Abstract
Cross-sectional studies support the role of serum uric acid (SUA) in inflammation, but evidence from cohort studies is scarce. Longitudinal associations between SUA and high-sensitivity C-reactive protein (hs-CRP) were examined in the general population. Data for participants from the Health Examinees-Gem cohort (n = 50,028; 40–69 years; 67% women) who were examined between 2004 and 2013 and followed up until 2016 were analyzed. SUA and hs-CRP were measured at baseline and during follow-up. SUA was evaluated as a continuous variable and was also divided into sex-specific quartiles. Mean hs-CRP levels at follow-up were evaluated using multivariable proportional odds regression, with non-linear smoothed baseline hs-CRP levels serving as a covariate. Selected pathological markers were also examined in relation to hs-CRP. Increased levels of SUA at baseline were related to increased levels of hs-CRP at follow-up [regression coefficient per mg/dL increase in baseline SUA (β) = 0.08, 95% confidence interval (CI), 0.040–0.128]. A dose–response relationship was observed, (P for linear trend = 0.0015). The mean values of hs-CRP were highest among participants with the highest follow-up but lowest baseline SUA levels. Elevated hs-CRP levels at follow up (> 3 mg/L) were positively related to fasting blood glucose levels, triglycerides levels, liver enzymes, and blood pressure, but negatively related to high density lipoprotein cholesterol levels per unit increase in baseline hs-CRP. High SUA levels were associated with high hs-CRP levels, suggesting a potential role of SUA in inflammation. However, additional research is needed to confirm these findings.
Similar content being viewed by others
Introduction
Low-grade, systemic chronic inflammation (SCI) is a significant contributor to the etiology of noncommunicable diseases. Globally, more than half of all deaths are attributed to chronic inflammatory diseases1, which underscores the need to identify exposures affecting SCI and therefore SCI-related diseases. C-reactive protein (C-RP) is a biomarker of low-grade SCI1,2. Specifically, high-sensitivity C-reactive protein (hs-CRP) is the most reliable marker of inflammation in clinical practice3. Accordingly, identifying multiple factors related to hs-CRP may help to elucidate the underlying determinants of SCI.
Serum uric acid (SUA), is a purine metabolite that has antioxidant effects within normal physiological ranges4, but may also exhibit prooxidant characteristics5,6,7. Elevated levels of SUA have been associated with cardiovascular disease (CVD), hypertension, metabolic syndrome, chronic kidney disease (CKD)8, cancer9,10, and all-cause mortality11,12,13. Several researchers have hypothesized that SUA may induce metabolic abnormalities via inflammatory pathways14, including activation of the IkB kinase/IkB alpha/NF-kB3, and AMPK (AMP-activated protein kinase)-mTOR (mammalian target of rapamycin) signaling pathways15.
However, few population-based studies have evaluated the association between SUA and inflammatory biomarkers such as hs-CRP. In a cross-sectional study of 2731 nondiabetic adults, SUA was positively associated with hs-CRP after adjusting for age, sex, and body mass index (BMI)3. In another cross-sectional study of 1107 individuals, SUA was correlated with serum hs-CRP after age and sex adjustment16. Raesi et al. reported a positive correlation between SUA and hs-CRP in 378 Iranian postmenopausal women17. SUA was also positively associated with C-RP in 957 community-dwelling elderly participants18. Only two longitudinal studies have investigated the association between SUA and hs-CRP; one included 3518 Brazilians, and the other included 892 Italians. Both studies reported positive associations between baseline SUA and hs-CRP at follow up19,20. The only clinical trial on this topic recruited 20 healthy volunteers, and reported that uric acid administration resulted in increased IL-6, but not C-RP14.
Most of the previous epidemiological studies on the relationship between SUA and hs-CRP have been cross-sectional3,16,17,19, and therefore causal conclusions could not be drawn. Moreover, previous studies were limited in sample size3,16,17,20, recruited participants with cardiometabolic conditions3,16, and adjusted for limited confounders3,16. In addition, none of those studies were conducted in an Asian population. To the best of our knowledge, only two longitudinal studies have evaluated the association between SUA and hs-CRP19,20. More longitudinal studies with large sample sizes taken from diverse populations are needed to determine whether SUA is a potential inflammatory biomarker.
Using a large population study of Korean adults, we evaluated the longitudinal association between baseline SUA and hs-CRP levels at follow up. In secondary analyses, the levels of selected pathological markers predicted from changes in hs-CRP were evaluated. This study hypothesised that baseline SUA was positively associated with hs-CRP at follow-up in individuals without elevated baseline hs-CRP (hs-CRP > 3 mg/L).
Materials and methods
The Health Examinees study
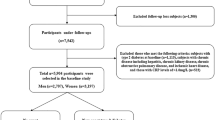
We conducted an observational longitudinal analysis of the Health Examinees study (HEXA) cohort, a prospective population-based substudy within the Korean Genome and Epidemiology study (KoGES) that was established to investigate the etiological factors of complex diseases21. The HEXA recruited participants between 2004 and 2013 at 38 health examination centers and training hospitals located in the eight regions of Korea and follow-up studies were conducted between 2007 and 2016. The study details have been published elsewhere22. Individuals who participated in both the baseline and follow up surveys of the HEXA study were included in the current analysis (n = 70,253, ≥ 40 years). From those individuals, we excluded participants who were recruited from invalid sites: (1) were recruited from sites that participated in the pilot study between 2004 and 2006; (2) were recruited from sites that did not meet the HEXA standards for biospecimen quality control; and (3) were recruited from sites that participated in the study for < 2 years (n = 219)23. Furthermore, participants who had missing data on baseline SUA (n = 9) and hs-CRP (n = 10,746), those who had missing data on hs-CRP at follow-up (n = 4497), those with follow-up hs-CRP equal to or exceeding 10 mg/L (considered as having acute inflammation, n = 664), and those with elevated hs-CRP (> 3 mg/L) at baseline (n = 4090) were excluded, yielding a final analytical sample of 50,028 participants (Fig. 1).
Measurement of clinical variables
After an overnight fast, a total sample of at least 19 cc of blood was drawn into a serum separator tube (SST) and two ethylenediamine-tetra-acetic acidtubes and placed into a conical tube for laboratory tests and storage. The biospecimens were given study IDs that were matched to the participants’ questionnaires and labeled with 2D bar code stickers. Biospecimens are kept in refrigerators at each medical institution until they are collected within 24 h by a courier from the commercial laboratory that processes specimens using various laboratory tests21. Blood test results were uploaded to the Korean Centers for Disease Control (KCDC) web-based database system. The extracted samples were subsequently transported to and stored at the National Biobank of Korea (https://biobank.nih.go.kr/eng). Baseline SUA, hs-CRP, fasting blood glucose (FBG), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and gamma glutamyl transferase (GGT) levels were measured using enzymatic calorimetric methods with automatic analyzers (ADVIA 1650 and ADVIA 1800 (Siemens, Tarrytown, NY, USA)24. Quality control of biospecimens in the Biobank of Korea has been described in detail elsewhere25,26.
Baseline covariates
Educational level, and household income were assessed in addition to demographic characteristics such as age and sex. Other covariates included lifestyle factors such as: smoking, where current smokers were defined as participants who had smoked more than four hundred cigarettes during their lifetime and were still smoking27; drinking categorized as current alcohol drinkers, and past drinkers/never drinkers, where current alcohol drinkers were those who reported that they had ever drunk alcohol and were still drinking alcohol at the time of the interview; and regular physical exercise, assessed by asking participants to report (1) whether they engaged in regular sweat-inducing physical exercise; (2) the number of times they engage in these exercises in a week (1–2 times/week to everyday), and (3) the duration of the exercise. Regular exercise was defined as engaging in activities that caused body sweating for at least five times a week lasting at least 30 min.
Weight and height were objectively measured at baseline by trained medical staff. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). BMI was categorized into four classes based on the WHO classification system of BMI for Asian adults: < 18.5, 18.5–22.9, 23–24.9 and ≥ 25 kg/m228. Blood pressure was measured using a mercury sphygmomanometer while the participant assumed a sitting position. Two measurements were used to compute average diastolic (DBP) and systolic (SBP) blood pressure.
The information about diseases and use of medication was obtained using a standardized questionnaire administered by trained staff and self-reported by participants. Diabetes was defined as FBG ≥ 126 mg/dL or drug treatment for elevated FBG, and hypertension was defined as SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or drug treatment for elevated blood pressure. Abdominal obesity was defined as a waist circumference ≥ 90 cm for men and ≥ 80 cm for women29. Prevalence of renal function decline was defined using estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2) that was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI)30. The number of morbidities was calculated based on the following chronic diseases defined based on self-reported doctor diagnosis and intake of medication: CVD, chronic gastritis, esophageal ulcers, gall bladder disease, fatty liver disease, chronic liver disease, chronic obstructive pulmonary disease, asthma, chronic bronchitis, pulmonary tuberculosis, depression, Parkinson’s disease, arthritis, osteoarthritis, hyper lipidemia, malignant neoplasms, and type 2 diabetes. Based on the number of morbidities, a disease score was created, and participants were categorized as having a disease score of zero (indicating absence of any of the above diseases) or ≥ 1 (indicating presence of any of the above diseases).
Statistical analysis
Participants were divided into sex-specific quartiles of SUA (Q1–Q4) for descriptive purposes. Participants with missing data on income (> 10%) were categorized as ‘unknown income’, whereas categorical variables with < 5% missing data were replaced by the mode (for education, marital status, smoking, drinking, physical exercise, and metabolic syndrome). Continuous variables with < 5% missing data were replaced by the median. The distribution of participant characteristics according to sex-specific quartiles of SUA was described using percentages for categorical variables or least square means with their standard errors (SE) for continuous variables.
Potential confounders were selected a priori based on previous literature1,19,20,31. To minimize potential regression to the mean bias, the change from baseline to follow up was not considered the primary outcome. The main outcome measure was follow up hs-CRP levels, adjusted for baseline hs-CRP and other covariates including age (spline); sex; marital status (married/cohabiting, single/separated/divorced/widowed/others); (≤ elementary school, middle school, high school, and ≥ university); family monthly income (< 1000, 1000–3000 and ≥ 3000 USD); smoking status (current or past/never smoker); drinking status (current or past/never drinker); regular physical exercise (yes or no); BMI (spline); metabolic syndrome and disease score.
The correlations between follow-up, baseline, and change from baseline to follow up of hs-CRP levels according to quartiles of baseline SUA were examined using scatter plots with loess nonparametric smoothers. To examine the longitudinal associations between baseline SUA and hs-CRP levels at follow-up, the proportional odds model was fit to estimate the mean values of hs-CRP at follow-up as a function of baseline SUA (per mg/dL increment), adjusted for baseline hs-CRP and the covariates described above. Smoothed nonlinear effects of baseline hs-CRP were fit using restricted cubic splines (RCS) with 4 knots, and allowing the nonlinear effects to differ by quartiles of SUA. Effect modification by sex was tested by including cross-product terms relating to sex and SUA in the model. There was no evidence of an interaction between sex and SUA; thus we did no presentstratified results. In secondary analyses, the odds ratios (ORs) and 95% confidence intervals (CIs) of incident elevation in hs-CRP (> 3 mg/L) were calculated using multivariable logistic regression, comparing the highest (Q4) to lowest (Q1) of baseline SUA.
The change in estimated mean hs-CRP values at follow-up according to quartiles of follow-up values of SUA, adjusted for baseline SUA levels, was also estimated using the proportional odds model, allowing for nonlinear smoothed effects of baseline SUA. To explore the clinical relevance of hs-CRP, the mean values of selected pathological markers (FBG, TG, HDL-C, liver enzymes, and blood pressure) at follow-up were estimated, adjusting for their baseline measurements, and using follow-up hs-CRP as the predictor. Baseline values of hs-CRP were also included as covariates, and were modeled as RCS to visualize predicted means according to baseline hs-CRP.
Descriptive analyses and logistic regression were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA), and proportional odds models and graphics were conducted using R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was used to define statistical significance.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Korean Health and Genomic Study of the Korean National Institute of Health and the Institutional Review Boards of all participating hospitals (IRB no. E-1503-103-657).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Results
A total of 50,028 participants including 33,593 women, were analyzed. The mean age (mean ± SE) was 53.6 ± 0.07 years. The median values (IQR) of baseline SUA was 4.5 (3.8–5.4) mg/dL and 4.4 mg/dL (3.7–5.0) at follow-up. The median hs-CRP at follow up was 0.6 (0.4–1.0) mg/L. After a median (IQR) follow-up of 5.0 (3.9–6.1) years, 2023 (4.0%) participants had elevated hs-CRP. Participants who were older, had low education attainment, were current smokers, current drinkers, or who had a high BMI were more likely to have high levels of SUA at baseline (Table 1). Participants with the highest levels of SUA were less likely to engage in regular physical exercise. On the other hand, individuals with high SUA levels were more likely to be diagnosed with metabolic syndrome, and chronic morbidity. Furthermore, hs-CRP, liver enzymes and creatinine tended to be elevated among participants in the highest quartile of SUA.
There was a strong correlation between baseline and follow up hs-CRP by SUA (Figure S1). Furthermore, as baseline hs-CRP increased, a greatest reduction in follow-up hs-CRP was observed in individuals with lower baseline SUA levels (Figure S1).
The regression coefficients and 95% CIs for hs-CRP at follow-up per mg/dL increase in baseline levels of SUA are shown in Table 2. Increased levels of SUA at baseline was related to increased levels of hs-CRP at follow-up (β, 0.08; 95% CI 0.040–0.128). The odds ratios and 95% CI for incident elevated hs-CRP according to quartiles of SUA are displayed in Table S1. After adjusting for baseline hs-CRP and potential confounders, increasing SUA was associated with increased odds of elevated hs-CRP [OR (95% CI) for highest vs. lowest SUA levels, 1.21 (1.06–1.38), P for trend = 0.010; OR (95% CI) per 1 mg/dL increment in SUA, 1.06 (1.02–1.11)].
The analyses of nonlinear relationships showed a linear increase in follow-up hs-CRP levels with increasing baseline levels of SUA (Fig. 2).
Regression coefficients for hs-CRP levels at follow-up according to baseline SUA levels. The dark line represents regression estimates, and bands represent 95% CIs. Coefficients were adjusted for baseline hs-CRP, age (spline), sex, education, income, marital status, alcohol consumption, smoking status, regular physical exercise, BMI (spline), metabolic syndrome, and disease score.
The longitudinal relationship between SUA and hs-CRP, with smoothed nonlinear effects of baseline hs-CRP are displayed in Fig. 3. Improvements in follow-up hs-CRP levels were observed with lower baseline SUA among individuals with baseline hs-CRP levels > 1.5 mg/L.
Estimated mean hs-CRP at follow up according to levels of SUA at baseline, allowing for non-linear effects of baseline hs-CRP. The model was fitted using the proportional odds model, and adjusted for baseline hs-CRP, age (spline), sex, education, income, marital status, alcohol consumption, smoking status, regular physical exercise, BMI (spline), metabolic syndrome, and disease score.
The predicted mean of hs-CRP levels at follow-up according to longitudinal changes in SUA from baseline to follow-up are shown in Fig. 4. Predicted mean values of hs-CRP at follow-up were highest among individuals who had the lowest baseline and highest follow-up SUA levels. However, sustained low levels of SUA were associated with the lowest mean values of hs-CRP (Fig. 4).
Predicted mean of hs-CRP at follow up according to SUA levels at follow up and baseline, with baseline SUA fitted as a smoothed nonlinear covariate, with additional adjustment for baseline hs-CRP, age (spline), sex, education, income, marital status, alcohol consumption, smoking status, regular physical exercise, BMI (spline), metabolic syndrome, and disease score.
We examined whether increased hs-CRP levels from baseline to follow-up was correlated with pathological markers, and the results are shown in Fig. 5 and Figure S2. Elevated follow-up hs-CRP (> 3 mg/L) was associated with the highest mean values of FBG, TG, liver enzymes, blood pressure; and reduced HDL-C levels, particularly at lower levels of baseline hs-CRP.
Predicted mean of pathological markers according to longitudinal change in hs-CRP, adjusted for baseline pathological markers, hs-CRP, age (spline), sex, education, income, marital status, alcohol consumption, smoking status, regular physical exercise, BMI (spline), metabolic syndrome, and disease score.
Discussion
This study used a large population-based cohort of 50,028 Korean adults, and multiple measurements of SUA and hs-CRP, to evaluate the longitudinal relationship between SUA and hs-CRP after 5 years. Individuals with the highest baseline SUA levels had the highest mean values of hs-CRP at follow-up. A linear dose–response relationship between SUA and hs-CRP was reported.
To discern the clinical relevance of elevated hs-CRP, the predicted means of FBG, TG, HDL-C, liver enzymes, and blood pressure were compared across levels of hs-CRP at follow up, adjusted for their baseline values. Serum hs-CRP level at follow-up was positively related to FBG, TG, liver enzymes, and blood pressure, and negatively related to HDL-C. The results suggest that SUA may be related to elevated hs-CRP levels, indicating SCI in the general Korean population.
The study findings are broadly supported by previous observational studies. Plasma SUA was positively associated with hs-CRP in a sample of 2731 outpatient individuals at a general hospital3. Raesi et al. reported a positive correlation between SUA and hs-CRP in 378 Iranian postmenopausal women17. SUA was positively associated with several inflammatory biomarkers including hs-CRP in community dwelling elderly participants18. In a cross-sectional study of 1107 participants, elevated SUA was related to increased levels of hs-CRP16. Elevated SUA was also associated with high circulating inflammatory markers including IL-6, CRP and TNF-α, and was negatively associated with IL-1β32. Consistent with findings from the present study, two previous longitudinal studies reported that baseline SUA levels predict increased levels of hs-CRP, and a high prevalence of elevated hs-CRP at follow-up in Brazilian and Italian populations19,20. One clinical trial reported null associations between SUA administration and C-RP in 20 volunteers14. Clinical trials with relatively large sample sizes are needed to confirm the associations between SUA and hs-CRP levels reported in observational studies.
Potential mechanisms have been suggested to explain the relationship between SUA and inflammation. SUA promotes a proinflammatory state by upregulating the production of soluble inflammatory mediators14, and increasing the production of reactive oxygen species33. SUA was found to exhibit proinflammatory effects by stimulating the expression of several inflammatory biomarkers via activation of the IkB kinase/IkB alpha/NF-kB signaling pathways in hepatic cells3. Furthermore, SUA promotes inflammasome-dependent inflammation by regulating the AMPK (AMP-activated protein kinase)-mTOR (mammalian target of rapamycin) signaling pathway15. Direct proinflammatory effects of SUA on vascular smooth muscle cells have also been reported31.
This study is the first to report on the longitudinal association between SUA and an SCI marker in the general Asian population. These results support the hypothesis that SUA levels may be linked to low-grade SCI, and may serve as a potential marker of SCI if validated with existing inflammatory markers.
Nevertheless, the results from this study should be contextualized within the following limitations. First, only hs-CRP was used to evaluate low-grade SCI. Although hs-CRP is a marker of inflammation, it is not a specific marker of chronic inflammation, and its optimal clinical use is still debated33. Moreover, interindividual variations in hs-CRP exist according to ethnicity and age due to genetic polymorphisms and lifestyle factors33, suggesting that our findings may not be generalizable to young individuals, or individuals of other ethnicities. In addition, short-term intraindividual variations in hs-CRP have been reported. However, we used multiple measures of hs-CRP to reduce possible misclassification of individuals resulting from the use of a single measurement33. Thus, to validate the role of SUA in inflammation, large population-based cohort studies that use inflammatory scores based on a wide array of inflammatory markers are needed1. Second, this was an observational study, which limits inference of causal relationships between SUA and SCI. Nevertheless, we employed a longitudinal modeling approach to minimize reverse causation. Finally, these results may be prone to residual confounding from factors that we could not control for, such as medication since data on prescribed medication could not be accessed. Nevertheless, we adjusted for presence of comorbidities based on self-reported diagnoses and current use of medication for existing chronic diseases.
The major strengths of our study include the use of a large, ethnically homogeneous sample of participants from all regions of Korea-which increases the generalizability of the findings to the entire adult population. The longitudinal design allowed us to examine the temporal relationships between SUA levels and SCI. Adjusting for multiple demographic, lifestyle, and medical-related factors that affect both SUA and hs-CRP levels contributed to the reliability of our estimates. Furthermore, the use of biochemical variables that were measured using standardized laboratory procedures, minimized the potential effects of measurement errors in the estimates.
Conclusion
High SUA levels were independently related to increased levels of hs-CRP-suggesting low-grade SCI, in a large population-based sample of Korean adults. This relationship was dose-dependent. Furthermore, high levels of hs-CRP positively predicted FBG, TG, liver enzymes and blood pressure, and negatively predicted HDL-C. Future studies are needed to validate SUA using other established inflammatory biomarkers. Nevertheless, these results contribute to further understanding of the role of SUA as a potential proinflammatory marker.
Data availability
The dataset used for the analysis in this study is maintained and managed by the Division of Population Health Research at the National Institute of Health, Korea Centers for Disease Control and Prevention. The Health Examinees Study dataset has been merged with the cancer registry data provided by National Cancer Center of Korea in a collaborative agreement. It contains some personal data that may potentially be sensitive to the patients, even though researchers are provided with an anonymized dataset that excludes resident registration numbers. Other researchers may request access to the data by contacting the following individuals at the Division of Population Health Research, National Institute of Health, Korea Centers for Disease Control and Prevention: Senior Staff Scientist Dr. Jung Hyun Lee (jaylee1485@korea.kr); Director Dr. Kyoungho Lee (khlee3789@korea.kr).
References
Johnson, R. J. et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 71(6), 851 (2018).
Kim, K. et al. Association between serum uric acid level and ESRD or death in a Korean Population. J. Korean Med. Sci. 35, 28 (2020).
Zuo, T. et al. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 16, 1 (2016).
Konta, T. et al. Association between serum uric acid levels and mortality: A nationwide community-based cohort study. Sci. Rep. 10(1), 1–7 (2020).
Wang, W. et al. Increased risk of cancer in relation to Gout: A review of three prospective cohort studies with 50,358 subjects. Mediat. Inflamm. 20, 15 (2015).
Strasak, A. M. et al. The role of serum uric acid as an antioxidant protecting against cancer: Prospective study in more than 28,000 older Austrian women. Ann. Oncol. 18(11), 1893–1897 (2007).
Liu, D. et al. What is the biological function of uric acid? An antioxidant for neural protection or a biomarker for cell death. Dis. Mark. 20, 19 (2019).
Itahana, Y. et al. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene 34(14), 1799–1810 (2014).
Sautin, Y. Y., Nakagawa, T., Zharikov, S. & Johnson, R. J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 293, 2 (2007).
Lanaspa, M. A. et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287(48), 40732–40744 (2012).
Corry, D. B. et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 26(2), 269–275 (2008).
Spiga, R. et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 37(6), 1241–1249 (2017).
Mi, S., Gong, L. & Sui, Z. Friend or foe? An unrecognized role of uric acid in cancer development and the potential anticancer effects of uric acid-lowering drugs. J. Cancer 11(17), 5236–5244 (2020).
Tanaka, T. et al. A double blind placebo controlled randomized trial of the effect of acute uric acid changes on inflammatory markers in humans: A pilot study. PLoS One 12, 8 (2017).
Kimura, Y. et al. Soluble uric acid promotes atherosclerosis via AMPK (AMP-activated protein kinase)-mediated inflammation. Arterioscler. Thromb. Vasc. Biol. 40, 570–582 (2019).
Nelson, S. H. et al. The association of the C-reactive protein inflammatory biomarker with breast cancer incidence and mortality in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 26(7), 1100–1106 (2017).
de Coutinho, T. A. et al. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am. J. Hypertens. 20(1), 83–89 (2007).
Raeisi, A. et al. Association of serum uric acid with high-sensitivity C-reactive protein in postmenopausal women. Climacteric 20(1), 44–48 (2017).
Keenan, T. et al. Relation of uric acid to serum levels of high-sensitivity C-reactive protein, triglycerides, and high-density lipoprotein cholesterol and to hepatic steatosis. Am. J. Cardiol. 110(12), 1787–1792 (2012).
Ruggiero, C. et al. Uric acid and inflammatory markers. Eur. Heart J. 27(10), 1174–1181 (2006).
Ruggiero, C. et al. Usefulness of uric acid to predict changes in C-reactive protein and interleukin-6 in 3-year period in Italians aged 21 to 98 years. Am. J. Cardiol. 100(1), 115 (2007).
Kim, Y. & Han, B.-G. Cohort profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 46(2), e20–e20 (2017).
Kang, D. et al. The health examinees (HEXA) study: Rationale, study design and baseline characteristics. Asian Pac. J. Cancer Prev. 16(4), 1591–1597 (2015).
Lee, H.-W. et al. Frequent low dose alcohol intake increases gastric cancer risk: The Health Examinees-Gem (HEXA-G) study. Cancer Biol. Med. 20, 20 (2022).
Yang, J. J., Song, M., Yoon, H.-S., Lee, H.-W., Lee, Y., Lee, S.-A., et al. What are the major determinants in the success of smoking cessation: Results from the health examinees study; 2015.
Pan, W.-H. & Yeh, W.-T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 17(3), 370 (2008).
Alberti, K. G. M. M., Zimmet, P. & Shaw, J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Med. 23(5), 469–480 (2006).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604 (2009).
Waring, W. S., McKnight, J. A., Webb, D. J. & Maxwell, S. R. J. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 55(11), 3127–3132 (2006).
Waring, W. S. et al. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin. Sci. (Lond). 105(4), 425–430 (2003).
Lyngdoh, T. et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One 6, 5 (2011).
Kanellis, J. et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41(6), 1287–1293 (2003).
Yousuf, O. et al. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link?. J. Am. Coll. Cardiol. 62(5), 397–408 (2013).
Acknowledgements
This research was supported by Regional Innovation Strategy (RIS) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2022RIS-005).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.K., methodology, A.K., S.A.-L., formal analysis, A.K., investigation, A.K., resources, S.A.-L., writing-original draft preparation, A.K., writing-review and editing, A.K., S.A.-L., supervision and project administration, S.A.-L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kityo, A., Lee, SA. Longitudinal changes in high sensitivity C-reactive protein associated with serum uric acid in the Korean Genome and Epidemiology Study. Sci Rep 14, 374 (2024). https://doi.org/10.1038/s41598-023-50951-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50951-2
- Springer Nature Limited