Abstract
Mining has led to severe environmental pollution in countries with exhaustive mining production and inadequate industrial waste regulation. Microorganisms in contaminated sites, like mine tailings, have adapted to high concentrations of heavy metals, developing the capacity of reducing or removing them from these environments. Therefore, it is essential to thoroughly characterize bacteria present in these sites to find different ways of bioremediation. In this regard, in this study, an enrichment and isolation procedure were performed to isolate bacteria with lower nutritional requirements and high tolerance to Cu(II) and Fe(II) from two Sonoran River basin mining tails. Two Staphylococcus species and a Microbacterium ginsengisoli strain were isolated and identified from the San Felipe de Jesús mining tail. Also, three strains were isolated from the Nacozari de García mining tail: Burkholderia cenocepacia, Sphingomonas sp. and Staphylococcus warneri. Significant microbiological differences were found between the two sites. All these species exhibited tolerance up to 300 mg/L for Cu (II)–Fe (II) solutions, indicating their capacity to grow in these conditions. Moreover, a consortium of isolated bacteria was immobilized in two different biocomposites and the biocomposite with larger pore size achieved greater bacterial immobilization showcasing the potential of these bacteria in biotechnological applications.
Similar content being viewed by others
Introduction
In the northwest of Mexico, particularly on the border between Arizona and Sonora states, mining activity is one of the priority industrial sectors. This is due to the geological and climatic conditions that allowed the formation of mineral deposits and their exploitation. Sonora is considered the leading state in mining and metallurgical production in Mexico, owing to its substantial deposits of metallic minerals such as copper, gold and molybdenum, which are currently in operation. Additionally, it is also an important producer of non-metallic minerals such as graphite and wollastonite. The Sonoran River and the Yaqui River basins have played a prominent role in mining activities, hosting the main mining companies in the state. As a result of the large mining production, significant contamination is also generated1.
Since mines are generally located in municipalities near to watercourses, the incorporation of mining contaminants remains a risk factor. This can occur through the leaching of contaminants from mine veins using solvents or the mobilization of these wastes through wind or water dispersion. There is also a risk of landslides during rainfall events when tailings accumulate in piles2. Common contaminants resulting from mining activities include heavy metals (HMs). The ecological impact of HMs is mainly due to their high toxicity and the fact that they cannot be degraded. Exposure to high concentrations of metals can cause damage to human health, manifested at the molecular level through toxicity mechanisms derived from their capacity as cations and their high affinity for binding to other elements. This includes the blocking of functional groups in biomolecules such as proteins, displacement of cationic centers in crucial enzymes, and the generation of reactive oxygen species. This results in irreversible damage to proteins, lipids, and nucleic acids3. One of the main drawbacks of these pollutants is their ability to resist degradation and their bioaccumulation, causing damage to the liver, nervous system, reproductive system, and carcinogenesis4. In the specific case of Sonora, Mexico the notable presence of copper, Cu (II), and iron, Fe(II), in sediments and water bodies is highlighted5,6. These two metals are found in higher quantities and can have detrimental effects on the environment and human health. Cu (II), for instance, can impact aquatic life and induce toxicity in sensitive organisms. On the other hand, excessive Fe(II) can contribute to the eutrophication of water bodies, negatively affecting water quality and local biodiversity. High concentrations of iron in drinking water have been associated also with potential health concerns, such as gastrointestinal issues7,8.
In this regard, efficient and sustainable ways to remove metals from the environment are priority9,10. A possible way to achieve metal bioremediation is using microorganisms, such as bacteria present in the contaminated sites, which are able to adapt to high concentrations of metals, and to develop mechanisms for removing HMs from the environment11,12,13,14. Therefore, isolation of bacteria from sites with high metal concentration is a key step to better understand the bioremediation processes and exploit them for biotechnological purposes. Until now, little is known about the diversity of bacteria in mining tails in Sonora. The aim of the present work was to isolate and identify a group of bacteria with lower nutritional requirements and high tolerance to Cu(II) and Fe(II) from two mining tails from the Sonoran River basin in Mexico, in order to be applied in a promising bioremediation system.
Results
Samples characterization
Soil samples obtained from each mining tail presented differences in metal concentration and pH (Table 1). San Felipe de Jesús had a more acid pH, and higher concentration of all the analyzed metals. The latter could allow the presence of bacteria more tolerant to high concentrations of metals. The higher concentration of Pb and Zn in SFJ mine tailings can also be attributed to the waste's original composition. Similarly, this phenomenon occurs with Nacozari de García (NG), where despite having lower metal concentrations, the Cu concentration is most akin to SFJ, and it is also due to the waste's original constitution.
Enrichment and isolation of bacteria
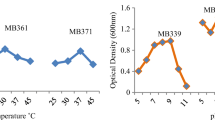
The bacteria isolation and the enrichment process were performed as reported by Majumder et al.15 with some modifications, as in this case bacteria were obtained from mining tails (Fig. 1). Bacteria with low nutritional requirements and tolerant to ascendant concentrations of Cu(II) and Fe(II) were isolated and analyzed from the consortium of each mining tails by optical density determination through time (Fig. 2). The isolated bacteria exhibited reduced growth with ascendant concentration of metals. Notably in NG bacteria, this trend was particularly pronounced in the concentration range between 10 and 20 mg/L, while from 15 to 20 mg/L the bacteria remained stable. In contrast, for the SFJ bacteria, bacterial proliferation was high at 10 mg/L, with a gradual increase in growth observed as the metal concentrations increased.
Once the acclimated consortium of each mining tail grew in 20 mg/L, regrowth in peptone yeast extract agar was performed in plates with 50 mg/L of each metal to be isolated and then identified. Three metallotolerant strains were isolated from SFJ mining tail, and three for NG. The strain name ID, gram stain and phenotype results of each isolate are shown in Table 2. SFJ bacteria were predominated gram positive, while NG predominated gram-negative bacteria. Additionally, SFJ exhibited more cocci morphology, while NG had more bacilli. The lowest concentration of the combination of metals that inhibited the growth of each isolated microorganism was found to be 300 mg/L. This concentration coincided with the level of Cu present in each mining tail. All isolated bacteria were identified based on their similarity percentage of identity using GenBank sequences by BLASTN (Table 3). The GeneBank accession numbers for the 16S rRNA sequences are PP157099-PP157104.
Immobilization of bacteria
A difference of almost 2 × 108 suspended cells were observed in the immobilization tests between the control used and the biocomposites over an extended incubation period lasting up to 72 h, where a lower concentration of suspended bacteria compared to the control indicates a higher bacteria immobilization within the material. Although there is no statistically significant difference in the immobilization tests performed between both materials, the biocomposite with a larger pecan nutshell particle size, which was B2, showed a stronger biofilm and better immobilization (Fig. 3), which was subsequently confirmed through SEM of samples cultivated in an extended incubation period of two months. In the case of B1, there was no visible proliferation in the SEM images even from the beginning of the experiment.
The SEM micrographs showed that B2 is a heterogeneous and porous material (Fig. 4A and B). The bacteria immobilized within the material correspond to the structure of the bacteria comprising the consortium (bacilli and cocci), with a major predominance of bacilli, which could match to the isolated Burkholderia cenocepacia and Sphingomonas sp., with an average size of 1–2 µm (Fig. 4C and D). They formed aggregates and secreted extracellular polymeric substances on the material’s pores that suggest that the biocomposite is a good support for the isolated bacteria.
Immobilization of the isolated consortium in the 400 μm pecan nutshell biocomposite. (A) Biocomposite structure. (B) Biocomposite pore size. Bacteria immobilized in the 400-mesh biocomposite after an incubation period of (C) 1.5 months and (D) 2 months. * Letter b within the image indicates the presence of bacillary shapes, letter c indicates the presence of coccoid shapes while EPS refers to extracellular polymeric substances.
Discussion
Our study exhibits that the conditions in both mining tails facilitated the presence of bacteria with tolerance to acidic environments and elevated concentrations of metals, with a particular emphasis on the conditions found in SFJ, which exhibit higher concentrations of metals and greater acidity. The elevated concentrations of metals and acidity in the mine tailing may be linked to the site’s age. Importantly, the predominant use of selective flotation of mineral extraction in these mine tailing could also significantly impact acidity. Additionally, it is crucial to recognize that acid mine drainages can originate in mines due to the oxidation of sulfate-bearing materials16.
Chemical composition has been previously characterized for both mine tailings. For SFJ, results were in the range obtained by Del Rio-Salas et al.17 for Cd (98–665 mg/L), Cu (338–5691 mg/L), and Pb (832–14,162 mg/L). These authors also analyzed As and Sb, obtaining results between 1306–16,756 and 82–492 mg/L, respectively. In the case of NG, results were comparable to the concentrations range of mine tailings on the outskirts of the municipality of NG analyzed by Peña-Ortega et al.18 for Cu (216.2–1194.5 mg/L) and Zn (49.7–394.7). In comparison with previous reports for this mine tailing, it seems that Pb concentration has increased.
Bacteria from SFJ likely avoided the toxic effects of the metals used, as they were present in sediments on a site with higher concentrations of metals. The resistance of tolerant bacteria to metals can be explained by the presence of specific genes in their genome that encode proteins such as metallothioneins19. These kinds of proteins enable the bacteria to withstand toxic effects or even utilize metals like Cu(I) as electron acceptor, acting as a source of energy13,20. Additionally, examples of bacterial resistance to heavy metals may involve features such as cell impermeability facilitated by the presence of exopolysaccharides that adsorb metals21. The resilience of bacteria to metal toxicity has been previously reported by Escamilla-Rodrigez et al.22 who observed an increase in bacterial concentration (CFU) in response to Cu and Hg. Bacteria tolerance to Cu and Fe can also be explained by the use of these metals as micronutrients for different metabolic pathways, or the facility of elements such as Cu for the formation of complexes with organic matter and its adsorption onto particulates (Fe–Mn-oxyhydroxides) such in the case of some Bacillus species23. Furthermore, the isolated bacteria may exhibit tolerance to Pb, a metal that is prominently present in both mine tailing, indicating a potential biotechnological use of the isolated species. Pb bioaccumulation in bacteria has been reported previously by Tiquia-Arashiro et al.24.
Common taxa in environments like mine tailings or even in rhizosphere environments include microorganisms from phyla Actinomycetota, Pseudomonadota, and Bacteroidota. The differences of bacteria groups could be associated to the abiotic factors in each site favoriting the development and prevalence of specific groups, Predominance of Actinobacteria has been observed in arid soils25. In the present study more bacteria from Bacillota and Actinomycetota phyla were identified. Three strains tolerant to metals were identified in the SFJ mining tail: Staphylococcus epidermidis, Staphylococcus pasteuri and Microbacterium ginsengisoli and three in NG: Burkholderia cenocepacia, Sphingomonas sp. and Staphylococcus warneri.
Escamilla-Rodriguez et al.22 also reported about three Staphylococcus species isolated from water of a mining area in Zacatecas, Mexico. Two of these bacteria had one hundred percent of growth when they were incubated in plates supplemented with Cu, in comparison with the initial population. They also had high growth rate in other metals tested (100 and 167% for Cr, 120% for Zn, 140% for Ag, 66 and 120% for Hg and 20 and 66.7% for Co, respectively). As indicated by Gadd26, the primary binding sites for metal cations in the cell walls of Gram-positive bacteria are the carboxyl groups within peptidoglycan. Furthermore, it has been reported that S. epidermidis has developed the ability to tolerate and grow in the presence of moderate concentrations of Cu. Resistance to Cu is partly attributed to the presence of specific genes in its genome encoding copper transport proteins and copper resistance proteins. These proteins enable the bacterium to withstand the toxic effects of Cu(I) and utilize such as a micronutrient or cofactor in key enzymes of important biological processes27. Such mechanisms suggest the adaptability of S. epidermidis to environmental copper stress, potentially explaining the presence of various Staphylococcus species in both locations in the present study, particularly due to the high copper concentrations at both sites.
In a similar way, Toribio-Jimenez et al.28, and Toledo-Hernández et al.29 isolated strains of Staphylococcus sp. from the rhizosphere of the mining tail El Fraile, located in Guerrero, Mexico, and in this case evaluated their capacity to bioaccumulate Ag(II). In the case of S. pasteuri isolated from Iranian mine calcareous soils has shown to remove 98.71% of Pb, 97.15% of Cd, and 94.83% of Zn by bioprecipitation due to their capacity to produce urea30, highlighting the potential biotechnological application of the Staphylococcus species isolated in the present work for metal recovery by different mechanisms.
On the other hand, regarding the Microbacterium sp isolated in the present work, a study by Jroundi et al.23, reported that Microbacterium genus accounted for approximately 1.03% of the relative abundance in deep-sea sediments from the westernmost Mediterranean. Furthermore, the study revealed a positive correlation between the presence of metals, such as Cu, Co, Zn, Pb, within this genus, as well as with other genera, (including Bacillus, Bacillales, Paenisporosarcina, Megasphaera, and the Enterobacteriaceae family). The authors suggested that this correlation may be attributed to changes in oxygenation induced by the presence of metals from anthropogenic activities, which may promote the proliferation of these bacteria. Similar conditions to those found in mining tailings such as those found in San Felipe de Jesús in the present study. Microbacterium as well as the strain of Sphingomonas isolated in the present work were also capable to develop pigments, offering another potential application for these bacteria.
In the case of Sphingomonas, it is an endophytic bacterium that has been previously isolated from Sedum alfredii roots and promote zinc extraction, so it could be used to enhance processes of phytoremediation31. Sphingomonas paucimobilis has been also used to degrade different organic pollutants, but in addition it has been proved that it serves as an adsorbent for the removal of Cu(II) ions from industrial effluents32,33,34. Furthermore, it has been reported that some strains of Sphingomonas and Microbacterium can produce yellow pigments as a result of their carotenoid synthesis metabolism, such as zeaxanthin and nostoxanthin35. These carotenoids have potential antioxidant properties, which can protect cells against oxidative stress, possibly explaining the tolerance of this strain to heavy metals, and even to gamma-ray irradiation, as reported by Asker et al.35 for a species of Sphingomonas isolated from freshwater samples collected at Misasa (Tottori, Japan), a region known for its high natural radioactivity, and designated as S. jaspsi. Furthermore, high concentrations of heavy metals can lead to the generation of free radicals in the environment, and carotenoids produced by these strains may neutralize them, preventing the harmful effects of oxidation. Therefore, the carotenoids of these species can be harnessed for their multifunctional roles, including applications in various industries such as food, pharmaceutical, cosmetic, and textile36. At the same time, these compounds make these species useful in bioremediation due to their antioxidant activity, allowing for increased bacterial tolerance and enabling subsequent remediation mechanisms like biosorption or bioprecipitation23,30.
In the case of the Burkholderia genus, it is a plant-associated genus that has previously been isolated from the rhizospheric soils in the same mining tail of Nacozari de García25. The genus Burkholderia is known for its capacity to remove metals. Jiang et al.37 isolated and identified a metal-resistant strain named Burkholderia sp. J62 from metal-contaminated soils, demonstrating its ability to solubilize metals such as Pb and Cd. Similarly, Yang et al.38 found that a Burkholderia sp. strain designated as Z-90 exhibited a removal efficiency of 44% for Zn, 32.5% for lead, 52.2% for Mn, 37.7% for Cd, 24.1% for Cu, and 31.6% for As. It was also observed that the removal capacity of these metals was attributed to mineral adhesion to the strain and the formation of a metallic complex with a glycolipid biosurfactant excreted extracellularly. The capacity to produce these extracellular polymeric substances makes bacteria potentially suitable for immobilization and application in bioremediation systems designed for metal removal. Similarly, Staphylococcus epidermidis strain that producing brown pigment, as observed in the present work, exhibit a greater capacity to form biofilms, as reported by Yao et al.39. Therefore, the isolation of bacteria with this capability enables their immobilization, a process that was subsequently analyzed and confirmed in the present work by SEM40,41.
The increased interest in the application of immobilized microorganisms in bioremediation processes arises from the higher biodegradation efficiency observed when compared to free microorganisms14,42,43,44,45. Additionally, as presented by Rahman et al.46, immobilized bacterial cells could be recovered and reused, lowering the cost of a possible remediation process, and when using a consortium or community of bacteria instead of individual strains in a biotechnological application, the removal rate increases42,47. In the present study, the selection of pecan nutshell biocomposite materials was motivated by their promising attributes, including renewability, biodegradability, and functionality, making them advantageous for various applications such as bioremediation, wastewater treatment, and sustainable biofilters48,49.
The proliferation of bacteria in the biocomposite with larger pecan nutshell particle size (B2) can be explained by the larger pore diameter in such material, which ranges between 100 and 200 µm (Fig. 4B). These pores can serve as accumulation channels, as they increase the surface area50,51 and, at the same time, can facilitate the transfer of ions for the removal of impurities or contaminants52. Darmayati et al.53 performed a study to analyze the immobilization of different bacterial strains on porous rock carriers after being stored at 25 °C for three months, conditions similar to those used in the current work. They attribute the differences in cell immobilization observed among the materials to variations in physical characteristics such as porosity and surface area, because materials like zeolites, which have a rough surface, tend to increase the surface area, leading to greater cell immobilization42,54.
It is also noteworthy to mention the presence of extracellular polymeric substances (biofilms) secreted by the bacteria upon adhering to the biocomposite. The formation of biofilms by bacteria could be a response to stressful environmental conditions and serves as a protective mechanism for bacteria. Additionally, it can indicate the tolerance of the isolated bacteria to adverse conditions, such as nutrient scarcity55. This tolerance may be attributed to the isolated bacteria’s capacity to thrive under minimal nutrient conditions.
Extended periods of time exceeding one month as in the present study, also promote the optimal growth of microorganisms, as demonstrated by Sarioglu et al.56. Their one-month immobilization of Morganella morganii STB5 on the surfaces of electrospun polystyrene and polysulfone webs resulted in a strong attachment of bacterial cells and this timeframe was considered adequate for conducting further studies on Cr (VI) removal.
In the case of immobilization on organic materials, Lupascu et al.57 presents the adsorption of Bacillus subtilis and Bacillus cereus on activated carbon derived from apricot kernels. In this case, immobilization occurs only on the exterior of the material since the pores generated are smaller than the size of the bacteria. Furthermore, they identify that bacteria are immobilized through interactions between basic groups from their structure (such as amines, amides, and pyrenes) with carboxylic, lactonic, and phenolic groups on the surface of the activated carbons. Similar interactions may be occurring between the immobilized bacteria in the present study and the pecan nutshell biocomposite, as well as in any organic material, especially those of plant origin with similar functional groups.
Despite this, few studies have focused on immobilizing bacteria in agro-industrial wastes. Gallardo-Rodríguez et al.14 utilized Furcraea andina fibers to immobilize Pseudomonas bacteria, employing them as a biofilter for lead removal. Their findings demonstrate the effective use of natural fibers from this plant for both Pseudomonas immobilization and metal removal. On the other hand, yeast cells such as Saccharomyces cerevisiae have been immobilized on Raphia farinifera fibers obtaining a high adsorption capacity for the removal of Pb (II). Therefore, the present study is one of the few that elucidates the potential application of all the isolated bacteria, immobilized within an organic material to enhance their bioremediation capabilities.
In conclusion, the present study demonstrated the disparity in the cultivated bacterial diversity found between two mining tailings within the Sonoran River basin. It identifies species that had not been previously reported from each site and demonstrates their tolerance to Cu (II) and Fe (II) solutions of up to 300 mg/L. The isolation process selectively yielded bacteria with high tolerance to Cu and Fe tolerance as well as those with low nutritional requirements. Among the isolated strains, Staphylococcus epidermidis, Staphylococcus pasteuri, and Microbacterium ginsengisoli from SFJ mine tailing, along with Staphylococcus warneri, Burkholderia cenocepacia, and Sphingomonas sp. from NG mine tailings, exhibit promising biotechnological potential for metal removal applications as previously presented in the literature. Furthermore, bacteria like Microbacterium and Sphingomonas, known for their pigment production capability, not only add value to these isolated bacteria for various applications but also have the potential to enhance their bioremediation activities. Moreover, the study successfully demonstrates the immobilization of these isolated bacteria through biofilms within an organic natural-origin biocomposite, making them ideal candidates for inoculating remediation systems aimed to heavy metal removal. Additionally, this work showcases the potential use of an agro-industrial waste product like pecan nutshell, which represents a cost effective and biodegradable product for possible remediation systems in which these bacteria can be applied. Hardest efforts have been performed in order to advance in these fields nevertheless, further research is needed to elucidate their mechanisms to decontaminate polluted environments.
Methods
Sampling sites
Mine tailings soil samples were collected during March 2021 from two abandoned sites located in two different historic mining areas in Sonora, Mexico: San Felipe de Jesus (SFJ) and Nacozari de García (NG) mine tailings. SFJ mine tailing is located in the San Felipe de Jesús municipality (N 29° 55′ 15″–29° 49′ 18″ and W 110° 260 12″–110° 10′ 02″), which is a semi-arid land with a mean temperature of 25.3 °C during July and August, the months with the highest temperatures, and an annual average precipitation of 468.8 mm. This tailing deposit has an estimated area of around 16,300 m2 and an approximate volume of 209,455 tons of mining wastes. Since 1900, the metallic recovery of Pb, Zn, Cu, Ag was carried out by selective flotation16. Additionally, it's important to note that the mine tailing is located at less than one kilometer from the town, which can potentially impact the population, particularly the existing agricultural sites and the Sonora River, which flows right alongside the town.
The second sampling site is located in Nacozari de García municipality (N 30° 21′ 24″–30° 21′ 29″ and W 109° 40′ 57″–109° 40′ 55″). This place has a predominant temperate semi-dry climate area with an average temperature of 35 °C during July, month with the higher temperature, and an average annual precipitation of 490 mm. Metal exploitation, mainly Cu, began in 1900 and continues to the present day, with the current estimated volume of around three million tons of mining wastes16,58. In this case, the mining site is located within the city, which increases the potential risks for the local population due to its proximity to mining waste.
The superficial sampling of sediments was performed according to the method described in NMX-AA-132-SCFI-20162,59. Five samples from each mining tail, with a minimum distance of 20 m, were collected at five randomly accessible sites in each mining area and pooled to obtain a composite sample of each mining tail. The samples were manually collected (30 cm) using a stainless-steel shovel and stored in sterile plastic bags until their immediately characterization. The collected mine tailing samples were completely homogenized, dried at 30 °C by 24 h and sieved through a 1 mm pore size to be used for physicochemical characterization and the isolation process.
Physicochemical samples characterization
For the samples characterization, The pH was measured by a potentiometer method using the supernatant suspension of a mixture with each composite mine tailing sample: water ratio of 1:2. The total metal composition was determined by Atomic Absorption Spectrometry (AAS)60,61. Firstly, 0.5 g of soil composite from each mine tailing was totally digested with 0.5 mL HNO3, 0.5 mL HF and 0.5 mL HClO4 due the presence of silicates in the mining tailings5. A second reduction was performed using 2 mL of HNO3 and 2 mL HF followed by heating at 95 ± 5 °C of 2 mL HNO3 for 30 min. Finally, the sample was heated in 10% H3BO3 for 20 min at 95 ± 5 °C and filtered on Whatman paper filters No. 40. The samples were diluted in 2% HNO3 before being analyzed in a Perkin Elmer 3100 atomic absorption spectrometer.
Enrichment and isolation of samples
For bacteria acclimatation process, 10 g of composite soil were exhaustively mixed with 100 mL of nutrient broth (0.5% peptone, 0.5% NaCl, 0.15% yeast extract, 0.15% meat extract) in 250 mL flasks per triplicate and with their respective blanks. The flasks were incubated at 30 °C and 150 rpm over 120 h. The supernatant from the flasks was then centrifuged in conical tubes (3200 g, 4 °C, 15 min), and the formed pellets were transferred to conical tubes. A process of enrichment of the bacterial culture with metals was performed as described by Majumder et al.15. This process consists in acclimation the obtained biomass to a mix of metals for six days. For this, 100 mL of minimal salt medium (MSM) (0.08% K2HPO4, 0.02% KH2PO4, 0.05% MgSO4, 0.005% CaSO4, 0.1% (NH4)2SO4, 0.001%FeSO4) was added to the pellets in the conical tubes with an initial concentration of glucose of 1 g/L (carbon source). The tubes were maintained in an incubator at 30 °C and 150 rpm during six days. Samples were centrifuged daily, discarding the supernatant, and progressively decreasing the concentration of glucose to reach 0 mg/L at the sixth day (1000, 800, 600, 400, 200 and 0 mg/L), and a concentration of metals of 20 mg/L Cu(II) and Fe(II) (0, 2, 5, 10, 15, and 20 mg/L) as pentahydrate copper sulfate and heptahydrate iron sulfate, respectively (Supplementary material Table 1). Each concentration was still stored at 30 °C and 150 rpm, and optical density determinations were performed after transitioning to the next concentration to determine the bacterial growth at each concentration.
After the enrichment, five tenfold dilutions on MSM of the bacterial culture with 20 mg/L of metals were prepared in MSM and 100 µl of each dilution was plated by using the spread-plate method in peptone yeast extract agar plates with 50 mg/L of each metal for the isolation. The plates were kept at 30 °C and analyzed every 24 h. When the isolated strains were obtained, the same process was repeated with 100 and 300 mg/L of each metal using plates with the same concentrations of metal15 as depicted in Fig. 1, to identify what was the maximum concentration that the bacteria can tolerate62. The isolates that grew up at each concentration of metals were preserved in culture broth and glycerol (50%) at − 80 °C.
Identification of isolated bacteria
Gram stain63 and molecular analyses using 16S rRNA gene amplification and sequencing were performed in order to identify the isolated colonies using the isolated bacteria grown in nutrient broth in an incubator at 30 °C after 48 h. DNA of each isolate was extracted by Fast DNA Spin Kit for Soil (MP Biomedicals, Solon, OH, US) following the manufacturer instructions.
The total DNA quality was performed using a NanoDrop 1000 spectrophotometer (Thermo Fisher, Waltham, MA, US) using 1 μL of sample. For the further analyses, the samples with an absorbance ratio of 260/280 nm in the range > 1.8–2.0 were used. Total DNA integrity was analyzed by 0.8% agarose gel electrophoresis stained with ethidium bromide and visualized using Bio-Imaging System MiniBis pro photodocumenter (Bio America Inc., CA, US). The electrophoresis conditions were 50 V for 50 min in 1X TAE.
For PCR, universal primers 27F and 1525R were used for the amplification of the 16S rRNA gene64. HPLC-purified oligonucleotides were purchased from Sigma, and GoTaq Flexi DNA Polymerase (Promega, Madison, WI, USA) was used for all PCRs, which were performed in a Bio-Rad T100 Thermal cycler (Bio-Rad, Hercules, CA, USA). The PCR mixture was 10 μl of GoTaq FlexiBuffer 1X, 3 μl MgCl2 1.5 mM, 2.5 μl DMSO 5%, 0.5 μl of each primer (0.8 μM), 1 μl dNTPs 0.2 μM, 0.2 μl of 2U/μl of Polymerase Taq. All reactions were performed using 4 μl of DNA in a reaction of 50 μl. The amplification conditions were a touchdown phase with an initial denaturation at 95 °C for 5 min; 10 cycles of denaturation at 94 °C for 30 s, an annealing from 65 to 55 °C for 30 s during the 10 cycles, and extension at 72 °C for 30 s, and then a second phase of 25 cycles with denaturation at 94 °C for 30 s, annealing at 55 °C for 45 s, extension at 72 °C for 2 min, and final extension at 72 °C for 5 min. DNA sequencing of the amplicons was performed at Macrogen (South Korea). The sequences obtained were first aligned in ClustalX65 and compared with GenBank sequences using BLAST66.
Immobilization of bacteria
The capacity of the isolated bacteria to be immobilized on a solid matrix was evaluated using a consortium of the isolated bacteria from the stocks of 50 mg/L of each metal. The evaluation employed the same proportion of each isolated strain as an inoculum to form a consortium with a concentration of 8.4 × 108 CFU/mL14. These tests were conducted using two biocomposites as immobilization materials, consisting of albumin-starch as the polymeric matrix, and an agro-industrial waste like pecan nutshell as the fiber filler. The two biocomposites varied as for the size of the pecan nutshell particles used, which were designated as B1 (< 200 µm of PNS particle size) and B2 (400 µm PNS particle size), with the main difference being greater roughness and pore size in B2.
For these tests, 0.5 g of each biocomposite was placed in tubes with 10 mL of sterile nutrient broth, which had 1 mL of the bacterial consortium, obtaining 8.4 × 107 CFU/mL14. The positive control had the same concentration of bacteria in nutrient broth without any biocomposite. The analysis of immobilization results was performed by measuring the optical density (600 nm) of the suspended bacteria and by means of CFU based on McFarland Standards over a 72 h period. Analyses were performed in triplicate and were compared: (a) with a positive control with culture medium with the consortium of bacteria, (b) a control using each biocomposite without inoculated bacteria, and (c) a negative control composed only of culture medium.
Additionally, to confirm the immobilization of bacteria over an extended period within each biocomposite, the formation of biofilms after six and eight weeks, where the material was kept in an incubator at 30 °C with weekly changes of culture medium (70% each week), carefully avoiding any disturbance of the material were analyzed using scanning electron microscopy (SEM) with a Tabletop Microscope TM3030Plus, (HITACHI, Tokyo, Japan), and analyzed by the provided software (Environmental Science Laboratory, ERNO, UNAM).
For these analyses, the samples were initially fixed with 5% v/v glutaraldehyde (Sigma Aldrich, San Luis, MO, US) in 1 X phosphate buffer saline (PBS) for 48 h at 4 °C. Subsequently they were washed with PBS and postfixed with 1% OsO4 for 2 h at 4 °C. After another wash with PBS, the samples were dehydrated using a series of increasing acetone solutions (ranging from 30, 50, 70, 90 and 100% acetone) before being subjected to analysis54.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
References
Meuly, R. J. & Gormith, D. Sonora state producer and strategic exporter of copper. Expert J. Econ. 5, 39–46 (2017).
Guadarrama-Guzmán, P., Fernández Villagómez, G. & Alarcón Herrera, M. T. Assessment of risk to health caused by the exposure to mining waste in Durango, Mexico. Ing. Investig. Tecnol. 22, 1–9 (2021).
Covarrubias, S. A. & Peña Cabriales, J. J. Contaminación ambiental por metales pesados en México: Problemática y estrategias de fitorremediación. Rev. Int. Contam. Amb. 33, 7–21 (2017).
Ramírez Calderón, O. A., Abdeldayem, O. M., Pugazhendhi, A. & Rene, E. R. Current updates and perspectives of biosorption technology: An alternative for the removal of heavy metals from wastewater. Curr. Pollut. Rep. 6, 8–27. https://doi.org/10.1007/s40726-020-00135-7 (2020).
Gómez-Alvarez, A., Meza-Figueroa, D., Valenzuela-García, J. L., Villalba-Atondo, A. I. & Ramírez-Hernández, J. Behavior of metals under different seasonal conditions: Effects on the quality of a Mexico-Usa border river. Water Air Soil Pollut. 225, 1–13 (2014).
León García, G. J. et al. Study of heavy metal pollution in arid and semi-arid regions due to mining activity: Sonora and Bacanuchi rivers. Int. J. Environ. Sci Nat. Resour. https://doi.org/10.19080/IJESNR.2018.10.555804 (2018).
Asrari, E. Heavy Metal Contamination of Water and Soil. Analysis, Assessment, and Remediation Strategies (CRC Press, 2014).
Sharma, S. K. Heavy metals in water. Presence, removal and safety. In Heavy Metals in Water P001–P004 (Royal Society of Chemistry, 2014). https://doi.org/10.1039/9781782620174-fp001.
UNEP. Global Environment Outlook—GEO-6: Healthy Planet, Healthy People (2019).
Babuji, P., Thirumalaisamy, S., Duraisamy, K. & Periyasamy, G. Human health risks due to exposure to water pollution: A review. Water https://doi.org/10.3390/w15142532 (2023).
Ndeddy Aka, R. J. & Babalola, O. O. Identification and characterization of Cr-, Cd-, and Ni-tolerant bacteria isolated from mine tailings. Bioremediat. J. 21, 1–19 (2017).
Aslam, F., Yasmin, A. & Sohail, S. Bioaccumulation of lead, chromium, and nickel by bacteria from three different genera isolated from industrial effluent. Int. Microbiol. 23, 253–261 (2020).
Sreedevi, P. R., Suresh, K. & Jiang, G. Bacterial bioremediation of heavy metals in wastewater: A review of processes and applications. J. Water Process Eng. 48, 102884 (2022).
Gallardo-Rodríguez, J. J., Rios-Rivera, A. C. & Von Bennevitz, M. R. Living biomass supported on a natural-fiber biofilter for lead removal. J. Environ. Manag. 231, 825–832 (2019).
Majumder, S., Gangadhar, G., Raghuvanshi, S. & Gupta, S. Biofilter column for removal of divalent copper from aqueous solutions: Performance evaluation and kinetic modeling. J. Water Process Eng. 6, 136–143 (2015).
Valenzuela, E. I., García-Figueroa, A. C., Amábilis-Sosa, L. E., Molina-Freaner, F. E. & Pat-Espadas, A. M. Stabilization of potentially toxic elements contained in mine waste: A microbiological approach for the environmental management of mine tailings. J. Environ. Manag. 270, 110873 (2020).
Del Rio-Salas, R. et al. Mineralogy and geochemistry of rural road dust and nearby mine tailings: A case of ignored pollution hazard from an abandoned mining site in semi-arid zone. Nat. Resour. Res 28, 1485–1503 (2019).
Peña-Ortega, M. et al. Environmental assessment and historic erosion calculation of abandoned mine tailings from a semi-arid zone of northwestern Mexico: Insights from geochemistry and unmanned aerial vehicles. Environ. Sci. Pollut. Res 26, 26203–26215 (2019).
Gupta, D., Satpati, S., Dixit, A. & Ranjan, R. Fabrication of biobeads expressing heavy metal-binding protein for removal of heavy metal from wastewater. Appl. Microbiol. Biotechnol. 103, 5411–5420 (2019).
Srivastava, N. K. & Majumder, C. B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 151, 1–8 (2008).
Raj, K. et al. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 199, 353–364. https://doi.org/10.1016/j.carbpol.2018.07.037 (2018).
Escamilla-Rodríguez, A., Carlos-Hernández, S. & Díaz-Jiménez, L. Evidence of resistance of heavy metals from bacteria isolated from natural waters of a mining area in Mexico. Water 13, 2766 (2021).
Jroundi, F., Martinez-Ruiz, F., Merroun, M. L. & Gonzalez-Muñoz, M. T. Exploring bacterial community composition in Mediterranean deep-sea sediments and their role in heavy metal accumulation. Sci. Total Environ. 712, 135660 (2020).
Tiquia-Arashiro, S. M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 102, 5437–5444. https://doi.org/10.1007/s00253-018-8969-6 (2018).
Romero, M. F. et al. Metagenomics of mine tailing rhizospheric communities and its selection for plant establishment towards bioremediation. Microbiol. Res. 247, 126732 (2021).
Gadd, G. M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 84, 13–28. https://doi.org/10.1002/jctb.1999 (2009).
Andrei, A. et al. Cu homeostasis in bacteria: The ins and outs. Membranes 10, 1–45. https://doi.org/10.3390/membranes10090242 (2020).
Toribio-Jiménez, J., Rodriguez-Barrera, M. A. & Segura, D. Production of biosurfactants by bacteria isolated from a mine tailing zone in Southern Mexico and their resistance to heavy metals. J. Bacteriol. Res. 6, 23–31 (2014).
Toledo-Hernández, E. et al. Aislamiento e identificación de bacterias tolerantes y bioacumuladoras de metales pesados, obtenidas de los jales mineros El Fraile, México. Rev. Terra Latinoam. 38, 67 (2020).
Jalilvand, N., Akhgar, A., Alikhani, H. A., Rahmani, H. A. & Rejali, F. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J Soil Sci. Plant Nutr. 20, 206–219 (2020).
Chen, B. et al. The endophytic bacterium, Sphingomonas SaMR12, improves the potential for zinc phytoremediation by its host, Sedum alfredii. PLoS One 9, e106826 (2014).
Wang, X. S., Huang, L. P., Li, Y. & Cheng, J. Removal of copper(II) ions from aqueous solution using Sphingomonas paucimobolis biomass. Adsorpt. Sci. Technol. 28, 137 (2010).
Corretto, E. et al. Comparative genomics of microbacterium species to reveal diversity, potential for secondary metabolites and heavy metal resistance. Front. Microbiol. 11, 1869 (2020).
Reis-Mansur, M. C. P. P. et al. Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci. Rep. 9, 9554 (2019).
Asker, D., Beppu, T. & Ueda, K. Sphingomonas jaspsi sp, nov,, a novel carotenoid-producing bacterium isolated from Misasa, Tottori, Japan. Int J. Syst. Evol. Microbiol. 57, 1435–1441 (2007).
Sebastian, L., Paul, A. M. & Jayanthi, D. Isolation and production of prodigiosin pigments from Streptomyces spp. in Actinobacteriology. In Methods in Actinobacteriology. Springer Protocols Handbooks (Humana, 2022).
Jiang, C. Y., Sheng, X. F., Qian, M. & Wang, Q. Y. Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72, 157–164 (2008).
Yang, Z. et al. Bioleaching remediation of heavy metal-contaminated soils using Burkholderia sp. Z-90. J. Hazard. Mater. 301, 145–152 (2016).
Yao, Y. et al. Factors characterizing Staphylococcus epidermidis invasiveness determined by comparative genomics. Infect. Immun. 73, 1856–1860 (2005).
Calderón, K., Rodelas, B., Cabirol, N., González-López, J. & Noyola, A. Analysis of microbial communities developed on the fouling layers of a membrane-coupled anaerobic bioreactor applied to wastewater treatment. Bioresour. Technol. 102, 4618–4627 (2011).
Guisado, I. M., Purswani, J., González-López, J. & Pozo, C. An extractive membrane biofilm reactor as alternative technology for the treatment of methyl tert-butyl ether contaminated water. Biotechnol. Prog. 32, 1238–1245 (2016).
Berrelleza-Valdez, F. et al. A novel process of the isolation of nitrifying bacteria and their development in two different natural lab-scale packed-bed bioreactors for trichloroethylene bioremediation. J. Environ. Manag. 241, 211–218 (2019).
Purnomo, C. W., Mellyanawaty, M. & Budhijanto, W. Simulation and experimental study on iron impregnated microbial immobilization in zeolite for production of biogas. Waste Biomass Valoriz. 8, 2413–2421 (2017).
Raval, N. P., Shah, P. U. & Shah, N. K. Adsorptive removal of nickel(II) ions from aqueous environment: A review. J. Environ. Manag. 179, 1–20 (2016).
Sanchez-Silva, J. M., González-Estrada, R. R., Blancas-Benitez, F. J. & Fonseca-Cantabrana, Á. Utilización de subproductos agroindustriales para la bioadsorción de metales pesados. TIP Rev. Esp. Cienc. Quím. Biol. 23 (2020).
Rahman, R., Mohamad Ghazali, F. & Basri, M. Biodegradation of hydrocarbon contamination by immobilized bacterial cells. J. Microbiol. 44(3), 354–359 (2006).
Cutright, T. J. & Meza, L. Evaluation of the aerobic biodegradation of trichloroethylene via response surface methodology. Environ. Int. 33, 338–345 (2007).
Agustin-Salazar, S. et al. Lignin and holocellulose from pecan nutshell as reinforcing fillers in poly (lactic acid) biocomposites. Int. J. Biol. Macromol. 115, 727–736 (2018).
Engel, J. B. et al. Reuse of different agroindustrial wastes: Pinhão and pecan nutshells incorporated into biocomposites using thermocompression. J. Polym. Environ. 28, 1431–1440 (2020).
Pörtner, R., Faschian, R. & Goelling, D. Fermentation of lactic acid bacteria: State of the art and new perspectives. In Applied Biocatalysis: From Fundamental Science to Industrial Applications 1st edn (eds Hilterhaus, L. et al.) 317–342 (Wiley-VCH Verlag GmbH & Co, 2016).
Dorado, A. D., Lafuente, F. J., Gabriel, D. & Gamisans, X. A comparative study based on physical characteristics of suitable packing materials in biofiltration. Environ. Technol. 31, 193–204 (2010).
Staroń, P. & Chwastowski, J. Raphia-microorganism composite biosorbent for lead ion removal from aqueous solutions. Materials 14, 7482 (2021).
Darmayati, Y., Wiranata, Y., Afianti, N. F. & Manurung, B. Comparison of viability and efficacy of an immobilized bacterial consortium in four different carriers to degrade oil. In IOP Conference Series: Earth and Environmental Science (OP Publishing Ltd, 2021).
Figueroa-Torres, G. M. et al. Kinetic studies of heavy metals biosorption by acidogenic biomass immobilized in clinoptilolite. J. Taiwan Inst. Chem. Eng. 61, 241–246 (2016).
Lemon, K. P., Earl, A. M., Vlamakis, H. C., Aguilar, C. & Kolter, R. Biofilm development with an emphasis on Bacillus subtilis. Curr. Top. Microbiol. Immunol. 322, 1–16. https://doi.org/10.1007/978-3-540-75418-3_1 (2008).
Sarioglu, O. F., Celebioglu, A., Tekinay, T. & Uyar, T. Bacteria-immobilized electrospun fibrous polymeric webs for hexavalent chromium remediation in water. Int. J. Environ. Sci. Technol. 13, 2057–2066 (2016).
Lupascu, L., Petuhov, O., Timbaliuc, N. & Lupascu, T. Study of the adsorption of Bacillus subtilis and Bacillus cereus bacteria on enterosorbent obtained from apricot kernels. C J. Carbon Res. 8, 38 (2022).
Meza-Figueroa, D. et al. The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere 77, 140–147 (2009).
Norma Mexicana. NMX-AA-132-SCFI-2016. Soil sampling for metals and metalloids identification and quantification, and samples handling. Official Journal of the Federation (2017).
León-García, G. J. et al. Assessment of heavy metal pollution in sediments of the Sonora River basin impacted by mining activities. Environ. Prog. Sustain. Energy 41, e13796 (2022).
Okewale, I. A. & Grobler, H. Assessment of heavy metals in tailings and their implications on human health. Geosyst. Geoenviron. 2, 100203 (2023).
Jafarzade, M., Mohamad, S., Usup, G. & Ahmad, A. Heavy-metal tolerance and antibiotic susceptibility of red pigmented bacteria isolated from marine environment. Nat. Resour. J. 03, 171–174 (2012).
Tripathi, N. & Sapra, A. Gram Staining (StatPearls Publishing, 2023).
Weisburg, W. G., Barns, S. M., Pelletier, D. A. & Lane, D. J. 6S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703 (1991).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Altschul, S. F., Gish, W., Miller, W. & Myers, E. W. Basic local alignment search tool (BLAST). J. Mol. Biol. 215, 403–410 (1990).
Acknowledgements
The authors are grateful to the Departamento de Investigaciones Científicas y Tecnológicas de la Universidad de Sonora (DICTUS) for providing financial support for this research (USO313007363). Jonathan Parades-Aguilar (605384) thank to the Consejo Nacional de Ciencia y Tecnología (CONAHCyT) for the scholarship received. We thank to the Departamento de Investigación en Polímeros y Materiales de la Universidad de Sonora, the Laboratorio de Ciencias Ambientales (LCA)-ERNO (UNAM), Dr. Ramón Dórame Miranda (UNISON), Dr. Rene Loredo Portales, and Dr. Daniel Ramos Pérez for their support with the SEM image analyses (UNAM). Finally, we also thank the participation of the students Diana Bárbara Sandoval Robles and Ana Paola Martínez Almada for their technical assistance.
Author information
Authors and Affiliations
Contributions
J. P.-A., K. C., and L. A. M.-J. conceived and designed the research. J. P.-A. and K. C. conducted the experiments. J. P.-A., K. C., and L. A. M.-J. analyzed the data. J. P.-A. and K. C. wrote the manuscript. S. A.-S., P. C., V. A., and N. G.-M. participated in drafting and critically revising the article. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parades-Aguilar, J., Calderon, K., Agustin-Salazar, S. et al. Isolation and identification of metallotolerant bacteria with a potential biotechnological application. Sci Rep 14, 3663 (2024). https://doi.org/10.1038/s41598-024-54090-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54090-0
- Springer Nature Limited