Table 1). Hypoplasia of the ACA A1 segment may significantly disturb perfusion relationships in the AComA area. This may be due to the suspected stress burden at the junction angle between ACA A1 and ACA A2. This alteration in the blood flow pattern may prompt the formation of an aneurysmal sac21. Another rare variation of the ACA is the azygos ACA, in which two ACA A1 segments of both hemispheres form a single ACA A2 trunk without an AComA8. The prevalence of this variation ranges from 1 to 2.3%7,8,25,26. The findings from the present study are similar to the previous reports, as we found azygos ACA A2 in 1.28% of the patients. Azygos ACA has been identified as a predictor of bilateral frontal strokes and midline central nervous system malformations, such as agenesis of the corpus callosum, holoprosencephaly, and intracranial arteriovenous malformation27,28. In contrast, some researchers have suggested that there is no direct evidence of any increased incidence of stroke associated with azygos ACA. However, symptoms resulting from occlusion of azygos ACA can be unique because it can cause ischemic infarction in both cerebral hemispheres and the corpus callosum28. Furthermore, saccular aneurysms of the azygos ACA are relatively common, with a prevalence rate between 13 and 71%27. The increased susceptibility for aneurysm formation may be attributed to the hemodynamic stress of heightened blood flow in the single azygos ACA, which is usually paired28.
The Triplicate A2 segment of the ACA, or “triple-A2”, is another uncommon anatomical variant suspected to mainly result from the persistence of the embryonic median artery of the corpus callosum29. The prevalence of triple-A2 in the general population without intracranial pathologies such as aneurysms and infarctions is between 1 and 4%25,29,30,31. Similar to our report, we registered triple-A2 in approximately 3% of the patients using MDCTA. On the contrary, Ferre and co-authors, using a similar image modality, reported a prevalence as high as 13%32. This may be due to selection bias; according to the authors, approximately 90% of the study population had aneurysms. Triple-A2 has been associated with a significantly higher risk of AComA aneurysms29. Knowledge of the triple-A2 variant in the setting of an AComA aneurysm is critical when planning and executing endovascular and open surgical treatment. If the third A2 segment is not anticipated and protected, it may be accidentally occluded during treatment, leading to neurologic complications29.
Fenestration is another interesting variation of the ACA commonly encountered in intracranial circulation. This phenomenon is characterized by the division of the lumen of an arterial segment into two distinct endothelial-lined parallel channels, which may or may not share a common adventitia28. The prevalence of ACA fenestration has been reported between 0 and 4%25,33,34. In agreement with previous reports, we found fenestrated ACA A1 and ACA A2 segments in 1.06% and 1.28% of the patients, respectively. Fenestrations of intracranial arteries are sometimes asymptomatic; however, they have been linked with a high tendency of developing aneurysms. Histological findings have revealed reduced smooth muscle and collagen fibres at the proximal and distal portions of the fenestrated segments28. We also found an absent/aplasia ACA A1 (Table 1) segment in 4.91% of the patients. Our report is similar to the prevalence reported in imaging studies 1–6%33,34,35. On the other hand, authors have reported a rate as low as 0.4% using cadaveric samples25. ACA A1 segment absent/aplasia has been associated with AComA aneurysms36. This may be due to compromised blood flow in the asymmetry or incomplete CoW. While some researchers have hypothesized that the frequency of the variation of CoW increases with age, the underlying cause of this relationship is not clear4,5,11,37. Hindenes and co-authors have suggested atherosclerosis as a possible cause. However, researchers using human embryo samples have reported incomplete CoW in 17 out of 20 samples, stating that the formation of atypical CoW is congenital19. This information suggests that age may not significantly affect the frequency of variation. Similarly, in the present study, age does not significantly affect the frequency of variation in all the segments of the CoW except for the right ACA A1 segment (Table 2). The absence of ACA segments and hypoplasia were more prevalent in the older patients (60–105 years), and fenestration was more prevalent in younger patients (19–59 years) (Table 2).
Authors have reported conflicting findings regarding the frequency of variation between males and females. According to some authors, complete CoW is common in females4,37, while some specific variations are mostly reported in males or females38. Others have reported no correlation between gender and frequency of CoW variation5,11. Similar to the present study, there was no statistically significant difference in the prevalence of variation in the morphology of the majority of the CoW arteries in both genders, except for the right ACA A1 and left ACA A2 segment (Table 3). The discrepancies reported in the above studies may be attributed to differences in sample sizes (predominantly male/female ratio). Differences in the choice of methodology may also make it difficult to compare the results of some morphological variations.
Researchers from different regions have hypothesized racial disparities in the incidence of CoW variation in their population samples. For instance, PCA hypoplasia has been reported across populations at different prevalence rates. Nyasa and co-authors reported an incident rate as high as 13% in the Malawi population3 compared to 5% reported in a USA study39. Others have reported an even lower rate of less than 5%, including samples from the South African population14,40,41. In the present study, hypoplasia of the PCA is higher than 20%, and most of the cases were reported in India, followed by the Black, and the least documented in the Caucasians. Similarly, we reported most of the MCA hypoplasia in India, followed by the Black and a few cases in the Caucasians (Table 4). The significant differences recorded across the racial group in the South African population may be a show of genetic influence since we defined hypoplasia by a similar value of less than 1 mm across the races. However, more data are required to corroborate these findings.
In the present study, the average diameter of the ACA, MCA, and PCA is similar to the values reported by Yeniçeri and co-authors in a Turkish population (ACA A1(R-L) 1.24–1.32, ACA A2(R-L) 1.32–1.40, MCA(R-L) 2.12–2.16 and PCA(R-L) 1.39–1.32)12. A slightly higher value was reported by El-Barhoun and co-authors, using samples from the Australian cohort13. Another important finding in the present study is that the diameter of ACA was significantly larger in females than in males (Table 6). Also, the bilateral MCA, PCA, and unilateral ACA A1 were significantly larger in the Caucasians compared with the Indians and the Blacks (Table 7). The average diameter seems significantly larger in the older patients for bilateral ACA A1 and PCA and unilateral ACA A2 and MCA (Table 8). We hypothesised that morphometric parameters such as diameter might vary across gender, race, and age. As most of the previous studies did not compare the average diameter of the cerebral arteries with these demographic factors, it is difficult to compare the findings in the present study with other studies in the literature. More studies with larger sample sizes are required to validate this hypothesis.
Study limitations
This study investigated variations of cerebral arteries and some arterial segments of the CoW but did not address the communicating arteries because of the imaging modality that was utilized. The AComA and PComA are rarely seen on CTA images due to their smaller sizes and were therefore excluded from this analysis.
Conclusion
Morphologic variations in the configuration of the cerebral arteries and the CoW are common in the South African population studied in this work. ACA is the most variable of all the arteries; hypoplasia was the most prevalent variation. There was a significant difference in the average diameter across demographic parameters such as gender, race, and age. Further studies are required to establish the morphometric discrepancies associated with the demographic factors, especially in the South African population. Knowledge of the configuration of the cerebral arteries and CoW plays a significant role in guiding therapeutic decision-making in treating various neurovascular pathologies. Awareness of these anatomical variations would be vital in patients' selection for preventative treatment and neurovascular procedures.
Data availability
Datasets included in the study are available from the corresponding author upon request.
Abbreviations
- CoW:
-
Circle of Willis
- ACA:
-
Anterior cerebral artery
- MCA:
-
Middle cerebral artery
- PCA:
-
Posterior cerebral artery
References
Singh, R., Kannabathula, A. B., Sunam, H. & Deka, D. Anatomical variations of circle of Willis- a cadaveric study. Int. Surg. J. 4(4), 1249–1258 (2017).
Jones, J. D., Castanho, P., Bazira, P. & Sanders, K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: A literature review and meta-analysis. Clin. Anat. 34, 978–990 (2020).
Nyasa, C., Mwakikunga, A., Tembo, L., Dzamalala, C. & Ihunwo, A. O. Distribution of variations in anatomy of the circle of Willis: Results of a cadaveric study of the Malawian population and review of literature. Pan Afr. Med. J. https://doi.org/10.11604/pamj.2021.38.11.27126 (2021).
Zaninovich, O. A., Ramey, W. L., Walter, C. M. & Dumont, T. M. Completion of the circle of Willis varies by gender, age, and indication for computed tomography angiography. World Neurosurg. 106, 953–963 (2017).
Hindenes, L. B. et al. Variations in the circle of Willis in a large population sample using 3D TOF angiography. The Tromso study. PLoS One 15(11), e0241373 (2020).
Fahy, P. et al. An experimental investigation of the hemodynamic variations due to aplastic vessels within three-dimensional phantom models of the circle of Willis. Ann. Biomed. Eng. 42, 123–138. https://doi.org/10.1007/s10439-013-0905-4 (2014).
Kapoor, K., Singh, B. & Dewan, I. J. Variations in the configuration of the circle of Willis. Anat. Sci. Int. 83, 96–106 (2008).
Ersoy, B., Gür, B., Çifcibaşı, K. & Ipsalali, H. O. Anterior cerebral circulation: A literature review. Turk. Med. Stud. J. 8(2), 44–9. https://tmsj.trakya.edu.tr (2021).
Pascalau, R., Padurean, V. A., Bartos, D., Bartos, A. & Szabo, B. A. The geometry of the circle of willis anatomical variants as a potential cerebrovascular risk factor. Turk. Neurosurg. 29(2), 151–158. https://doi.org/10.5137/1019-5149.JTN.21835-17.3.PMID:29484629 (2019).
Youssef, A., Emad, S., Mahfouz, Y., Raafat, T. & Elkiki, H. A. Normal variant of the cerebral circulation at MR angiography. Eur. Radiol. ECR 2014/C-1544. https://scholar.cu.edu.eg. (2014).
Ansari, S. et al. A simple technique for morphological measurement of cerebral arterial circle variations using public domain software (Osiris). Anat. Cell Biol. 44(4), 324–330 (2011).
Yeniçeri, I. Ö., Çullu, N., Deveer, M. & Yeniçeri, E. N. Circle of Willis variations and artery diameter measurements in the Turkish population. Folia Morphol. 76(3), 4 (2016).
El-Barhoun, E., Gledhill, S. & Pitman, A. Circle of Willis artery diameters on MR angiography: An Australian reference database. J. Med. Imaging Radiat. Oncol. 53(3), 248–260 (2009).
Cilliers, K., Vorster, W. & Page, B. J. The anatomical variation of the circulus arteriosus cerebri in a cadaver cohort representing the population dynamics of the Western Cape. Br. J. Neurosurg. 32(1), 61–67 (2018).
Eftekhar, B. et al. Are the distributions of variations of circle of Willis different in different populations?—Results of an anatomical study and review of literature. BMC Neurol. 6(22), 1471–2377 (2006).
Omotoso, B. R., Harrichandparsad, R., Satyapal, K. S., Moodley, I. G. & Lazarus, L. Radiological anatomy of the intracranial vertebral artery in a select South African cohort of patients. Sci. Rep. 11(1), 1–9 (2021).
Ayre, J. R., Bazira, P. J., Abumattar, M., Makwana, H. N. & Sanders, K. A. A new classification system for the anatomical variations of the human circle of Willis: A systematic review. J. Anat. 240(6), 1187–1204 (2022).
Cilliers, K. & Page, B. J. Review of the anatomy of the distal anterior cerebral artery and its anomalies. Turk. Neurosurg. 26(5), 653–661 (2016).
Furuichi, K. et al. Variations of the circle of Willis at the end of the human embryonic period. Anat. Rec. 301(8), 1312–1319 (2018).
De Silva, K. R. D., Silva, R., Amaratunga, D., Gunasekera, W. S. L. & Jayesekera, R. W. Types of the cerebral arterial circle (circle of Willis) in a Sri Lankan Population. BMC Neurol. 17, 11:5 (2011).
Stojanović, N. N., Kostić, A., Mitić, R., Berilažić, L. & Radisavljević, M. Association between circle of Willis configuration and rupture of cerebral aneurysms. Medicina (Kaunas) 55(7), 338. https://doi.org/10.3390/medicina55070338 (2019).
Trandafilović, M. et al. Hypoplastic arteries of the cerebral arterial ring in the blind spot of computed tomography angiography. Folia Morphol. 3, 7 (2023).
Karatas, A. et al. Assessment of the circle of Willis with cranial tomography angiography. Med. Sci. Monit. 21, 2647–2652. https://doi.org/10.12659/MSM.894322 (2015).
Pennekamp, C. W. A. et al. Incompleteness of the circle of Willis is related to EEG-based shunting during carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 46(6), 631–637 (2013).
Yokuş, A. et al. Anterior cerebral artery and anterior communicating artery variations: Assessment with magnetic resonance angiography. World Neurosurg. 55, e203–e209. ISSN 1878-8750 https://doi.org/10.1016/j.wneu.2021.08.027 (2021).
Yılmaz, A. & Ozkul, A. Anatomical variations of anterior circulation in the brains of patients with and without intracranial aneurysm. Turk. J. Cerebrovasc. Dis. 24, 8–13 (2018).
Huh, J. S., Park, S. K., Shin, J. J. & Kim, T. H. Saccular aneurysm of the azygos anterior cerebral artery: Three case reports. J. Korean Neurosurg. Soc. 42(4), 342–345 (2007).
Kathuria, S., Gregg, L., Chen, J. & Gandhi, D. Normal cerebral arterial development and variations. Semin. Ultrasound CT MRI 32(3), 242–251. https://doi.org/10.1053/j.sult.2011.02.002 (2011).
Jalali, A., Visish, V. M., Kan, P. & Duckworth, E. A. M. Association of AComA aneurysms with triplicate A2 segment of the anterior cerebral artery. World Neurosurg. 140, e234–e239. ISSN 1878-8750 https://doi.org/10.1016/j.wneu.2020.05.005 (2020).
Jiménez-Sosa, M. S. et al. Anatomical variants of anterior cerebral arterial circle: A study by multidetector computerized 3D tomographic angiography. Int. J. Morphol. 35, 1121–1128 (2017).
Krzyżewski, R. M. et al. Anatomical variations of the anterior communicating artery complex: Gender relationship. Surg. Radiol. Anat. 3, 81–86 (2015).
Ferre, J. C. et al. Anatomical variations of the anterior cerebral arterial circle visualized by multidetector computed tomography angiography: Comparison with 3D rotational angiography. J. Neuroradiol. 40, 112–120 (2013).
Şahin, H. & Pekçevik, Y. Anatomical variations of the circle of Willis: Evaluation with CT angiography. J. Anat. 12(1), 20–26 (2018).
Uchino, A., Nomiyama, K., Takase, Y. & Kudo, S. Anterior cerebral artery variations detected by MR angiography. J. Neuroradiol. 48, 647–652 (2006).
Karatas, A., Yilmaz, H., Coban, G., Koker, M. & Uz, A. The anatomy of circulus arteriosus cerebri (circle of Willis): A study in Turkish population. Turk. Neurosurg. 26(1), 54–61 (2016).
Krzyżewski, R. et al. Variations of the anterior communicating artery complex and occurance of anterior communicating artery aneurysm: A2 segment consideration. Folia Med. Crac. 54(1), 4 (2014).
Krabbe-Hartkamp, M. et al. Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology 207(1), 103–111 (1998).
Horikoshi, T., Akiyama, I., Yamagata, Z., Sugita, M. & Nukui, H. Magnetic resonance angiographic evidence of sex-linked variations in the circle of Willis and the occurrence of cerebral aneurysms. J. Neurosurg. 96(4), 697–703. https://doi.org/10.3171/jns.2002.96.4.0697PMID:11990810 (2002).
Riggs, H. E. & Rupp, C. Variation in form of circle of Willis. Arch. Neurol. 8, 8–14 (1963).
Du Toit, F. Circulus Arteriosus Cerebri: Anatomical Variations and Their Correlation to Cerebral Aneurysms (Thesis). University of Cape Town, Faculty of Health Sciences, Division of Clinical Anatomy and Biological Anthropology. http://hdl.handle.net/11427/16481 (2015).
Klimek-Piotrowska, W. et al. A multitude of variations in the configuration of the circle of Willis: An autopsy study. Anat. Sci. Int. 91(4), 325–333 (2016).
Funding
This work was supported by National Research Foundation (NRF) (Grant number MND210710621715).
Author information
Authors and Affiliations
Contributions
All persons listed as authors have contributed substantially to the study's conception and design. G.S: protocol development, project design, data collection, and data analysis, manuscript writing. B.O: conceptualized project, protocol development, data collection, data analysis, manuscript writing and editing. R.H: project design, data collection, manuscript writing and editing. L.L: protocol development, data collection, manuscript writing and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sibiya, G., Omotoso, B.R., Harrichandparsad, R. et al. Exploring the anatomical configurations of the cerebral arteries in a cohort of South African patients. Sci Rep 14, 6060 (2024). https://doi.org/10.1038/s41598-024-56767-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56767-y
- Springer Nature Limited