Abstract
Reliable real-world data on direct acting anti-retroviral (DAA) uptake and treatment outcomes are lacking for patients with hepatitis C virus (HCV) in sub-Saharan Africa. This study provides data on HCV DAA-based treatment outcomes, mortality, loss-to-follow up, and associated factors among patients in Eritrea. A multicenter retrospective observational cohort study was conducted in two tertiary hospitals in Asmara, Eritrea. A structured checklist was used to collect data from patient’s cards. Descriptive and inferential statistics used included means (± Standard deviation (SD), medians (Interquartile range (IQR), chi-squire (χ2), Kaplan–Meier estimates, and multivariate Cox proportional hazard models. A total of 238 patients with median age of 59 years (IQR 50–69 years) were enrolled in the study. Out of the 227 patients initiated on treatment, 125 patients had viral load measurements at 12 weeks after end of treatment (EOT) whereas 102 patients had no viral load measurements at 12 weeks EOT. Among the patients with HCV RNA data post-EOT 12, 116 (92.8%) had sustained viral response (SVR). The prevalence of death and loss-to-follow up (LTFU) were (7.5%, 95% CI 1.7–4.1) and 67 (28.1%, 95% CI 22.3–33.9) translating into an incidence of 1.1 (95% CI 0.8–1.5) per 10,000 person days. Independent predictors of LTFU included the enrollment year (2020: aHR = 2.2, 95% CI 1–4.7; p value = 0.04); Hospital (Hospital B: aHR = 2.2, 95% CI 1–4.7; p value = 0.03) and the FIB-4 score (FIB-Score < 1.45: aHR = 3.7, 95% CI 1.2–11.5; p value = 0.02). The SVR rates achieved in this cohort were high. However, high LTFU and high mortality driven largely by late presentation and suboptimal population screening/case finding, were uncovered. These challenges can be addressed by test-and-treat programs that simultaneously prioritize programmatic screening, decentralization of care, and better patient tracking in the HCV care cascade.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV), a predominantly blood-borne hepatotropic RNA virus with ~ 7 genotypes (Gt1-7) and > 67 confirmed subtypes1, has emerged as one of the leading causes of mortality and morbidity worldwide2. According to a recent World Health Organization (WHO) global estimate, the number of people with chronic HCV infection is approximately 71 million (95% confidence interval (CI): 62–79 million) people (1%)3 and only 20% are aware of their condition4. In terms of new infections, the data suggest that approximately 1.5 million new infections are registered per year (global incidence: 23.7 per 100,000)5. Most of these new infections have been attributed to iatrogenic causes, injection drug use, vertical transmission, body piercings/traditional scarification, and occupational exposure (e.g. needle-stick injuries), among others6.
In general, spontaneous clearance of the virus can occur within 6 months in approximately 30% (95% CI 15–45%) of infected persons7. However, 70% (95% CI 55–85%) progress to chronic HCV infection that can remain asymptomatic, and therefore unnoticeable, for decades7. Chronic hepatitis C (CHC) viremia is associated with multiple hepatic and extra hepatic sequelae which can lead to mortality8,9,10. Collectively, these complications were responsible for approximately 580,000 HCV-related deaths in 2017 and substantial impairments in multiple health-related quality of life (HRQL) indices11,12. Additional data suggests that unlike, Human Immunodeficiency virus (HIV), or tuberculosis (TB); mortality has trended upward in the last two decades. This upward trend is projected to increase in low- and middle-income countries (LMICs) if testing and subsequent treatments are not scaled-up13.
Fortunately, the development of multiple direct-acting antivirals (DAAs) that can achieve up to 95% cure rate with few adverse reactions has revolutionized treatment2,14. The main current therapeutic goal for HCV and prevention of liver disease progression is the sustained viral response (SVR)15,16. Beyond cure, SVR is associated with several solid clinical endpoints such as reduced likelihood of decompensated cirrhosis12,17. Additional public health benefits include better utility values and a reduction in community transmission rates12. In 2016, the potential benefit of effective screening programs and prompt treatment prompted the WHO to develop an improved public health action strategy to eliminate HCV infection by 203018. This strategy calls for the diagnosis of 90% of infected individuals and the treatment of 80% of eligible patients.
Emerging evidence suggests that these goals are unlikely to be met14. First, some scholars have noted that the prevalence of HCV in LMIC in SSA is poorly researched3,19. Of note, the per-capita quantity and quality of real-world studies on DAA effectiveness or data on HCV care cascade performance are extremely rare. In Eritrea, real-world data on therapeutic outcomes is lacking. However, existing data point to low-to-moderate level of HCV infection20,21. These circumstances create a strong demand for updated high-quality data on a range of HCV-related issues in Eritrea. Therefore, this study was designed to investigate the treatment outcome of DAA therapy, estimate the frequency loss-to-follow up (LTFU), mortality and incidence rates, and associated factors in two pilot treatment centers in Asmara, Eritrea.
Methods
Study design and settings
This observational retrospective cohort study was conducted on patients followed from 2018 to 2021 in the two major chronic HCV care centers in Asmara, Eritrea. See Fig. 1. At present, they serve as the major treatment centers for patients with HCV in Eritrea. Since the inception of the program in 2018, a total of 238 patients have received treatment and follow-up care in the two facilities. Treatment, in general, is guided by the Eritrean Ministry of Health (EMoH) Guideline for Chronic Hepatitis B and C infection (2018). Pan-genotypic DAA regimens recommended in the guideline include Sofosbuvir (SOF) (an NS5B Polymerase Inhibitor-nucleotide analogue), Daclatasvir (DCV) and Velpatasvir (VEL) (NS5A replication complex inhibitors) with SOF/VEL or SOF/DCV for 12 weeks being the preferred combination (See “Supplementary data” Page 2 for clinical laboratory assessments). The HCV viral load count was evaluated using HCV RNA assay. After initiation of DAA, HCV-RNA viral load assays are quantified at 12 and 24 weeks. Of note, these assessments are undertaken at the discretion of the attending physician/clinician and the costs of treatment are covered by the government.
Map of Eritrea, Zoba Maekel (central zone) and locations of the treatment centers and HCV viral load testing center. Note: The map was created using ArcGIS software (ArcMap version 10.7.1 (Esri, Redlands, CA, USA) and google search [https://www.google.com/maps/place/Asmara,+Eritrea/@15.3329318,38.918554,16.25z/data=!4m6!3m5!1s0x166df23bb4c933a9:0xb8c1b327af63f5c5!8m2!3d15.3228767!4d38.9250517!16zL20vMGZuejg].
Participants
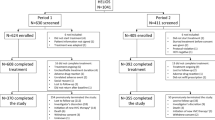
Patients in the two treatment centers are pooled from the entire country. In general, most patients are referred by clinicians in centers across the country or by transfusion centers following HCV antibody positivity on serological testing. In 2019, several patients were referred following screening campaigns among specific subgroups22. All patients aged > 18 years, registered in the two treatment centers were enrolled in this study. See Fig. 2 for additional details.
Data collection tool
Data have been collected via a structured checklist from each patient’s clinical card that is routinely documented form for every patient upon enrollment and follow-up. The checklist was structured in a systematic way that would enable data collectors to retrieve data in an orderly fashion and detect systematic errors.
Clinical and biochemical parameters
The following laboratory and clinical parameters were collected from the patient’s medical records: sex, age at enrollment, address, enrollment year, marital status, DAA regimen, HCV-RNA (baseline, 12 and 24 weeks), Platelets (PLTs), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), serum bilirubin (BIL), HIV status, Hepatitis B virus (HBV), and drug-side effect. Follow-up outcomes were also collected from clinical cards.
Outcome measures and formulas
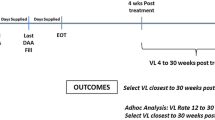
Patients were considered to have SVR if they had undetectable serum HCV RNA 12 weeks after EOT16. End of treatment (EOT) was estimated from the last day covered by prescription of SOF/DCV or SOF/VEL. Further, treatment nonresponse was defined as detectable HCV-RNA after EOT. Survival funtions included death and loss-to-follow up (LTFU). Death event was defined as all-cause mortality occurring during the patient’s follow up. In turn, loss-to-follow-up (LTFU) was defined as nonattendance of scheduled clinic appointments after enrollment into care23.
Cirrhosis probability was defined using FIB-4 (Non-invasive Fibrosis Evaluation in Hepatitis C) formula ([Age (years) × AST (U/L)]/[PLT (× 109/L) × ALT1/2 (U/L)]. Computed scores were grouped as follows: Less likely (< 1.45 points), Indeterminate (1.45–3.25 points), and highly likely (> 3.25 points)20. In addition, the AST level with platelet ratio index (APRI) was also calculated: APRI = [(AST/upper limit of the normal AST level) × 100]/PLT (× 109/L). Cirrhosis was defined as a cut-off > 0.524.
Geographical data and mapping
Zonal boundary data and latitude, and longitude coordinates were obtained from the National Statistical and Geography office in Eritrea. For coordinates that could not be obtained from this office, alternate sources ArcGIS software (ArcMap version 10.7.1 (Esri, Redlands, CA, USA)) and a Google search (See links in supplementary file 1) were employed.
Data processing and analysis
Analysis was conducted using IBM SPSS (version 26) and STATA version 12.0 (STATA Corporation, College Station, TX). Descriptive statistics for categorical variables were analyzed using chi-square (χ2)/Fishers exact test and summarized using counts (frequency), proportions (percentages), means (± SD) and medians (interquartile range (IQR). Normality test (Kolmogorov–smirnov test) was conducted prior to any statistical computation and the appropriate parametric (t-test, ANOVA) and nonparametric statistics (Mann–Whitney U and Kruskal Wallis) were used to evaluate differences. Kaplan–Meier curves were used to estimate survival rates and failure rates at different intervals of follow-up. All LTFUs were censored on the date of their last visit. Multivariate Cox regression model was implemented for assessing the variables that predict LTFU. The final results are presented as adjusted hazard ratios (aHR) with a 95% CI. Two-sided p value < 0.05 was considered significant.
Ethical consideration
Ethical approval was obtained from the Ministry of Health research ethics and protocol review committee with a letter of reference (Approval Number: Ref: 01/22). All the information gathered was de-identified, and at most confidentiality was upheld. As the study also included data based on patients’ clinical card records, consent for the data access was waived by the ethical committee in place of the patients. All procedures of the study also followed the recommendation of the Declaration of Helsinki Convention.
Results
Inter-facility analysis of cohort clinical and demographic characteristics
A total of 238 patients [Hospital A: 142(59.7%) vs. Hospital B: 96(40.3%)], treatment naïve patients, were enrolled for care from 2018 to 2021(94 (39.5%) ≤ 2019; 74(31.1%) = 2020; 70(29.4%) ≥ 2021). The median (IQR) age at diagnosis was 59 (IQR 50–69) years. HCV/HIV and HCV/HBV co-infections were observed in 9 (3.8%) and 3 (1.3%) patients, respectively. The mean (± SD) hemoglobin (Hgb) and PLTs count were 14.3 (± 1.7) g/dL and 181.9 (± 92.3) × 109/µL, respectively. Moreover, anemia (Hgb < 12 g/dL in women and Hgb < 13 g/dL in men) and thrombocytopenia were present in 12 (5%) and 76 (31.9%) patients, respectively. According to FIB-4 score estimates, cirrhosis was highly likely in 81 (34%) patients. In contrast, 81 (34%) of the patients were in the indeterminate category whereas 47 (19.5%) of the patients had low likelihood of cirrhosis. See Table 1 for pairwise comparisons of means, medians and/or proportions in the two facilities.
Specific host factors and liver Fib-4 score stages
Compared to participants with FIB-4 > 3.25 points, participants with FIB-4 < 3.25 points were younger (50 ± 12 vs. 60 ± 10 years, p value < 0.001); had lower median AST (IQR) (27(20–34) vs. 66 (45–133) IU/L, p value < 0.001); higher baseline Hgb (14.7 ± 1.7 g/dL) vs. 13.6 ± 1.6 g/dL) p value < 0.02; and higher mean PLT counts (255(± 99) × 109/µL) vs. 135(± 48) × 109/µL), p value < 0.001. Moreover, majority of patients with cirrhosis (FIB-4 score > 3.25) were initiated on SOF/VEL (77.9%). (See additional information in Table 2).
Treatment outcomes of DAA Therapy
Out of 238 patients enrolled in the study, 227 were initiated on treatment. The median (IQR) duration of treatment was 90 (IQR: 60–114) days. Of 227 patients who were placed on treatment with DAA, 125 (55%) had viral load measurements at 12 weeks EOT whereas 102 patients had no viral load measurements at 12 weeks EOT. In addition, 54 were LTFU and 18 died before SVR12 while 19 patients were on treatment during the study period. Of the 125 patients with HCV RNA data post EOT, 116 (92.8%) had SVR12. According to the data, there was no statistical difference in SVR rates between the SOF/VEL and SOF/DCV groups (93.9% and 88.9% respectively, p value = 0.6). Moreover, 58 (96.7%) and 12 (85.7%) of patients on SOF/VEL and SOF/DOC with FIB-4 score < 3.25 attained SVR, respectively. Among patients with a FIB-4 score > 3.25 (Cirrhosis), 27 (87.1%) of patients on SOF/VEL and 11 (100%) of patients on SOF/DOC attained SVR. No significant difference was identified in the rate of SVR between the FIB-4 ≤ 3.25 and FIB-4 > 3.25 groups (94.5% and 90.5%, respectively, p value = 0.4). (See Fig. 3 for details).
Pre- and Post-treatment values of specific variables
Pre-treatment and post-treatment analysis of specific laboratory variables demonstrated that AST and ALT were significantly lower than pre-treatment values (41(IQR: 30–68) vs. 30(IQR: 24–35), p value < 0.001) and (33(IQR: 20–60) vs. 18(IQR: 14–26), p value < 0.001), respectively. Moreover, FIB-4 score and APRI score were significantly lower following treatment with DAA (2.1(IQR: 1.4–4.1) vs. 1.9 (IQR: 1.2–2.9), p value = 0.003) and (0.5(IQR: 0.3–1) vs. 0.3 (IQR: 0.2–0.5), p value < 0.001, respectively. However, PLT count demonstrated limited improvement following treatment (See Table 3 for details).
Loss to follow-up and Mortality in the HCV care cascade
Death occurred in 18 (7.5% (95% CI 1.7–4.1) patients while 67 cases were LTFU (28.1%, 95% CI 22.3–33.9). Analysis of the proportion of LTFU and death through HCV Cascade of Care demonstrated that majority of LTFU and mortality occurred prior to treatment completion—28 (41.7%) and 11 (61.2%)), respectively. Furthermore, 26 (38.8%) were LTFU − 11 (16.4%) LFTU occurred at a follow-up appointment after diagnosis; 28 (41.7%) occurred before treatment completion; 26 (38.8%) occurred before SVR and 2 (2.9%) occurred post SVR care (Fig. 4).
Factors associated with mortality and LTFU
Table 4 presents factors associated with mortality and LTFU. In this analysis, patients who died had a significantly shorter median duration on treatment with DAA of 31 days (IQR 21–227). In contrast, patients with positive outcomes and LFTU had longer duration of treatment—90 days (IQR 80–144) vs. 84 days (IQR 42–92), respectively (p value = 0.002). Moreover, patients who died had a higher median FIB-4 score, 6.9 (IQR 4.5–6.9) as compared to positive outcome 3.1 (IQR 1.6–6.2) and LTFU 1.3 (IQR: 0.9–6); p value < 0.001. Similarly, mortality was associated with significantly higher median APRI and baseline AST. In contrast, LTFU was higher in both patients who had not started treatment and had been on follow-up for ≤ 12 weeks while Fib-score was relatively lower.
Incidence rates, rate ratio and Kaplan–Meier survival estimates for mortality
Following 29,786 person-days of follow-up,incidence rate of death was 6.04 (95% CI 3.8–9.5) per 10,000 person-days. Kaplan–Meier survival analysis demonstrated that patients enrolled in Hospital B had a significantly shorter mean duration of survival (548 days (95% CI 403–694) vs. 578 days (95% CI 533–624), p value = 0.02) (Fig. 5c) and higher risk of death, 3.3(95% CI 1.1–10.1). Moreover, patients with FIB-4 ≥ 3.25 score had a significantly shorter mean survival duration as compared to FIB-4 < 3.25 (472 days (95% CI 402–542) vs. (649 (95% CI 537–761) days), p < 0.001) (Fig. 5d). Lastly, patients from outside central zone had a shorter mean duration of survival, 358 days (95% CI 283–433) vs. 623 days (95% CI 547–669) for those from central zone, p < 0.05) (See Table 5 for details). Overall survival curve for mortality is displayed in Fig. 5a.
Kaplan–Meier curves for cumulative survival, LTFU and mortality of chronic HCV patients followed in the two major treatment centers in Eritrea from 2018-to 2021. (A) Overall cumulative proportion of death; (B) Overall cumulative proportion of LTFU; (C) Cumulative proportion of survival by hospital (D) Cumulative mortality curve by FIB 4 score.
Incidence rates and Kaplan–Meier survival estimates for LTFU
The number of LTFU events was 67 events translating into an incidence rate of 1.1(95% CI 0.8–1.5) per 10,000 person-days. In the Kaplan–Meier analysis, patients enrolled at Hospital B had a shorter mean duration of survival, 304 days (95% CI 215–392) vs. 736 days (95%:515–956) in Hospital A, p < 0.001. Moreover, patients not initiated on DAA had significantly shorter mean survival duration than those on treatment, 169 days (95% CI 1–458) vs. 371 days (95% CI 308–434) for patients on SOF/DCV and 830 days (95% CI 659–1001) for patients on SOF/VEL, p < 0.001 (Table 6). Overall survival curve for LTFU is displayed in Fig. 5b.
Independent predictors of LTFU in chronic hepatitis C patients in Eritrea
Table 7 presents unadjusted and adjusted hazard ratios for variables associated with LTFU in 238 chronic HCV patients followed from 2018 to 2021. In the adjusted model, independent predictors of LTFU included enrollment year (2020: aHR = 2.2, 95% CI 1–4.7; p value = 0.04); Hospital (Hospital B: aHR = 2.2, 95% CI 1–4.7; p value = 0.03) and FIB-4 score (≥ 3.25: aHR = 3.7, 95% CI 1.2–11.5; p value = 0.02).
Discussion
Real-world data for treatment programs in SSA is hard to locate in the published literature. Unlike participants in phase 3 randomized controlled trials (RCTs); real-world study cohorts typically include patients with unfavorable conditions. Furthermore, DAAs outcomes can be compromised by clinicians’ limited expertise25. In this study, all patients had unknown genotypes and unknown fibrosis status. Although consistent details were not available for all patients, the overall SVR rate was 116 (92.8%) for a subset of patients. These results are in line with data from multiple seminal RCT studies (the Phase 3 ASTRAL-1, ASTRAL-2, ASTRAL-3 and ASTRAL-5 trials and POLARIS-3 trials); which reported SVR rates of 93–100%26. Even more important, other real world studies and RCTs have similarly shown that DAAs are well tolerated by patients27.
Where possible, we computed FIB-4 and APRI scores and evaluated SVR for SOF/VEL and SOF/DCV along cirrhosis strata. In this analysis, our results demonstrated that patients with FIB-4 score < 3.25 had higher SVR (94.5%) compared to patients with FIB-4 score > 3.25(90.5%) for both regimens. Generally, our findings are consistent with the observation that fibrosis may not compromise SOF/VEL and SOF/DCV efficacy, but decompensated cirrhosis may undermine SVR28. To illustrate these points, Abdul and colleagues noted a significant difference in treatment outcomes between patients with disparate Child-Turcotte-Pugh (CTP) classification (95.5% in CTP A vs. 90.8% in CTP B, p value = 0.010)29. Literature also suggests that specific HCV genotypes (e.g., HCV-GT4 subtype 4k, 4q, 4p, and 4r) and the emergence of resistance-associated mutations (RAM)30 may contribute to virological failure. Unfortunately, the possible contribution of these factors to SVR rates in this population is difficult. Therefore, further studies will be required to address this gap.
Remarkably, most of the non-responders had high FIB-4 scores, relatively low PLT count (thrombocytopenia can be a surrogate marker of portal hypertension), and high ALT and AST (see “Supplementary data” page 1). Many of these findings align with previous literature which suggested that decompensated liver cirrhosis (CTP B and C), and elevated transaminase levels, among others, are associated with lower SVR31,32. Interestingly, post-treatment AST, ALT, FIB-4 score, and APRI score were significantly lower in a sub-set of patients. Furthermore, PLT counts demonstrated limited improvement following treatment. Much of this information concurs partially with literature relating to the clinical benefits of SVR31,32,33 such as the possible restoration of the liver functional reserve34. Altogether it’s our conclusion that for most patients, genotype-blind treatment with SOF/VEL or SOF/DCV regimens is largely satisfactory. Importantly, this outcome reinforces the fact that these regimens can have utility in population-level scale-up measures directed at the elimination of HCV in resource-poor settings in SSA.
Despite the favorable SVR rates, mortality rate was high [18 deaths (7.5% (95% CI 1.7–4.1)] with a significant proportion of deaths occurring in the first 8 weeks after initiation of treatment. The high mortality rate observed in this cohort is probably linked to late presentation of patients. Indeed, patients in this cohort were older (Median (IQR): 60 years (50–69 years) suggesting long-term exposure to HCV. At present, reports suggest that HCV-related cirrhosis can be observed in 5–20% of patients after 20–30 years of chronic infection35. Others have noted that cirrhotic patients are at high risk of hepatic decompensation (27.7–39.5% risk over five years) and hepatocellular carcinoma (HCC) (2.8–7.4% in the first year, and 8–16.1% over 5 years) and liver-related mortality36. In most countries in SSA, liver transplantation and the cost associated with the management of patients with End-Stage Liver Disease (ESLD) is prohibitive, therefore, the condition is invariably fatal. This implies that screening of all at-risk populations is a more affordable option for most countries in the region. Therefore, the need for early detection or scale-ups in screening/case-finding along with robust treatment of patients should be prioritized.
In general, experts agree that determination of the severity of liver fibrosis is a challenging but essential component of HCV management28. Highlighting this issue, some have noted that limited provider experience; lack of technology for fibrotic staging (e.g. Fibroscan), confirmatory HCV-RNA testing/or genotype determination, as well as clinical chemistry infrastructure, is compromising treatment in LMIC in SSA37. Our data corroborate this position. First, the number of hepatologists or specialized internists is severely limited in Eritrea. Clearly, the lack of clinical expertise may compromise hepatic staging-informed care or management of advanced fibrosis/cirrhosis (an outcome which appears to be common in this setting). In addition, confirmatory HCV-RNA testing is highly centralized and periodic reagent stock-outs have been reported. More importantly, the existing HCV/HBV standard-of-care guidelines for Eritrea highlight the possible use of APRI, FIB-4 score, and liver elastography for fibrosis determination without specifying the preferred approach. On the latter, we can conclude that the FIB-4 score appears to have a good discriminatory capacity for non-SVR and likelihood of mortality. Overall, better diagnostic performance for FIB-4 score has been reported by multiple investigators37.
Lastly, it should be noted that successful completion of treatment is critical for long-term HCV elimination goals. Previous work has shown that LTFU-associated DAA treatment interruptions can be linked to avoidable morbidity and mortality, increased health care costs, preventable HCV transmission, and the development of drug resistance mutations. However, despite overwhelming evidence demonstrating the importance of retention in care; our data demonstrate that LTFU was disproportionately high (67(28.1%, 95% CI 22.3–33.9) and that it was the most important non-virological reason for non-attainment of SVR. This outcome stands in stern contrast to reports from a study in Rwanda which reported no LTFUs38. Low proportions of LTFUs were also reported by workers in other countries39,40. At present, we have to concede that the observed results were largely unexpected because treatment in these facilities is offered gratis or with minimal out-of-pocket cost. This aside, it should be noted that a significant number of these patients were categorized as LTFUs. It is likely that many of these patients were not cured and had some level of viremia when they left care. From a public health concern, this outcome can undermine the progress towards the achievement of HCV eradication.
Although consistent details were not available for most patients on a large number of factors with a potential link to LTFU; several variables independently demonstrated significant effects on LTFU rate—treatment center, years of enrollment (2020), and cirrhosis status were independent predictors of LTFU. The link between years of enrollment and LTFU is probably connected to the COVID-19 pandemic and the lockdowns of 2020. On the other hand, the connection between LTFU and treatment centers (Hospital B) may be connected to proximity to laboratories services. For example, viral load testing center is located near Hospital A but is at a considerable distance from the hospital B (see Fig. 1). Moreover, difference in quality of services in either institution may also account for the difference in LTFU. In general, the number of specialized clinicians is severely limited in hospital B. Interestingly, patients who had less likelihood of cirrhosis (FIB-4 score < 1.45) had higher hazards of LTFU. The health belief model predicts this outcome—the perception that a disease is not dangerous can undermine health seeking behavior. Poor patient awareness, cost of treatment/lack of insurance, housing instability/or address changes and distance to care facilities can also drive the incidence of LFTUs41. Altogether, our result underscores the fact that even in settings where DAA treatment and essential laboratory services are offered gratis or with minimal out-of-pocket fee; LTFU can still be a formidable barrier.
Limitations of the study
Although this study addresses an important information gap on HCV literature in SSA, it has some limitations. Firstly, poor documentation of baseline history, laboratory data and post-treatment surveillance data undermined our analyses. Lastly, FIB-4 and APRI scores have not been validated for populations in SSA.
Conclusion
These results provide the first primary data on treatment outcomes in two HCV treatment programs in Eritrea. Important insights included the fact that SOF/VEL and SOF/DCV are highly effective even in settings where genotypes are unknown. Secondly, mortality rates were relatively high, an outcome that was largely associated with the large proportion of patients with cirrhosis. The high incidence of LTFU was surprising given that care was offered for free. Therefore, more work is needed to monitor and understand the factors behind the high proportion of LTFU in this setting. Going forward, more emphasis should be placed on decentralization of care services and better monitoring during and post-EOT.
Data availability
The dataset supporting the conclusions of this article is available from the corresponding author on reasonable request.
Abbreviations
- aHR:
-
Adjusted hazards rate
- ALT:
-
Alanine aminotransferase
- APRI:
-
AST level with platelet ratio index
- AST:
-
Alanine aminotransferase
- CHC:
-
Chronic hepatitis C
- cHR:
-
Crude hazards rate
- CI:
-
Confidence interval
- DAA:
-
Direct acting antivirals
- DCV:
-
Daclatasvir
- EOT:
-
End of treatment
- FIB-4 :
-
Non-invasive fibrosis evaluation in hepatitis C
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- Hgb:
-
Hemoglobin
- HIV:
-
Human immunodeficiency virus
- IQR:
-
Interquartile range
- LMIC:
-
Low and middle income countries
- LTFU:
-
Loss to follow up
- Plt:
-
Platelets
- RCT:
-
Randomized controlled trial
- RNA:
-
Ribonucleic acid
- SOF:
-
Sofosbuvir
- SSA:
-
Sub-Saharan Africa
- SVR:
-
Sustained virological response
- TB:
-
Tuberculosis
- VL:
-
Viral load
- WHO:
-
World Health Organization
References
Thong, V. D., Akkarathamrongsin, S., Poovorawan, K., Tangkijvanich, P. & Poovorawan, Y. Hepatitis C virus genotype 6: Virology, epidemiology, genetic variation and clinical implication. World J. Gastroenterol. 20, 2927 (2014).
Martinez, M. A. & Franco, S. Therapy implications of hepatitis C virus genetic diversity. Viruses 13, 41 (2020).
Meshram, R. J., Kathwate, G. H. & Gacche, R. N. Progress, evolving therapeutic/diagnostic approaches, and challenges in the management of hepatitis C virus infections. Arch. Virol. 167, 1–20 (2022).
World Health Organization. Global hepatitis report 2017, https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (2017).
Brunner, N. & Bruggmann, P. Trends of the global hepatitis C disease burden: Strategies to achieve elimination. J. Prev. Med. Public Health 54, 251 (2021).
Dennis, B. B., Naji, L., Jajarmi, Y., Ahmed, A. & Kim, D. New hope for hepatitis C virus: Summary of global epidemiologic changes and novel innovations over 20 years. World J. Gastroenterol. 27, 4818 (2021).
World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection (2018).
Torre, P., Aglitti, A., Masarone, M. & Persico, M. Viral hepatitis: Milestones, unresolved issues, and future goals. World J. Gastroenterol. 27, 4603 (2021).
Chan, S.-W. Establishment of chronic hepatitis C virus infection: Translational evasion of oxidative defence. World J. Gastroenterol. 20, 2785 (2014).
Falade-Nwulia, O. & Sulkowski, M. S. Hepatitis C virus treatment: Simplifying the simple and optimizing the difficult. J. Infect. Dis. 222, S745-s757. https://doi.org/10.1093/infdis/jiaa534 (2020).
Afendy, A. et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment. Pharmacol. Ther. 30, 469–476 (2009).
Manns, M. P. et al. Hepatitis C virus infection. Nat. Rev. Disease Primers 3, 1–19 (2017).
Scheel, T. K., Simmonds, P. & Kapoor, A. Surveying the global virome: identification and characterization of HCV-related animal hepaciviruses. Antivir. Res. 115, 83–93 (2015).
Taherkhani, R. & Farshadpour, F. Global elimination of hepatitis C virus infection: Progresses and the remaining challenges. World J. Hepatol. 9, 1239 (2017).
Green, V. & Roytman, M. Treatment-resistant hepatitis C viral infection: A case report and literature review. Case Rep. Hepatol. 2022, 3556780 (2022).
Pearlman, B. L. & Traub, N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: A cure and so much more. Clin. Infect. Dis. 52, 889–900 (2011).
Simmons, B., Saleem, J., Heath, K., Cooke, G. S. & Hill, A. Long-term treatment outcomes of patients infected with hepatitis C virus: A systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin. Infect. Dis. 61, 730–740 (2015).
World Health Organization. Combating hepatitis B and C to reach elimination by 2030, https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf (2016).
Hill, A. M., Nath, S. & Simmons, B. The road to elimination of hepatitis C: Analysis of cures versus new infections in 91 countries. J. Virus Erad. 3, 117–123 (2017).
Apata, I. W. et al. Progress toward prevention of transfusion-transmitted hepatitis B and hepatitis C infection—Sub-Saharan Africa, 2000–2011. Morb. Mortal. Wkly. Rep. 63, 613 (2014).
Siraj, N. et al. Seroprevalence of transfusion-transmissible infections among blood donors at National Blood Transfusion Service, Eritrea: A seven-year retrospective study. BMC Infect. Dis. 18, 264. https://doi.org/10.1186/s12879-018-3174-x (2018).
World Health Organization. WHO welcomes Egypt’s support to 14 African countries in their fight against hepatitis C, https://www.afro.who.int/news/who-welcomes-egypts-support-14-african-countries-their-fight-against-hepatitis-c (2019).
van Dijk, M. & Drenth, J. P. H. Loss to follow-up in the hepatitis C care cascade: A substantial problem but opportunity for micro-elimination. J. Viral Hepat. 27, 1270–1283. https://doi.org/10.1111/jvh.13399 (2020).
Karic, U. et al. FIB-4 and APRI scores for predicting severe fibrosis in chronic hepatitis C—A developing country’s perspective in DAA era. J. Infect. Dev. Ctries 12, 178–182. https://doi.org/10.3855/jidc.10190 (2018).
Arias, A. et al. Rate and predictors of treatment failure to all-oral HCV regimens outside clinical trials. Antivir. Ther. 22, 307–312. https://doi.org/10.3851/imp3061 (2017).
Mariantonietta, P., Antonio, R., Lorenzo, O. & Nicola, C. Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: A meta-analysis. Acta Bio Med. Atenei Parm. 90, 187 (2019).
Mushtaq, S. et al. Efficacy and safety of generic sofosbuvir plus daclatasvir and sofosbuvir/velpatasvir in HCV genotype 3-infected patients: Real-world outcomes from Pakistan. Front. Pharmacol. 11, 550205. https://doi.org/10.3389/fphar.2020.550205 (2020).
Huang, Y.-T. et al. Sofosbuvir/velpatasvir is an effective treatment for patients with hepatitis C and advanced fibrosis or cirrhosis in a real-world setting in Taiwan. BMC Gastroenterol. 21, 1–9 (2021).
Mohammed Abdul, M. K., Snyder, H. S., Chunduru, M., Lee, S. M. & Satapathy, S. K. Hepatitis C virus in the elderly in the direct-acting antiviral era: From diagnosis to cure. Curr. Treat. Options Infect. Dis. 12, 296–309 (2020).
Gupta, N. et al. Treatment of chronic hepatitis C virus infection in Rwanda with ledipasvir–sofosbuvir (SHARED): A single-arm trial. Lancet Gastroenterol. Hepatol. 4, 119–126 (2019).
Mir, F., Kahveci, A. S., Ibdah, J. A. & Tahan, V. Sofosbuvir/velpatasvir regimen promises an effective pan-genotypic hepatitis C virus cure. Drug Des. Devel. Ther. 11, 497–502. https://doi.org/10.2147/dddt.S130945 (2017).
Ahmed, R. et al. Sofosbuvir/Velpatasvir—A promising treatment for chronic hepatitis C virus infection. Cureus 13, e17237. https://doi.org/10.7759/cureus.17237 (2021).
Younossi, Z. M. et al. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: Results from ASTRAL-1 placebo-controlled trial. J. Hepatol. 65, 33–39. https://doi.org/10.1016/j.jhep.2016.02.042 (2016).
Atsukawa, M. et al. Real-world clinical application of 12-week sofosbuvir/velpatasvir treatment for decompensated cirrhotic patients with genotype 1 and 2: A prospective, multicenter study. Infect. Dis. Ther. 9, 851–866. https://doi.org/10.1007/s40121-020-00329-y (2020).
Ge, P. S. & Runyon, B. A. Treatment of patients with cirrhosis. N. Engl. J. Med. 375, 767–777 (2016).
Dustin, L. B., Bartolini, B., Capobianchi, M. R. & Pistello, M. Hepatitis C virus: Life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin. Microbiol. Infect. 22, 826–832. https://doi.org/10.1016/j.cmi.2016.08.025 (2016).
Sonderup, M. W. et al. Hepatitis C in sub-Saharan Africa: The current status and recommendations for achieving elimination by 2030. Lancet Gastroenterol. Hepatol. 2, 910–919 (2017).
Umutesi, J. et al. Screening a nation for hepatitis C virus elimination: A cross-sectional study on prevalence of hepatitis C and associated risk factors in the Rwandan general population. BMJ Open 9, e029743. https://doi.org/10.1136/bmjopen-2019-029743 (2019).
Min Thaung, Y. et al. Treatment outcomes and costs of a simplified antiviral treatment strategy for hepatitis C among monoinfected and HIV and/or hepatitis B virus-co-infected patients in Myanmar. J. Viral Hepat. 28, 147–158. https://doi.org/10.1111/jvh.13405 (2021).
Yek, C. et al. Effectiveness of direct-acting antiviral therapy for hepatitis C in difficult-to-treat patients in a safety-net health system: A retrospective cohort study. BMC Med. 15, 1–8 (2017).
Ziff, J. et al. Predictors of hepatitis C treatment outcomes in a harm reduction-focused primary care program in New York City. Harm. Reduct. J. 18, 38. https://doi.org/10.1186/s12954-021-00486-4 (2021).
Acknowledgements
The authors would like to thank the clinical staff who supported this work in the two treatment centers in Eritrea.
Funding
Data was collected by the Chronic Viral Hepatitis care center in ONRH and HNRH focal personnel with incentive and material support obtained from the Eritrean Ministry of Health, CDC division.
Author information
Authors and Affiliations
Contributions
Conceptualization: G.G.G. and M.B.S. Study design & proposal writing: G.G.G., A.B.M., and M.B.S. Data curation: G.G.G., M.B.S., and R.F.A. Data analysis and result interpretation: G.G.G., S.T.M., M.B.S., and O.O.A. Writing, original draft: G.G.G., M.B.S., S.T.M., and O.O.A. Writing, review & editing: G.G.G., M.B.S., O.O.A., A.B.M., S.T.M., and M.E.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghebremeskel, G.G., Berhe Solomon, M., Achila, O.O. et al. Real-world treatment outcome of direct-acting antivirals and patient survival rates in chronic hepatitis C virus infection in Eritrea. Sci Rep 13, 20792 (2023). https://doi.org/10.1038/s41598-023-47258-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47258-7
- Springer Nature Limited