Abstract
Glycolate oxidase (GLO) is an FMN-containing enzyme localized in peroxisomes and performs in various molecular and biochemical mechanisms. It is a key player in plant glycolate and glyoxylate accumulation pathways. The role of GLO in disease and stress resistance is well-documented in various plant species. Although studies have been conducted regarding the role of GLO genes from spinach on a microbial level, the direct response of GLO genes to various stresses in short-season and leafy plants like lettuce has not been published yet. The genome of Lactuca sativa cultivar ‘Salinas’ (v8) was used to identify GLO gene members in lettuce by performing various computational analysis. Dual synteny, protein–protein interactions, and targeted miRNA analyses were conducted to understand the function of GLO genes. The identified GLO genes showed further clustering into two groups i.e., glycolate oxidase (GOX) and hydroxyacid oxidase (HAOX). Genes were observed to be distributed unevenly on three chromosomes, and syntenic analysis revealed that segmental duplication was prevalent. Thus, it might be the main reason for GLO gene diversity in lettuce. Almost all LsGLO genes showed syntenic blocks in respective plant genomes under study. Protein–protein interactions of LsGLO genes revealed various functional enrichments, mainly photorespiration, and lactate oxidation, and among biological processes oxidative photosynthetic carbon pathway was highly significant. Results of in-depth analyses disclosed the interaction of GLO genes with other members of the glycolate pathway and the activity of GLO genes in various organs and developmental stages in lettuce. The extensive genome evaluation of GLO gene family in garden lettuce is believed to be a reference for cloning and studying functional analyses of GLO genes and characterizing other members of glycolate/glyoxylate biosynthesis pathway in various plant species.
Similar content being viewed by others
Introduction
Lettuce (Lactuca sativa L.), is an annual, self-fertilizing, cool-season green leafy vegetable that belongs to the tribe Cicoreae and family Compositae, also called Asteraceae1,2,3,4. It requires an optimal temperature range of 13 °C to 18 °C and can be grown in regions with mild winter5. In genus Lactuca, cultivated lettuce (Lactuca sativa L.) is the only member grown commercial scale6. Horticulturally, lettuce cultivars with high consumer demand are classified based on the morphological differences. The cultivar under study, L. sativa cv. salinas, is marketed as Iceberg lettuce, also known as Crisphead lettuce, and has a large spherical head7,8. The genome sequence of Lactuca sativa cultivar ‘Salinas’ has recently been released. Lettuce has a diploid genome with 2n = 2x = 18 chromosomes, and the genome size is estimated to be 2.5 Gb9. The hybrids of L. sativa are self-fertile10. Hydroponics is one of the most common production systems and addresses many issues regarding lettuce production11,12,13. Lettuce is nutrient-rich and contains potentially beneficial compounds such as vitamin A, antioxidants and minerals like calcium and iron. High levels of antioxidant compounds, namely polyphenols and vitamin C, as well as fiber determine the healing properties of lettuce14. It is demonstrated in a recent study that lettuce has beneficial effects on rats and humans for the prevention of cardiovascular diseases15. In an experiment, the internalization of human pathogens was demonstrated using lettuce as a model vegetable mainly because of its commercial importance16.
Glycolate oxidase (GLO) is important in plant photorespiration as the only enzyme that catalyzes glycolate oxidation into glyoxylate and hydrogen peroxide (H2O2) in peroxisomes17,18. Photorespiration is an essential pathway in plants for resistance against abiotic stresses, thus it supports plant growth in stress conditions, and it is caused by high light intensity, salinity and drought19,20. Hydroxy acid oxidase (HAOX) is quite similar to GLO and is mostly present in peroxisomes21 and because of the high similarity, this group is considered as a subgroup of GLO family22. GLO genes have been identified in many plant species such as A. thaliana, N. benthamiana, S. oleracea, and O. sativa. GLO family in A. thaliana and S. oleracea consists of five genes namely GOX1, GOX2, GOX3, HAOX1 and HAOX2, whereas, rice genome contains only four functional GLO genes, OsGLO1-4, and GLO family of N. benthamiana consist of seventeen genes. Out of these seventeen genes, seven genes are identified as NbGOXs and ten as NbHAOXs, and it is the highest number of GLO genes ever reported in a plant22,23,24. The five genes in A. thaliana GLO family have high similarity among each other and have similar protein size that ranges from 364 to 374 amino acids. In rice, there is complexity in the interactions for GLO isozymes,which are controlled by OsGLO1 and OsGLO424.
A wide variety of physiological processes are regulated by GLO genes, such as the involvement of GLO genes in response against drought and salt stress25,26, and strongly regulate photosynthesis, possibly through Rubisco activase inhibition by feedback mechanism27. A GLO in rice has been shown to affect plant growth28, and the survival of maize in ambient air is dependent on the high activity of glycolate oxidase29. The reaction catalyzed by GLO produces glyoxylate and H2O2, which are known to be involved in plant disease resistance. Therefore, GLO supposedly may have crucial role in plant disease resistance22,30. Oxalate, biosynthesized by glyoxylate31, plays a positive role in the interaction between plants and pathogens such as Sclerotinia sclerotiorum and Botrytis cinerea32,33,34. Glyoxylate also has importance in biosynthesis of amino acids35. However, the pathway through which oxalate accumulates in plants and its regulation is independent of GLO36. H2O2, an important reactive oxygen species (ROS), plays a role as a signaling molecule in the interaction between plants and pathogens37,38,39,40. Nonetheless, there is direct evidence reported in Arabidopsis, O. sativa, and N. benthamiana that supports the role of GLO in plant disease resistance22,23,41.
Lettuce grown under low temperatures is associated with decreased fresh weight42,43. Quick changes in basic physiological processes can occur due to decreased ambient temperature44. Low temperature also results in reduced enzyme activity45. Freezing temperatures can cause blisters and peeling of leaves, leading to rot and decay, and can also act as sites of entrance for plant pathogens5. Drought and low temperature are factors that affect the cyclic electron flow (CEF) in plants46. Cyclic electron flow is pivotal for photorespiration, a mechanism initiated by glycolate oxidase, and helps the plant to resist abiotic stresses. A study revealed expression of CBF/DREB1 genes in lettuce for resistance against low temperature (4 °C). However, it did not explain the action and function of glycolate oxidases under such a stressed environment.
Temperate leafy vegetables, when grown under hot climates, can develop a condition known as bolting. Bolting is a condition that occurs when a plant shifts from the vegetative to the reproductive stage in its lifecycle, but this doesn’t mean that bolting occurs in all plants. Iceberg lettuce is susceptible to bolting, whereas varieties such as summer crisp or French crisp, butter head and loose leaves are resistant to bolting47. High temperature promotes bolting, which results in rapid stem elongation, making lettuce bitter and unfit for marketing48. The activity of glycolate oxidases increased gradually during the vegetative stage, and it was maximum at 1st leaf stage of flowering in sunflower49. Research has been conducted to understand the mechanism and regulation of bolting in lettuce, but the activity of glycolate oxidases under its regulation is still unknown.
Heavy metal (Cd) soil contamination greatly affects lettuce growth and development50. Cadmium is easily absorbed by lettuce plants when grown in cadmium contaminated soils1,51. Cd stress reduces the contents of photosynthetic pigment in the leaf significantly and suppresses growth by decreasing the photosynthetic potential of the lettuce plant52. Light absorption is affected by leaf area; hence, leaf area is a critical factor in photosynthesis53. It has been seen that large and flat leaves show more photosynthetic activity than other leaf types54. The intensity of light is an important environmental factor that greatly affects the growth and development of a plant. Light acts as a source of energy and plant physiology adaptation is regulated by light55. Photosynthesis requiress photorespiration, and metabolic interaction exists between photosynthetic carbon fixation and photorespiration56. Studying the fluctuations in the expression of GLO genes under heavy metal (Cd) and light stress might provide a clear understanding of the role played by glycolate oxidases in improving lettuce tolerance to abiotic stresses.
Circular RNA (circRNA) is an RNA molecule through which transcriptional regulation of gene expression occurs in many species, but the functions of most circular RNAs are still unknown57. Targeting glycolate oxidase genes with light-responsive circRNAs might provide an understanding of light-regulated expression of GLO genes in lettuce.
Bremia lactucae, also known as the downy mildew of lettuce, is an oomycete known worldwide for causing devastating diseases in lettuce58,59. According to research, this pathogen caused disease in cultivated lettuce (Lactuca sativa), but wild lettuce (Lactuca saligna) resisted this pathogen60. The pathogen (Bremia lactucae) has been widely studied in Lactuca serriola, and it was found that disease severity and prevalence had close relationship with the type of habitat or size and density of populations (L. serriola)58,61,62. Since glycolate oxidase genes have been previously reported for strengthening plant resistance against various diseases, it would be interesting to show differential expression of glycolate oxidases in healthy and infected lettuce plants.
MicroRNAs, or simply miRNAs are important plant regulators that regulate various biological processes such as growth and development, defense against pathogens, and maintenance of proper internal conditions63,64,65,66. Among multiple species, miRNAs remain highly conserved, which means that the function of miRNA is very specific regardless of the species under observation. Targeted miRNA analysis can provide valuable insights into the expression and functions of glycolate oxidase genes in lettuce.
As mentioned earlier, glycolate oxidase enzyme is crucial for photorespiration, photorespiration goes simultaneously with photosynthesis, and photosynthetic inhibition occurs due to suppressing glycolate oxidase genes67. In C3 plants, glycolate oxidase is estimated to produce approximately 70% of the total respiratory hydrogen peroxide (H2O2)68, H2O2 acts as a key signaling molecule in stress-related signal transduction pathways69, and is widely known for its role in plant disease resistance22,30. Lettuce is considered one of the most important leafy vegetable crops in the world2,70, and like any other crop, profitable production depends on optimal photosynthesis and resistance to diseases and stresses. Lettuce is a suitable specimen for genetic studies due to its characteristic features, including a relatively short life cycle, high rate of natural self-pollination with full self-fertility, the possibility to carry out multiple crosses on one plant, and less space requirement for individual plant4. This research aimed to determine the role of glycolate oxidases in lettuce plant resistance to various biotic and abiotic stresses and to understand whether or not the results support the previously documented functions and significance of glycolate oxidases in various plant species.
Results
Identification of GLO gene family in L. sativa
To identify GLO genes in lettuce, the protein sequence of SoGLO1/Spo19861 (S. oleracea) retrieved from Spinachbase database was blast against the whole genome of lettuce (Lactuca sativa v8/Lactuca sativa cv. salinas). An initial analysis led to the identification of fourteen GLO proteins. Proteins encoded by the same gene isoforms and proteins containing truncated FMN-dependent dehydrogenase domain, were excluded from the analysis. Finally, five non-redundant LsGLO genes were identified and used for further analysis. The five proteins identified as glycolate oxidase in lettuce consisted of 1-3 LsGOXs, and 1-2 LsHAOXs.
Identification of conserved domains in LsGLO genes
The non-redundant GLO protein sequences from lettuce exhibit highly conserved domains, i.e., FMN-dependent dehydrogenase (FMN-dh), long chain alpha-hydroxy acid oxidase (alpha_hydroxyacid_oxid_FMN), and L-lactate dehydrogenase (LldD) were specific. Other non-specific domains were PLN02493, PLN02493 superfamily, PLN02535, L_lactate_LldD, and L_lactate_LldD superfamily, and these are typical features of glycolate oxidase proteins in general (Fig. 1, Supplementary material Table S1). Within the highly conserved sequence of alpha_hydroxyacid_oxid_FMN domain, there exists an amino acids gap from 165 to 210 in LsGOXs, and from 168 to 210 amino acids in LsHAOXs, and none of the protein had additional fragment in the middle of alpha_hydroxyacid_oxid_FMN domain sequence as reported in N. benthamiana. The same amino acids gap as LsGOXs was also observed in AtGOXs. A gap from 166 to 208 amino acids exists in AtHAOXs (Supplementary material Fig. S1). All 5 LsGLO proteins contain nine active sites, five substrate binding sites, seven FMN binding sites, and three putative catalytic residues. These sites were also reported in A. thaliana and N. benthamiana (Fig. 1).
Identification of conserved domains in LsGLOs through NCBI CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) in comparison with Arabidopsis GLO genes.
Physio-chemical properties of GLO genes and prediction of nuclear and sub-cellular localization signals
LsGLO genes encode proteins ranging from 365 to 369 amino acids with a molecular weight ranging from 40.2 to 40.6 kD. LsHAOX1 and LsHAOX2 are the smallest and LsGOX1 and LsGOX2 are the largest GLO proteins (Table 1). The isoelectric point (pI) of LsGLO proteins ranged from 8.7 to 9.1 (Table 1).
No nuclear localization signals (NLS) were found in any of the lettuce GLO genes (Supplementary material Table S2). Sub-cellular location of lettuce GLO genes was predicted in cytoplasm, mitochondria, peroxisomes, and plastid etc., with LsGOXs being highly predicted in cytoplasm and LsHAOXs mainly in peroxisomes (Fig. 2, Supplementary material Table S2).
Heat map showing predicted subcellular localization of LsGLO proteins. Green indicates the lowest and red indicates the highest number of signals in LsGLOs displayed with hierarchy. ‘Cyto’, ‘Pero’, ‘Chlo’, ‘Mito’, ‘Plas’, and ‘Cysk_nucl’ represent cytosol, peroxisomes, chloroplast, mitochondria, plasma membrane, and cytoskeleton and nucleus.
Comparative phylogenetic analysis of GLO proteins
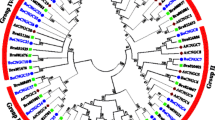
The phylogenetic analysis of L. sativa GLO genes was conducted compared with S. oleracea, S. lycopersicum, S. tuberosum and Arabidopsis GLO genes. An outgroup was added to create a more defined tree. To investigate the evolutionary relationships between GLO genes, Neighbor-Joining (NJ) phylogenetic tree was constructed through MEGA X v10.2.4 by aligning full-length protein sequences (Supplementary material Table S3). The result showed two groups that were named GOX and HAOX (Fig. 3). Group GOX consisted of total fifteen members in which three GOX proteins of L. sativa, S. oleracea, S. lycopersicum, S. tuberosum, and Arabidopsis were present. Group HAOX consisted of thirteen members in which two HAOX proteins of L. sativa, S. oleracea, and Arabidopsis were present along with three and four HAOX proteins of S. lycopersicum and S. tuberosum, respectively (Fig. 3). It was noticed that LsGOX1 and LsGOX2 genes and LsHAOX1 and LsHAOX2 genes were present in the same clade in their respective groups, however, LsGOX3 was present in an entirely different clade in GOX group, and the same pattern was also observed in Arabidopsis GLO genes (Fig. 3). Also, LsGOX1 and LsGOX2 genes were present in the same clade with AtGOX1 and AtGOX2 in group GOX, whereas, LsHAOX1 and LsHAOX2 were present in a different clade from AtHAOX1 and AtHAOX2 in group HAOX (Fig. 3). All the L. sativa GLO genes showed close relationship with S. lycopersicum and S. tuberosum GLO genes (Fig. 3).
Phylogenetic relationships of LsGLO genes with other plant species( MEGA X v10.2.4). Using the UPGMA method to infer the evolutionary history with 1000 Bootstrap, and phylogenetic tree was constructed using an online tool, iTol v6 (Interactive Tree Of Life) (https://itol.embl.de). ‘At’, ‘So’, ‘Sl’, and ‘St’ represent Arabidopsis, S. oleracea, S. lycopersicum, and S. tuberosum genes, respectively. HPR, AGT, UAH, GLYR, MLS, and CAT were used as outgroups and represent hydroxypyruvate reductase, glyoxylate aminotransferase, ureidoglycolate amidohydrolase, glyoxylate reductase, malate synthase, and catalase, respectively. Different numbers indicate the bootstrap values on each node.
Analysis of LsGLOs gene structure
The organization of exon and intron provides the backbone of genes and can assist in studying evolutionary relationships between genes or organisms71. The number and distribution pattern of exon and intron are considered an evolutionary mark for a gene family. A comprehensive demonstration of the exon–intron structures of lettuce GLO genes and phylogeny analysis revealed that the gene structure pattern was consistent with the phylogenetic grouping (Fig. 4, Supplementary material Table S4). The LsGOX and LsHAOX genes showed similar gene structure patterns. An Almost similar number of introns and gene structure was displayed by Arabidopsis, spinach, tomato, and potato compared with lettuce (Fig. 4, Supplementary material Table S4). The number of introns varied from eight to ten in lettuce. LsGOX1 and LsGOX3 genes possessed nine introns, LsGOX2 possessed eight introns, and LsHAOX1 and LsHAOX2 included ten introns. All GOX genes contain eight to ten introns, except SoGOX genes, whereas all HAOX genes consist of ten introns, except SoHAOX2 (Fig. 4).
Gene structure of L. sativa (Ls) GLO genes compared to S. oleracea (So), S. lycopersicum (Sl), S. tuberosum (St), and A. thaliana (At) GLO genes. Pink arrows indicate exons, blue lines indicate introns, and purple lines represent phylogram (GSDS) at (http://gsds.cbi.pku.edu.cn/).
Identification of conserved motifs in L. sativa GLO genes
The identification and distribution of fifteen motifs within all the GLO proteins were studied in L. sativa, S. oleracea, S. lycopersicum, S. tuberosum, and Arabidopsis using the MEME program (Fig. 5, Supplementary material Fig. S2, Supplementary material Table S5). The number and position of motifs were observed to be 100% conserved in all the LsGLO genes, except motif 15 which was present in only LsHAOX genes (Fig. 5). Similar to LsHAOX genes, motif 14 was present in the same position in most S. lycopersicum, S. tuberosum and S. oleracea HAOX genes. Glycolate oxidase genes in all species showed similar pattern except S. oleracea (Fig. 5).
The distribution of 15 motifs in GLO proteins of various species. GLOs are displayed in hierarchy and ‘At’, ‘So’, ‘Sl’, and ‘St’ represent Arabidopsis, S. oleracea, S. lycopersicum, and S. tuberosum GLO genes, respectively. Bars with color gradient represent various motifs with a unique color code for each motif(TB tool v1.120).
Identification of LsGLO orthologs in arabidopsis
Functional prediction of a gene through identifying characterized orthologs is extensively used as a powerful tool in computational biology72,73. Orthologous proteins in different species have been observed to show similar biological functions73. LsGOX1 and LsGOX2 showed maximum percentage homology with AtGOX1. LsGOX1 showed 90.51, and LsGOX2 showed 89.97 percentage homology with AtGOX1. AtGOX1 was found to be expressed in cotyledon, fruit, leaf, guard cell, juvenile leaf during the seedling development stage, and non-host resistance, it regulates signal transduction pathway through reactive oxygen species (Table 2). LsGOX3 expressed maximum percentage homology of 85.08 with AtGOX3. AtGOX3 has previously shown expression in roots and mature to aging leaves during various developmental stages such as the flowering stage, differentiation, and expansion stage of the petal and embryo stage. AtGOX3 metabolizes l-lactate to pyruvate when l-lactate is lower in concentration inside the cell (Table 2). LsHAOX1 and LsHAOX2 showed the highest homology of 75.55 and 74.01, respectively, with AtHAOX1. AtHAOX1 was found to be expressed in seed and guard cells, and it encodes medium and long-chain hydroxy acid proteins as substrates (Table 2).
Chromosomal location and synteny analysis of lettuce GLO genes
Chromosomal distribution analysis of L. sativa GLO genes demonstrated that out of ten lettuce chromosomes (Lg0-Lg9), LsGLO genes were present on only three chromosomes. The maximum of three GLO genes were located on Lg5, and these were LsGOX2, LsHAOX1, and LsHAOX2, whereas, LsGOX1 and LsGOX3 genes were found to be present on Lg9 and Lg4, respectively (Fig. 6). Furthermore, syntenic analysis was performed for LsGLO genes to gain insight about the probability of segmental or tandem duplication of GLO gene family in lettuce (Fig. 6). In LsGLO genes, three paralogous gene pairs were distributed non-uniformly in the lettuce genome, which suggested that they might be emerged from whole-genome duplication or segmental duplication, whereas, one paralogous gene pair located too close to each other on the same chromosome might have emerged as a result of tandem duplication. Synteny analysis revealed that LsGOX1, LsGOX2, and LsGOX3 gene emerged as a result of the whole genome duplication (WGD) or segmental duplication, and LsHAOX1, and LsHAOX2 genes emerged as a result of tandem duplication (Fig. 6).
Distribution of LsGLO genes. (a) Position of LsGLO genes on chromosomes within the lettuce genome. (b) Syntenic relationship of LsGLO genes. Vertical (a) or curved (b) yellow blocks represent lettuce chromosomes, and labels on the right or top of each block represent the chromosome number. Rust colored lines represent the position of LsGLO genes on respective chromosomes, and red lines indicate segmental or tandem duplication of LsGLO genes(TB tool v1.120).
Dual synteny plot of lettuce GLO genes
To understand the evolution of LsGLO gene family, the syntenic relationship of LsGLO genes with A. thaliana, S. oleraceae, S. lycopersicum, and S. tuberosum was analyzed. Several GLO genes in L. sativa were revealed as orthologous genes in A. thaliana, S. oleraceae, S. lycopersicum, and S. tuberosum through collinearity analysis (Fig. 7, Table 3). Four LsGLO genes (80%) generally had syntenic relationships with GLO genes in other species. LsGOX1 and LsHAOX2 showed a syntenic relationship with AtGOX2 and AtHAOX1 on chromosome 3 (Chr3) in Arabidopsis. LsHAOX2 showed synteny to Spov3_chr4.04560 on chromosome 4 (Spov3_chr4) in S. oleraceae. Syntenic relationship of LsGOX1 and LsGOX2 genes with SlGOX1 on chromosome 7 (SL4.0ch07), LsGOX3 with SlGOX3 on chromosome 10 (SL4.0ch010), and LsHAOX2 with SlHAOX1 on chromosome 3 (SL4.0ch03) was observed in S. lycopersicum. Syntenic blocks were revealed in LsGOX1 and LsGOX2 with StGOX3 on chromosome 7 (chr07), LsGOX1 with StGOX2 on chromosome 10 (chr10), and LsHAOX2 with StHAOX2 on chromosome 3 (chr03) in S. tuberosum (Fig. 7, Table 3). However, LsHAOX1 did not show a syntenic relationship with any observed species.
Dual synteny of L. sativa GLO genes with other plant species. Green bars represent L. sativa chromosomes, and orange bars represent chromosomes of (a) Arabidopsis, (b) S. oleracea, (c) S. tuberosum, and (d) S. lycopersicum. Labels on top or bottom of the bars represent chromosome number of the respective plant species. Syntenic GLO gene pairs are highlighted by red lines and gray lines in the background indicate other collinear blocks among respective genomes(TB tool v1.120).
Evaluation of duplication event of lettuce GLO genes
The values of Ks, Ka, and Ka/Ks ratio of LsGLO genes were estimated through TBtools using simple Ka/Ks calculator (Fig. 8). Ks depicts the number of synonymous substitutions per synonymous site, whereas Ka shows the number of nonsynonymous substitutions per nonsynonymous site and the ratio of nonsynonymous (Ka) versus synonymous (Ks) mutation was represented by Ka/Ks. This ratio ranged from 0.03 in LsGOX1/LsGOX2 pair to 0.29 in LsHAOX1/LsHAOX2 pair (Fig. 8). All four paralogous pairs in lettuce had Ka/Ks ratio less than 0.3 which suggested the probability of limited functional divergence in the duplication process due to purifying selection.
Analysis of LsGLOs cis-regulatory elements
The presence and organization of various cis-regulatory elements present on the promoter region at the binding site of transcription factors affect the spacio-temporal transcriptomic expression of the genes. Therefore, to evaluate the putative functions of LsGLO genes, an in-silico analysis was conducted through the PlantCare database. Lettuce GLO genes consisted of light responsive, endosperm specific, hormone specific, meristem specific, metabolism related, and stress and defense related cis-regulatory elements (Fig. 9, Supplementary material Table S6). GLO family in lettuce contained nine cis-regulatory elements that are responsive to light, these were AE-box, Box 4, GATA-motif, I-box, GT1-motif, MRE, Sp1, G-box, and TCT-motif. Box 4 was present in all LsGLOs, and I-box was in all LsGLOs, except, LsHAOX2. GT1-motif was present in LsGOX3, LsHAOX1 and LsHAOX2. G-box was present in LsHAOX1 and LsHAOX2 only. AE-box was only present in LsGOX1, GATA-motif was present only in LsGOX2, MRE and Sp1 were present in LsGOX3 only, and TCT-motif was solely present in LsHAOX2 (Fig. 9, Supplementary material Table S6).
Identification of cis-acting elements in LsGLO genes. (a) Position of various cis-acting elements on the promoter region of LsGLO genes is represented on a scale of 1000 bps. Colored legends at the bottom represent various cis-elements found in each LsGLO gene, and each element is represented by a specific color. (b) Heat map representing the number of each cis- element found in LsGLO genes via a color gradient from green, representing the lowest, to red, representing the highest number of occurrences, and beige represents the absence of these elements.
Three defense related cis-regulatory elements in LsGLOs were CGTCA-motif, TGACG-motif, and TCA-element. CGTCA-motif and TGACG-motif are involved in response to methyl jasmonate and TCA-element is involved in response towards salicylic acid as a result of biotic stress, and these elements were limited to LsGOX1 and LsGOX2. Abiotic stress-related four cis-regulatory elements, ARE, GC-motif, MBS, and TC-rich repeats, were also present in LsGLOs. ARE, which is essential for anaerobic induction, was absent only in LsHAOX1, GC-motif, which is involved in anoxic specific inducibility, was present only in LsGOX2 and LsHAOX1, MBS, which is involved in the induction of drought, was present in LsGOX2, LsHAOX1, and LsHAOX2. TC-rich repeats involved in response to stress and defense were limited to LsHAOX2 (Fig. 9, Supplementary material Table S6).
GCN4_motif, which is involved in endosperm expression was present only in LsHAOX1, and CAT-box, which is related to meristem expression was present only in LsGOX2. O2-site involved in regulation of zein metabolism and MBSI involved in the regulation of flavonoid biosynthetic genes, was present only in LsHAOX1 and LsHAOX2, respectively. AT-rich element, which acts as the binding site of AT-rich DNA binding protein (ATBP-1), was present only in LsGOX1, and CCAAT-box, which acts as MYBHv1 binding site, was present in LsGOX2 and LsHAOX2. TATA-box and CAAT-box which are highly common cis-regulatory elements, were present in all lettuce GLO genes (Fig. 9, Supplementary material Table S6). The cis-regulatory elements identified among five LsGLOs, and their functional annotation, are shown in Supplementary material Table S6.
Analysis of protein–protein interaction network
Interaction among proteins reflects various important plant functions and processes such as signal transduction pathway, plant developmental, physiological, and pathological processes74. In lettuce GLOs, protein–protein interaction was studied through STRING database. Glycolate oxidase proteins showed interaction among various GLO proteins and with various other proteins in Lactuca sativa genome (Fig. 10).
Protein–protein interactions of LsGLO proteins predicted through STRING database(https://string-db.org). (a) Each colour represents a highly significant GO enrichment and type of interaction identified within the network. (b) Comparison of protein–protein interacting structure in L. sativa (Ls) and A. thaliana (At). HPR, AGT, UAH, GLYR, MLS, and CAT represents hydroxypyruvate reductase, glyoxylate aminotransferase, ureidoglycolate amidohydrolase, glyoxylate reductase, malate synthase, and catalase, respectively.
The STRING database provided various functional enrichments such as molecular functions, biological processes, and KEGG pathway etc. present in LsGLOs proteins (Table 4). Biological processes related to lactate oxidation, photorespiration, and oxidative photosynthetic carbon pathway showed the highest significance at p-value of 3.48 × 10−13, 2.08 × 10−14, and 2.39 × 10−15, respectively. Molecular functions that showed high enrichment are L-lactate dehydrogenase activity and long-chain 2-hydroxy acid oxidase activity at p-value of 4.15 × 10−13. Phosphoglycolate phosphatase activity and purine metabolism were identified as significantly enriched GO terms in local network cluster (STRING) at p-value of 1.82 × 10−20 and 5.16 × 10−21, respectively. KEGG signaling pathway revealed glyoxylate and dicarboxylate metabolism as most enriched functional category at p-value of 4.82 × 10−18. These significantly enriched GO terms showed presence in all LsGLO protein–protein interactions (Fig. 10, Table 4). Hydrogen peroxide biosynthetic process (biological process) and glycolate oxidase activity (molecular function) were specific to only LsHAOXs and LsGOXs, respectively (Table 4). Interestingly within the local string network cluster, butanoate metabolism showed presence only in LsGOX1 and LsGOX3 (Table 4).
Partner proteins, namely hydroxy pyruvate reductase (HPR), alanine glyoxylate aminotransferase (AGT), ureidoglycolate amidohydrolase (UAH), and glycolate reductase (GLYR1) showed presence in the protein–protein interaction network other than lettuce GLO proteins (Fig. 10, Table 4). HPR1 revealed interactions such as co-expression with LsGOX1 and LsGOX2 (Fig. 10). HPR2 and UAH showed only known interactions from curated databases with all LsGLO genes. AGT1 showed co-expression with all LsGLO genes and gene co-occurrence with LsGOX1 and LsGOX2. GLYR1 represented co-expression with LsGOX1 and LsGOX2 (Fig. 10). The genes representing these proteins in L. sativa, its ortholog, and the functions of these orthologs in Arabidopsis thaliana were provided in Table 5.
Comparing the protein–protein interaction of A. thaliana and L. sativa glycolate oxidase proteins revealed interesting results (Fig. 10). A. thaliana GLO genes showed complex interaction with other proteins compared to L. sativa. Contrary to L. sativa GLO genes mainly showing co-occurrence and gene co-expression with AGT1 and GLYR1, A. thaliana GLO genes showed co-expression with HPR (AT1G68010.2), AGT (AT2G13360.1), CAT (catalase) (CAT1/AT1G20630.1, CAT2/AT4G35090.1), and MLS (malate synthase/AT5G03860.1) (Fig. 10). Catalase occurs mainly in all aerobically respiring organisms and plays a role in protecting cells from the toxic effects of hydrogen peroxide by reducing hydrogen peroxide into water and oxygen using heme group as cofactor (TAIR).
Expression analysis of LsGLO genes in various organs under stress environment
Differential expression patterns of all LsGLOs, when exposed to various biotic, abiotic and developmental stresses, were analyzed using the available lettuce expression data on NCBI GEO. These expression data include the following stresses; low temperature, heavy metal (Cd), light, bolting, over-expression of LsAP2, and Bremia lactucae infection.
Low temperature (4 °C) stress affecting LsGLO genes
When the lettuce plant was exposed to low temperature (4 °C) for 0 h, 4 h, 24 h and 7 days, all LsGLO genes showed statistically significant (p < 0.05) results in above-ground part of lettuce, except LsHAOX1. The expression level was quite high for LsGOX1 and LsGOX2 and very low, almost negligible, for LsGOX3 and LsHAOX2 (Fig. 11). The graph showed that initially, the expression of LsGOX1 and LsGOX2 gene decreased rapidly when lettuce plants were exposed to 4 °C temperature for 4 h as compared to the control group (0 h) (Fig. 11). The expression of these genes then started to increase and reached its peak after 24 h. At this point, the expression level of LsGOX1 and LsGOX2 exceeded the expression in the control group (Fig. 11). The expression, then, started to decrease, and after 7 days it was lower than the control group but still higher than expression of LsGLOs in 4 h group (Fig. 11).
Expression of lettuce GLO genes under low temperature (4 °C) stress. The expression in control group (20 °C/0 h) and experimental group (exposure to 4 °C for 4 h, 24 h, and 7 days) lettuce plants was analyzed through multiple comparisons of means using Tukey HSD test at a significance level of 0.05. Different letters indicate significant differences and error bars indicate standard error of 3 replicates. LsGLO genes are represented by different colours as shown in legends.
Gene expression during various developmental stages in bolting-sensitive and bolting-resistant lines of L. sativa
The expression data generated to study the regulation of bolting in lettuce showed the expression of all LsGLO genes except LsHAOX2, however, only the expression showed by LsGOX1 was statistically significant (p < 0.05). It was observed that high expression was given by LsGOX1 in SAM cells of bolting-sensitive line S39 as compared to bolting-resistant line S24 of lettuce (Fig. 12). In bolting-sensitive line S39, the expression of LsGOX1 increased gradually in all four stages and reached a maximum level of expression at flowering stage (Fig. 12). However, in bolting-resistant line S24 of lettuce, the expression of LsGOX1 showed sudden decrease initially in bolting stage from the vegetative stage, but then gradually increased from bolting stage and reached its peak at flowering stage (Fig. 12). Collectively, the flowering stage showed the highest expression of LsGOX1, and difference in expression of LsGOX1 was highest at bolting and inflorescence stage in both bolting-sensitive line S39 and bolting-resistant line S24 of lettuce (Fig. 12).
Expression of LsGOX1 under bolting stress in in bolting-sensitive S39 and bolting resistant S24 lettuce lines. Different letters indicate significant differences and these differences were determined by Tukey HSD test at a significance level of 0.05 and error bars indicate standard error in biological replicates.
Differential expression of LsGLO genes in leaves and roots of L. sativa under cadmium (Cd) stress
In expression profiling of Italian lettuce (Lactuca sativa L. var. ramose Hort.) where melatonin treated and non-treated groups were grown under cadmium (Cd) stress for 0 day (control group), 1 day, and 5 days, all LsGLO genes showed statistically significant expression (p < 0.05) in roots and leaves, except, LsHAOX2 in leaves. The expression of LsGLO genes in leaves was significant than the expression in roots (Fig. 13).
Expression pattern of LsGLO genes under cadmium (Cd) stress. (a) Expression in treated and non-treated lettuce leaves. (b) Expression in treated and non-treated lettuce roots. 0, 1, and 5 represent duration of cadmium stress in days. Error bars indicate standard error in biological replicates. Significant differences in expression of LsGLO genes were determined by Tukey HSD test (p < 0.05), and the differences are indicated by letters a, b, and c. LsGLO genes are represented by different colours as shown in legends.
In leaves, melatonin-treated, and non-treated group showed the same level of expression in the control group (0 day), then at day 1 the expression of LsGLO genes was significantly reduced in both groups, but melatonin treated group showed higher expression than non-treated group, however at day 5, the expression of all LsGLO genes was lower in melatonin treated group than non-treated group. At day 5, expression in both groups was relatively higher than in day 1 (Fig. 13).
In roots, the control group (day 0) showed higher expression of GLO genes in the melatonin-treated group than the non-treated group. At day 1, the expression level of LsGLO genes in non-treated roots was comparatively high than control group, however, melatonin-treated roots showed decrease in expression level from control (day 0) (Fig. 13). At day 5, non-treated group showed same level of expression as a control group; however, expression of melatonin-treated group was lower than the control group (0 day) (Fig. 13).
Over-expression of APETALA2 gene affects the expression of LsGLO genes
Leaf expression profiling of one-month old wild type (WT) and LsAP2 over-expressed (LsAP2-OE) lettuce plants revealed significant expression (p < 0.05) of only two LsGLO genes, LsGOX1 and LsGOX2. The expression of both LsGOX1 and LsGOX2 genes was high in LsAP2 over-expressed lettuce group compared to wild lettuce (Fig. 14). A significant increase in expression was showed by LsGOX1, whereas, LsGOX2 showed very minor increase in expression in LsAP2 over-expressed lettuce group (Fig. 14).
Expression profiling of LsGLOs in wild type (WT) and LsAP2 overexpressed (LsAP2-OE) lettuce S39 cultivar. Different letters indicate significant differences determined by one-way ANOVA with Tukey HSD test (p < 0.05) and error bars represent standard error in biological replicates. LsGLO genes are represented by different colours as shown in legends.
Light regulated expression of LsGLOs
RNA sequencing was performed on 24 days old lettuce leaves grown under treatments of varying light intensities (low, medium, and high light intensity). The data revealed that not a single LsGLO gene was targeted by light-responsive circular RNAs (circRNAs).
Expression of LsGLO genes in downy mildew-infected lettuce
Small RNAs (sRNA) derived from lettuce flowers and healthy and infected (Bremia lactucae) lettuce leaves targeted all LsGLO genes in both healthy and infected lettuce leaves (Fig. 15). Number of sRNA targets were transcribed as expression of LsGLO genes. Graph showed that LsGLO genes showed higher expression in heathy leaves than infected lettuce leaves, however, LsGLOs showed highest expression in flowers (Fig. 15). LsHAOX1 showed similar level of expression as LsGOX3 in healthy leaves. However, expression of LsGOX1 was lower than LsGOX3 in infected leaves. LsHAOX1 and LsHAOX2 showed similar level of expression in healthy leaves and flowers as LsGOX3, however, expression of these genes was lower than LsGOX3 in infected leaves (Fig. 15).
Putative miRNA targets in Lactuca sativa
Plant MicroRNA Encyclopedia database was used to retrieve the miRNA sequences, and through an online tool, psRNATarget, miRNAs were identified that potentially target lettuce (Lactuca sativa v8) GLO genes. As a result, six miRNAs were found targeting all five LsGLO genes. These miRNAs were single-stranded nucleotides with 20 to 21 nucleic acids (Table 6). The number of miRNAs targeting lettuce GLO genes were one miRNA per LsGLO genes, and only one miRNA, Lsa-miR414, targeted two LsGLO genes. Lsa-miR168a and Lsa-miRN1713 targeted LsGOX1. LsGOX2 and LsGOX3 were targeted by only one miRNA, Lsa-miR2109 and Lsa-miRN1723, respectively. LsHAOX2 was targeted by two miRNAs, Lsa-miR414 and Lsa-miRN1644. LsHAOX1 was targeted by only one miRNA, Lsa-miR414 (Table 6). This analysis indicated that LsGOX1 and LsHAOX2 were the only genes that were targeted by more than one miRNA, and group GOX was targeted the most since this group was targeted by four mature miRNAs, whereas only two miRNAs targeted HAOX group.
The expression of these miRNAs in leaf and flower was also studied, which revealed that miR168a showed the highest expression in both leaf and flower and only miR168a and miR2109 showed expression in both leaf and flower, however, the expression showed by miR2109 was too low in comparison with miR168a (Fig. 16). Apart from miR168a and miR2109, no other miRNA was expressed in the flower. miRN1713, miRN1723, and miRN1644 showed expression in the leaf to some extent, but it was very low compared to miR168a (Fig. 16).
Expression of LsGLOs targeting miRNAs in leaf and flower of mature lettuce plants. The expression values are represented as log2 fold-change. Color gradient represents the expression levels of miRNAs with blue indicating the lowest, red indicating the highest, and yellow indicating the absence of expression in lettuce.
Discussion
This study identified five GLO family genes in lettuce (Table 1). Three domains, namely FMN-dependent dehydrogenase (FMN-dh), long chain alpha-hydroxy acid oxidase (alpha_hydroxyacid_oxid_FMN), and L-lactate dehydrogenase (LldD) were found to be 100% conserved in all lettuce GLO genes along with various binding and catalytic sites (Fig. 1, Supplementary material Table S1). In-silico analysis predicted the absence of nuclear localization signals (NLS) in lettuce GLO proteins and sub-cellular localization signals confirmed the presence of abundant cytosolic signals in LsGOX1-3 and peroxisomal signals in LsHAOX1-2 (Fig. 2, Supplementary material Table S2).
Phylogenetic grouping of LsGLO genes was based on the grouping of Arabidopsis GLO genes, and the grouping was confirmed by predicted sub-cellular localization signals, since, cytosolic signals were dominant in GOX. Peroxisomal signals were dominant in HAOX group (Fig. 3). In GOX group, GOX1 and GOX2 genes of lettuce and Arabidopsis shared the same clade. Protein–protein analysis revealed that LsGOX1 and LsGOX2 showed maximum percentage homology with AtGOX1 (Table 2). LsGOX3 was present in an entirely different clade from StGOX1 and SlGOX2, and the same pattern was observed for AtGOX3, which shared the clade with StGOX2 and SlGOX3. LsGOX3 expressed maximum percentage homology with AtGOX3. In HAOX group, HAOX1 and HAOX2 genes of lettuce and Arabidopsis were present in different clades. LsHAOX1 and LsHAOX2 showed the highest homology with AtHAOX1 (Table 2). The presence of HAOX genes of L. sativa and Arabidopsis far away from each other in comparison with GOX genes of these species suggests that HAOX genes are evolutionarily more distant as compared to GOX genes (Fig. 3). Proteins present in the same clade usually exhibit similar structure and functions. In Arabidopsis, only AtGOX1 and AtGOX2 genes are involved in the photorespiratory pathway, whereas, AtGOX3, AtHAOX1, and AtHAOX2 genes play a role in non-photosynthetic functions75,76. The high protein homology of lettuce and Arabidopsis GLO genes suggested that LsGLO genes might perform in the same pattern (Table 2).
The knowledge about the presence and position of exons and introns in a gene can be used for understanding the gene’s evolutionary relationship with other genes or organisms71,77. Analysis revealed that all five LsGLO genes contain introns on the upstream end of the GLO domain, and similar exon–intron structure was shared among all lettuce GLO genes in the same group (Fig. 4, Supplementary material Table S4). Similar exon–intron association noticed in S. oleracea, S. lycopersicum, S. tuberosum, and Arabidopsis suggests that these structures remain preserved during the evolutionary process and might protect gene integrity (Fig. 4).
Assessment of conserved motifs in LsGLO genes identified twelve motifs conserved in all LsGLO genes, except motif 15, which was only present in LsHAOX genes (Fig. 5, Supplementary material Fig. S2, Supplementary material Table S5). The presence of motifs in the same pattern and position suggests that these motifs might play an essential part in activities specific to GLO proteins. The presence of motif 15 only in LsHAOX genes and motif 12 only in S. lycopersicum and S. tuberosum HAOX genes indicates that these motifs are specific to HAOX genes and could be one of the controlling factors that distinguish HAOX genes from GOX genes in these species (Fig. 5).
The location of a gene on the chromosome can assist in the prediction of gene duplication events. Gene duplication event could be called tandem duplication if two or more than two genes are present on the same chromosome, whereas the presence of a gene group on different chromosomes could occur as a result of segmental or whole genome duplication (WGD)78. Three LsGLO genes were located close to each other on chromosome 5, and syntenic analysis revealed the dominance of segmental duplication (Fig. 6). NCBI protein blast showed 97.02 and 83.97 percent homology of LsGOX1 with LsGOX2 and LsGOX3, respectively, and 95.43 percent homology of LsHAOX1 with LsHAOX2. High protein homology and the presence of LsGOX1, LsGOX2, and LsGOX3 on different chromosomes suggests that these genes might be segmentally duplicated, and LsHAOX1 and LsHAOX2 might have duplicated through tandem duplication as these genes are present on the same chromosome (Fig. 6). The duplication of LsGOX3 gene appeared to be dispersed according to synteny analysis, and could mean that this gene might have emerged through replicative transposition from LsGOX1. This also explains the position of LsGOX3 in a different clade than LsGOX1 and LsGOX2 in phylogram (Fig. 3). The expansion of a gene family is mainly driven through the phenomenon of gene duplication, and the increase in GLO gene member in higher plants (S. lycopersicum, N. benthamiana, and S. tuberosum) may be mainly due to the duplication of FMN-dependent dehydrogenase (FMN-dh), alpha hydroxyacid oxidase, and L-lactate dehydrogenase domain during evolution of eukaryotic plants79,80.
Comparative syntenic maps can be used further to explore the potential evolutionary mechanism of a gene family81. Syntenic maps revealed collinear blocks between LsGOX1, AtGOX2, SlGOX1, StGOX2, and StGOX3, whereas, LsHAOX2 showed syntenic gene pair with AtHAOX1, SlHAOX1, and StHAOX2 (Fig. 7, Table 3), which suggests the origin of these genes from a common ancestor, and explain expansion of GLO gene family. The high number of collinear gene pairs between L. sativa and S. lycopersicum/S. tuberosum and the presence of GLO genes of these species in the same clades points to their close evolutionary relationship. Syntenic gene pairing of lettuce GOX and HAOX genes being consistent with GOX and HAOX genes in respective plant genomes, except in S. oleracea, further proves that LsGOX genes might be segmentally duplicated HAOX genes might have duplicated through tandem duplication. In S. oleracea, LsHAOX2 showed syntenic gene pair with Spov3_chr4.04560 gene, which is not a glycolate oxidase gene, but rather a nuclear pore complex protein Nup107 (NUP107, NUP84) (Table 3). Interestingly, we found that a transcript of AtGOX1 (AT3G14120.2) is also a nuclear pore complex protein NUP107 which suggests the possibility that GLO genes might have evolved from nuclear pore complex proteins, or they might have evolved into nuclear pore complex proteins. Because no LsGLO gene or gene isoform is a nuclear pore complex protein, no other LsGLO gene showed a syntenic relationship with nuclear pore complex protein, and none of the lettuce GLO genes exhibit nuclear localization signals (NLS), the former hypothesis seems more convincing, however, it’s not quite supported by evidence.
The selection pressure on the substitution of amino acids can be understood through the ratio of Ka/Ks (Fig. 8). The ratio of Ka/Ks > 1 suggests the possibility of purifying selection, whereas, Ka/Ks ratio > 1 suggest the likelihood of positive selection82,83. Generally, the evaluation of selective pressure provides selective lead for amino acid sequence altered in a protein and is also necessary for interpreting functional residues and protein shifts84. The estimated values of Ka/Ks ranged from 0.03 to 0.29, which being less than 1, suggests that lettuce GLO genes undergo strong purifying selection pressure and positive selection might have acted on a few sites only during the process of evolution (Fig. 8).
The presence of hormone-responsive, stress and defense responsive cis- acting elements suggest that these genes might play important roles in lettuce plant growth and development and might be involved in lettuce plant’s defense mechanisms as well (Fig. 9). Highest number, twelve, of cis- acting elements were found in LsHAOX genes, and a range of eight to ten cis- acting elements were found in LsGOX genes (Fig. 9). Light, hormone, and stress-responsive cis- acting elements were dominant and defense related cis- acting elements were absent in LsHAOX genes, whereas, only light and defense related cis- acting elements were dominant in LsGOX genes. Overall, light responsive cis- acting elements were highly dominant, and defense-related cis- acting elements were least dominant in lettuce GLO genes (Fig. 9, Supplementary material Table S6).
Protein–protein interaction is crucial in understanding the functions of a gene in silico85. During cell signaling and metabolism, enzyme activity is known to be regulated through protein–protein interaction86. Specificity of the hydrogen peroxide biosynthetic process to only LsHAOX1-2 genes, and glyoxylate bypass and glycolate oxidase activity being specific to only LsGOX1-3 genes further confirms the hypothesis that LsGOX1 and LsGOX2 play role in photorespiratory pathway and LsGOX3, LsHAOX1, and LsHAOX2 genes are involved in non-photosynthetic functions (Table 4). Butanoate metabolism produces signal molecule GABA (g-Aminobutyric acid) that rapidly accumulates in plant tissues in response to biotic and abiotic stress and elevates plant stress tolerance87. It is predicted only in LsGOX1 and LsGOX3, which suggests that these genes trigger GABA synthesis by activating butanoate metabolism thus, indirectly enhancing plant defenses in response to plant-animal and plant–microbe interactions (Table 4). Surprisingly, LsGOX1 and LsGOX2 did not show any type of interaction with each other. The same thing was observed between LsHAOX1 and LsHAOX2 (Fig. 10). The respective gene pairs in A. thaliana have a well-established interaction, co-occurrence and co-expression of AtGOX1/AtGOX2 and co-occurrence, of AtHAOX1/AtHAOX2, which implies that these gene pairs in L. sativa (LsGOX1/LsGOX2 and LsHAOX1/LsHAOX2) are not regulated or expressed similarly. Glycolate oxidase (GLO) is the first enzyme in glycolate pathway88,89, and co-expression of LsGLO genes with other members of this pathway (GLYR1, HPR1, and AGT1) emphasizes their strong functional association with LsGLO proteins and implies that disruption in this protein will probably result in disruption of the whole glycolate/glyoxylate pathway, photorespiration, and photosynthesis, so the implications would be quite destructive (Fig. 10, Table 5). Co-expression of catalases with GLO genes prevent hydrogen peroxide mediated stress in plant due to activity of glycolate oxidases in A. thaliana. It is quite surprising that GLO genes in L. sativa did not interact with catalases despite containing the same number of GLO genes as A. thaliana and no such stress is reported to date. This suggests that the absence of co-expression interaction between LsGOX1/LsGOX2 and LsHAOX1/LsHAOX2 might be directly linked with low hydrogen peroxide mediated stress due to limited activity of glycolate oxidases in lettuce (Fig. 10).
Expression data analysis provides important information to predict the functions of a gene or gene family in an organism and revealed interesting findings. LsGOX1 and LsGOX2 genes were up regulated when lettuce plants were grown under low (4 °C) temperature for 24 h as compared to control group (0 h) (Fig. 11). The initial decrease in expression could be the result of shock caused by sudden temperature decrease in plant environment. During short-term chilling stress (4 h), hydrogen peroxide up regulates cyclic electron flow (CEF) and alternative oxidase (AOX) pathways through signal transduction46. Alternative oxidase (AOX) optimizes photosynthesis during low temperature, and drought stress90. Photorespiration is pivotal for photosynthesis and oxidative stress response was induced, therefore, the expression of the GLO genes was increased and coordination among these two processes helps plant to adapt in cold environment. When the stress lasted for longer, i.e., 7 days, the expression of GLO genes was decreased due to reduced enzyme activity. The expression of LsGLO genes was down regulated in non-treated leaves, melatonin-treated leaves and melatonin-treated roots, and up-regulated in non-treated roots under cadmium stress (Fig. 13). Melatonin is a plant signaling molecule that restricts cadmium (Cd) transport from roots to shoots to protect the photosynthetic apparatus of the plant. Exogenous melatonin stimulates the production of endogenous melatonin. Since melatonin is a ubiquitous antioxidant it aids plants in stress tolerance by enhancing the production of antioxidants and activates the genes involved in redox reactions91,92. Movement of glycolate oxidases from other plant parts to roots, increased activity of LsGLOs in non-treated roots, and reduced activity in melatonin-treated roots under the same conditions suggests that LsGLOs might also play a role in increasing stress tolerance of lettuce through an increase in redox reactions and H2O2-mediated signal transduction under heavy metal (Cd) stress. These results support the role of LsGLO genes in lettuce tolerance to abiotic stresses.
The expression of LsGOX1 was up regulated during the vegetative, bolting, and inflorescence stage in bolting-sensitive line S39 as compared to bolting-resistant line S24 (Fig. 12). High temperature promotes bolting and also enhances photorespiration which explains high expression of LsGOX1 and suggests that LsGOX1 might be a key player in plant developmental processes such as inflorescence and flower initiation. The down regulation of LsGOX1 at a bolting stage in bolting-resistant line S24 could be due to changes in lettuce plant gene expression to resist bolting (Fig. 12). Expression pattern of LsGOX1 and LsGOX2 in APETALA2 over-expressed lettuce plants was significantly higher as compared to wild type lettuce (Fig. 14). This suggests that LsGOX1 might play role in leaf development. The number of sRNAs targeting LsGLO genes in flowers, healthy and infected lettuce leaves can be interpreted as the expression level of LsGLO genes in these organs. sRNA targets demonstrated that GLO genes were down regulated in infected (downy mildew) lettuce leaves (Fig. 15). Lettuce plant, when exposed to Bremia lactucae activates SAR (Systemic acquired resistance) signaling in which known compound such as abscisic acid, methyl-jasmonate, ethylene, sodium-salicylate are involved. Sodium-salicylate is a salicylic acid derivative and salicylic derivatives have been found to inhibit glycolate oxidases through binding to the substrate site, decreasing GLO gene expression93,94. Significantly high expression level of LsGLO genes in flowers as compared to healthy lettuce leaves suggests the involvement of these genes in various processes and functions related to flowering. These results suggest that LsGOX1 might play vital role in flowering initiation and leaf and flower developmental processes.
LsGLO genes are not regulated by light intensity since no GLO gene was targeted by light-responsive circular RNAs (circRNAs). Six miRNAs were found to target the GLO genes in mature lettuce plants, but only miR168a was significantly expressed in mature lettuce flowers and leaves that specifically targeted LsGOX1 (Fig. 16, Table 6). According to previous studies, MIR168 has been most commonly detected as stress inducible gene and MIR168a is abundant in rice95,96. 21 nucleotide MIR168 showed effective response to fluctuations in plant environment97. MIR168 regulates the function of miRNAs, since it regulates the expression of AGO1 (Argonaute RISC Component 1) and in Arabidopsis, and it is involved in post-transcriptional gene silencing by maintaining AGO1 homeostasis98,99,100,101. High expression of miR168a in leaf and flower might result ofresult from the post-transcriptional gene silencing of LsGOX1 to regulate its expression. MIR168a regulates flowering time and is involved in plant hormone signal transduction and plant-pathogen interaction in rice95,96. This supports the previously stated roles of LsGOX1 during inflorescence and flowering stage and in various stresses suggesting that LsGOX1 might play role in regulating flowering, plant signaling and interaction with pathogens.
The results of all these expression analyses suggest that LsGOX1 and LsGOX2 might play role in plant resistance mechanism by regulating ROS-mediated signaling during various environmental stresses, LsGOX1 seems to contribute in various stages of plant development through oxidative photosynthetic carbon pathway and both of these functions are also performed by AtGOX1 (Table 6). miR168a targeting LsGOX1 might regulate gene expression of LsGOX1 through AGO1-mediated negative feedback and avoid abnormality in plant functioning or any developmental defects.
In Arabidopsis, the combined expression of GOX1 and GOX2 was reported to be higher than GOX3 in leaves. Lower in roots under normal conditions@@102,103, and the same results were observed in lettuce (Figs. 11, 13). However, Arabidopsis leaves infected with Botrytis cinerea showed reduction in combined expression of GOX1 and GOX2. It became comparable to the expression of GOX3 and same pattern in LsGOX genes was observed in B. lactucae-infected lettuce leaves (Fig. 16). In Arabidopsis, AtGOX1 and AtGOX2 genes were highly expressed in green organs, and these genes are co-expressed with other AtGLO genes@@21,104. The same pattern was observed in lettuce GLO genes. In conclusion, lettuce glycolate oxidase (GLO) might be a constitutive gene, as it is expressed in all organs. The significance of its expression include uninterrupted photorespiration essential for photosynthesis in C3 plants like lettuce, plant signaling in response to plant–microbe interactions, developmental role in leaf and flower, and tolerance to abiotic stresses.
Material and methods
It has been confirmed that the experimental data collection complied with relevant institutional, national, and international guidelines and legislation with appropriate permissions from authorities of the Department of Horticulture, Faculty of Agricultural Sciences, University of the Punjab, Lahore, Pakistan.
Database search and retrieval of sequence
The GLO protein amino acid sequence was retrieved from S. oleracea (Accession no SoGLO1/Spo19861) through SpinachBase database105,106. 341 AA sequence of FMN-dependent dehydrogenase domain was retrieved from SoGLO1 (Accession no Spo19861) through Pfam database. The peptide sequence of SoGLO1/Spo19861 was used to identify GLO protein encoding genes in lettuce (Lactuca sativa cv. Salinas/L. sativa v8) proteome database at Phytozome v13 using BLAST-P 2.6.0 + (Protein- basic local alignment search tool) program107,108. Furthermore, the protein sequences of the reported Arabidopsis GLO family genes were retrieved from TAIR at Phytozome v13, which consisted of three AtGOX (AT3G14420, AT3G14415 and AT4G18360), and two AtHAOX (AT3G14130 and AT3G14150) genes. These sequences were then used to BLAST-P (Protein- basic local alignment search tool) search in Lactuca sativa v8 (Lactuca sativa cv. Salinas) proteome database at Phytozome v13 to reconfirm the retrieved GLO proteins in lettuce.
Identification of conserved domains in LsGLO genes
The retrieved amino acid sequences were subjected to NCBI CDD (Conserved Domain Database) with the default parameters109,110. The proteins lacking FMN-dependent dehydrogenase (PF01070) conserved domain were removed.
Determining physio-chemical properties of LsGLO proteins
The length of proteins (amino acid), weight of protein molecules, and theoretical isoelectric point of LsGLO proteins were predicted through an online tool, ProtParam111. The information regarding gene identity, position on chromosomes and gene and protein sequences were retrieved from Phytozome. The renaming of LsGLO genes was done based on the order of their physical position in lettuce genome database.
Prediction of nuclear and sub-cellular localization signals
The nuclear localization signals (NLS) in lettuce GLO proteins were predicted through an online server, nuclear localization signals database (NLSdb)112,113,114. Subcellular localization of LsGLO proteins was predicted using an online tool WoLF PSORT115.
Phylogenetic analysis
The amino acid sequence of GLO proteins in L. sativa, S. oleracea, S. lycopersicum, S. tuberosum, and A. thaliana along with HPR, GLYR, AGT, UAH, MLS, and CAT protein sequences of L. sativa and A. thaliana were aligned using Clustal W version 2.1116,117. Neighbor joining method (NJ) in MEGA X v10.2.4 program created the phylogenetic tree with bootstrap set at 1000 replications and other default parameters118,119. HPR, GLYR, AGT, UAH, MLS, and CAT proteins were used as outgroup.
Gene structure analysis
Genomic and coding sequence of LsGLO genes were retrieved from Phytozome database and were used to draw the gene structure through Gene Structure Display Server (GSDS v2.0)120,121.
Recognition of conserved motifs
Multiple EM for Motif Elicitation (MEME) program was used to analyze motifs in the retrieved LsGLO protein sequences with maximum number of motifs set as 15, and minimum 6 and maximum 50 width of motif was set as default values along with other factors122,123. Visual representation of the motifs was created using the Gene Structure View (Advanced) software in TBtools124.
Identification of LsGLO gene orthologs in arabidopsis
Protein homologs of L. sativa GLO genes were identified in A. thaliana through protein–protein BLAST (blastp) on NCBI, a web-interface125,126. The analysis was conducted using protein sequences of LsGLO genes against the whole genome of A. thaliana (taxonomy id: 3702) with all parameters set as default.
Chromosomal location and synteny analysis
The information regarding chromosome length and location of gene on chromosome was extracted from the lettuce genome database, and gene location was visualized through TBtools. Gene pairs of lettuce GLO genes were created through TBtools, and synteny analysis was conducted using the Advanced Circos program in TBtools. The genome and gff3 files of L. sativa, A. thaliana, S.oleraceae, S. lycopersicum and S. tuberosum were retrieved from Phytozome database to display the syntenic blocks of L. sativa GLO genes in respective genomes. The syntenic maps were constructed by using the One Step MCScanX and Dual Systeny Plot in TBtools comparative genomics program with default parameters124,127,128.
Analysis of gene duplication event
The lettuce GLO gene family duplication event was studied using Ks and Ka values. The number of non-synonymous (Ka) and synonymous (Ks) substitution rates, and Ka/Ks ratios were calculated using the simple Ka/Ks calculator through TBTools Software124. The parameters were set as described in the software package manual. The Ka/Ks ratios were used to predict the rates of molecular evolution of each paralogous gene pair5. Generally, a Ka/Ks ratio greater than one indicates positive selection, a ratio close to 1 indicates neutral selection, whereas, a ratio less than 1 indicates the probability of purifying selection which leads to limited functional divergence of the duplicated genes5.
Analysis of cis-acting elements
A sequence of 1000 bps upstream was retrieved from the initiation codon of putative LsGLO genes to analyze the promoter region. PlantCare database was then used to predict the cis-regulatory elements in these sequences129,130,131,132. The number and position of the predicted cis-regulatory elements were visualized using a heat map and Basic Biosequence view program in TBtools133,134,135.
Analysis of protein–protein interaction network
Interactions among the lettuce GLO proteins were studied by constructing protein interaction networks using the Search tool in STRING database v11.5 with a medium confidence score of 0.400 as the default value, along with other settings. Functional associations in interacting networks consisted of biological processes, molecular functions, KEGG pathways, and local network clusters created by STRING database v11.5136,137.
Transcriptome analysis
To study the external stimuli-specific expression profile of LsGLOs in various organs, we analyzed previously generated RNA-seq data of lettuce (L. sativa) plant under several biotic, abiotic, and developmental stresses from NCBI GEO (Gene Expression Omnibus). These stresses include low temperature, light, heavy metal (Cd), bolting, over-expression of APETELA2, and lettuce downy mildew (Bremia lactucae) infection. Statistical analysis of the gene expression data was conducted through Statistix v8.1 (Analytical software 2005).
Effect of low temperature (4 °C) stress on LsGLO genes
Lettuce (Salinas) plants were grown at 20 °C in a growth chamber, and 18-day old plants were exposed to low temperatures (4 °C) for different time intervals, i.e., 4 h, 24 h, and 7 days5. ‘0 h’ sample represents controlled group lettuce plants grown at 20 °C. Three replicates of lettuce plant were taken for each time interval, so the total number of sample plants taken was 12. RNA sequencing was performed in above-ground lettuce plant tissues, and expression data was calculated in log2FPKM. Statistical analysis was done using ANOVA, genes showing p < 0.05 were considered statistically significant and Tukey HSD test determined the significant differences in expression.
Lettuce plant under bolting stress
An experiment was conducted to understand the underlying processes in inflorescence development and the putative role of LsFT (FLOWERING LOCUS T) in the regulation of bolting in lettuce. Two leafy lettuce lines, bolting-sensitive line S39, and bolting-resistant line S24 of lettuce (L. sativa) were cultivated in a growth chamber at 25 °C day and 15 °C night temperature. Expression profiling of shoot apical meristem (SAM) cells was carried out at four critical developmental stages in lettuce i.e., vegetative stage from 0 to 35 days after planting (DAP), bolting stage from 35 to 75 DAP, inflorescence stage from 75 to 95 DAP, and flowering stage from 95 to 125 DAP. Two replicates of shoot apical meristem (SAM) cells were taken from each developmental stage of both lettuce lines, so 16 replicates were created for experiment48. Expression values were calculated in log2FPKM. Significance in LsGLO expression was determined by p-value, genes showing p-value < 0.05 were considered differentially expressed.
Leaves and roots of L. sativa under cadmium (Cd) stress
A study was conducted to understand the relationship between exogenous melatonin treatment and relative resistance shown by Italian Lettuce (L. sativa var. ramose Hort.) against cadmium (Cd) stress environment138. The seeds were germinated in a growth chamber and after 20 days, the seedlings were divided into two groups based on the treatments applied i.e., seedlings cultivated with a nutrient solution containing 1 μmol/L (Sigma-Aldrich, USA) melatonin, and seedlings cultivated with a nutrient solution without melatonin (control group). Then after two days, both groups were treated with 50 μmol/L cadmium chloride (CdCl2) to create Cd stress environment. The leaves and roots of seedlings in two groups were sampled at 0-, 1-, and 5-days interval and three replicates were taken for each treatment. Expression levels were calculated in log2FPKM. Significant expression was determined through multiple comparisons of means were performed using Tukey HSD test at significance level of 0.05.
Over-expression of APETALA2 in lettuce leaves
Wild type (WT) and LsAP2 overexpressed (LsAP2-OE) S39 cultivar of leaf lettuce (Lactuca sativa L.) plants were cultivated in a growth chamber at 16/8 h light/dark and 25/18 °C day/night temperature. Three replicates were taken from each treatment, and RNA-seq experiment was conducted on leaves of each sample after one month139. Expression data was calculated in log2FPKM. Significance in gene expression was determined by one-way ANOVA (p-value < 0.05) and Tukey HSD test.
Lettuce plant under light stress of various intensities
RNA sequencing (RNA-Seq) was performed on 24 days old lettuce leaves grown in 3 different light intensity treatments, low light (Las_WL, 60 ± 2 µmol m−2 s−1), medium light (Las_ML, 175 ± 2 µmol m−2 s−1), and high light intensity (Las_SL, 340 ± 2 µmol m−2 s−1) in a growth chamber57. Expression data was calculated in log2FPKM. Differentially expressed genes were selected based on log2(fold-change) > 1, and statistical significance (p < 0.05) was determined by using ANOVA.
Expression profiling of LsGLO genes in various organs through sRNAs targets
An experiment was designed in which lettuce leaves infected with fungus Bremia lactucae strain CA-III were harvested 7 days after inoculation and small RNA libraries were derived from flowers, heathy leaves of lettuce (Lactuca sativa cv. ‘Salinas’) and infected (Bremia lactucae) lettuce leaves (GEO accession: GSE28322). Analysis was conducted by uploading the sequence of small RNAs and CDS sequences of all lettuce GLO genes on an online tool, psRNATarget with default parameters64,140. The expression pattern of LsGLO genes was visualized through bar graph.
Analysis of putative microRNAs targeting LsGLO genes
The lettuce plant's micro-RNA (miRNA) dataset was retrieved from PmiREN (Plant miRNA Encyclopedia)141. To find out the miRNAs which targets the lettuce GLO genes, the CDS sequences of all GLO genes of lettuce were searched against the complementary sequences of miRNAs with the help of psRNATarget with default parameters64,140. Expression levels of miRNA in leaves and flowers were presented in log2 change. Heatmap illustrator in TBtools was used to display expression patterns of miRNAs in lettuce plants with hierarchical clustering124,133.
Data availability
All the data generated or produced during the study has been given in the manuscript and its related supplementary file. Gene expression data studies in this paper were downloaded from NCBI GEO (Gene Expression Omnibus) (Series Accession: GSE134012, GSE108260, GSE143675, GSE168886, GSE148578, GSE28322).
References
Aderinola, O. & Kusemiju, V. Heavy metals concentration in Garden lettuce (Lactuca sativa L.) grown along Badagry expressway, Lagos. Nigeria. Transnatl. J. Sci. Technol. 2, 115–130 (2012).
Shatilov, M., Razin, A., & Ivanova, M. Analysis of the world lettuce market. Paper presented at the IOP Conference Series: Earth and Environmental Science (2019).
Hasan, M., Tahsin, A., Islam, M., Ali, M. A. & Uddain, J. Growth and yield of lettuce (Lactuca sativa L.) influenced as nitrogen fertilizer and plant spacing. J. Agric. Vet. Sci. 10, 62–71 (2017).
Pink, D. & Keane, E. M. Lettuce: Lactuca sativa L. In Genetic Improvement of Vegetable Crops 543–571 (Elsevier, 1993).
Park, S., Shi, A. & Mou, B. Genome-wide identification and expression analysis of the CBF/DREB1 gene family in lettuce. Sci. Rep. 10, 1–14 (2020).
Koike, S. T., Gladders, P. & Paulus, A. O. Vegetable Diseases: A Color Handbook (Gulf Professional Publishing, 2007).
Fertet, A. et al. Sequence of the mitochondrial genome of Lactuca virosa suggests an unexpected role in Lactuca sativa’s evolution. Front. Plant Sci. 12, 697136 (2021).
Simko, I. et al. Identification of QTLs conferring resistance to downy mildew in legacy cultivars of lettuce. Sci. Rep. 3, 1–10 (2013).
Reyes-Chin-Wo, S. et al. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat. Commun. 8, 1–11 (2017).
Thompson, R. C., Whitaker, T. W. & Kosar, W. F. Interspecific genetic relationships in Lactuca. J. Agric. Res. 63, 91–107 (1941).
Gómez, C. & Jiménez, J. Effect of end-of-production high-energy radiation on nutritional quality of indoor-grown red-leaf lettuce. HortScience 55, 1055–1060 (2020).
Resh, H. M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower (CRC Press, 2012).
Zhou, C. et al. Light quality affected the growth and root organic carbon and autotoxin secretions of hydroponic lettuce. Plants 9, 1542 (2020).
Materska, M. et al. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 245, 733–744 (2019).
Nicolle, C. et al. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 23, 605–614 (2004).
Jongen, W. Improving the Safety of Fresh Fruit and Vegetables (Elsevier, 2005).
Rojas, C. M. et al. Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24, 336–352 (2012).
Foyer, C. H., Bloom, A. J., Queval, G. & Noctor, G. Photorespiratory metabolism: Genes, mutants, energetics, and redox signaling. Ann. Rev. Plant Boil. 60, 455–484 (2009).
Florian, A., Araújo, W. & Fernie, A. New insights into photorespiration obtained from metabolomics. Plant Biol. 15, 656–666 (2013).
Wingler, A., Lea, P. J., Quick, W. P. & Leegood, R. C. Photorespiration: Metabolic pathways and their role in stress protection. Philos. Trans. Roy. Soc. Lond. Ser. B Biol. Sci. 355, 1517–1529 (2000).
Reumann, S., Ma, C., Lemke, S. & Babujee, L. AraPerox. A database of putative Arabidopsis proteins from plant peroxisomes. Plant Physiol. 136, 2587–2608 (2004).
Xu, Y.-P., Yang, J. & Cai, X.-Z. Glycolate oxidase gene family in Nicotiana benthamiana: Genome-wide identification and functional analyses in disease resistance. Sci. Rep. 8, 1–11 (2018).
Rojas, C. M. & Mysore, K. S. Glycolate oxidase is an alternative source for H2O2 production during plant defense responses and functions independently from NADPH oxidase. Plant Signal. Behav. 7, 752–755 (2012).
Zhang, Z. et al. Glycolate oxidase isozymes are coordinately controlled by GLO1 and GLO4 in rice. PLoS One 7, e39658 (2012).
Lin, C. C. & Kao, C. H. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 30, 151–155 (2000).
Moran, J. F. et al. Drought induces oxidative stress in pea plants. Planta 194, 346–352 (1994).
Xu, H. et al. Inducible antisense suppression of glycolate oxidase reveals its strong regulation over photosynthesis in rice. J. Exp. Bot. 60, 1799–1809 (2009).
Chern, M., Bai, W., Chen, X., Canlas, P. E. & Ronald, P. C. Reduced expression of glycolate oxidase leads to enhanced disease resistance in rice. PeerJ 1, e28 (2013).
Zelitch, I., Schultes, N. P., Peterson, R. B., Brown, P. & Brutnell, T. P. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol. 149, 195–204 (2009).
Kachroo, A. et al. Induction of H2O2 in transgenic rice leads to cell death and enhanced resistance to both bacterial and fungal pathogens. Transgenic Res. 12, 577–586 (2003).
Yu, L. et al. Glyoxylate rather than ascorbate is an efficient precursor for oxalate biosynthesis in rice. J. Exp. Bot. 61, 1625–1634 (2010).
Foster, J., Kim, H. U., Nakata, P. A. & Browse, J. A previously unknown oxalyl-CoA synthetase is important for oxalate catabolism in Arabidopsis. Plant Cell 24, 1217–1229 (2012).
Sun, G. et al. The dual role of oxalic acid on the resistance of tomato against Botrytis cinerea. World J. Microbiol. Biotechnol. 35, 36 (2019).
Williams, B., Kabbage, M., Kim, H.-J., Britt, R. & Dickman, M. B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 7, e1002107 (2011).
Sinha, S. & Cossins, E. The importance of glyoxylate in amino acid biosynthesis in plants. Biochem. J. 96, 254–261 (1965).
Xu, H.-W. et al. Oxalate accumulation and regulation is independent of glycolate oxidase in rice leaves. J. Exp. Bot. 57, 1899–1908 (2006).
Li, W., Xu, Y.-P., Yang, J., Chen, G.-Y. & Cai, X.-Z. Hydrogen peroxide is indispensable to Xanthomonas oryzae pv. oryzae-induced hypersensitive response and nonhost resistance in Nicotiana benthamiana. Austral. Plant Pathol. 44, 611–617 (2015).
Pastor, V. et al. Fine tuning of reactive oxygen species homeostasis regulates primed immune responses in Arabidopsis. Mol. Plant–Microbe Interact. 26, 1334–1344 (2013).
Scheler, C., Durner, J. & Astier, J. Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 16, 534–539 (2013).
Suzuki, N. et al. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 14, 691–699 (2011).
Skelly, M. J. & Loake, G. J. Synthesis of redox-active molecules and their signaling functions during the expression of plant disease resistance. Antioxidants Redox Signal. 19, 990–997 (2013).
Choi, K. Y., Paek, K. Y. & Lee, Y. B. Effect of air temperature on tipburn incidence of butterhead and leaf lettuce in a plant factory. In Transplant Production in the 21st Century 166–171 (Springer, 2000).
Thompson, H. C., Langhans, R. W., Both, A.-J. & Albright, L. D. Shoot and root temperature effects on lettuce growth in a floating hydroponic system. J. Am. Soc. Horticult. Sci. 123, 361–364 (1998).
Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E. & Mittler, R. Abiotic and biotic stress combinations. New Phytologist 203, 32–43 (2014).
Zhou, S., Chen, F.-C., Nahashon, S. & Chen, T. Cloning and characterization of glycolate oxidase and NADH-dependent hydropyruvate reductase genes in Pachysandra terminalis. HortScience 41, 1226–1230 (2006).
Sunil, B., Saini, D., Bapatla, R. B., Aswani, V. & Raghavendra, A. S. Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth. Res. 139, 67–79 (2019).
Gauslaa, Y. & Solhaug, K. High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria—Interactions of irradiance, exposure duration and high temperature. J. Exp. Bot. 50, 697–705 (1999).
Chen, Z. et al. Inflorescence development and the role of LsFT in regulating bolting in lettuce (Lactuca sativa L.). Front. Plant Sci. 8, 2248 (2018).
Sarkar, U. & Choudhuri, M. Glycolate content, glycolate oxidase and catalase activity in intact sunflower plant during ageing and development. Biochemie und Physiologie der Pflanzen 175, 23–28 (1980).
Hu, W. et al. Accumulation and health risk of heavy metals in a plot-scale vegetable production system in a peri-urban vegetable farm near Nanjing, China. Ecotoxicol. Environ. Saf. 98, 303–309 (2013).
Baldantoni, D., Morra, L., Zaccardelli, M. & Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Saf. 123, 89–94 (2016).
Sun, G., Wang, Y., Wei, X., Xiao, Y., Xu, X., & Tang, Y. Effects of exogenous indole-3-acetic acid on the photosynthesis characteristics of lettuce under cadmium stress. Paper Presented at the E3S Web of Conferences (2019).
Gonzalez, N., Vanhaeren, H. & Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 17, 332–340 (2012).
Zhu, X.-G., Long, S. P. & Ort, D. R. Improving photosynthetic efficiency for greater yield. Ann. Rev. Plant Biol. 61, 235–261 (2010).
Cheng, Y.-L. & Tu, S.-L. Alternative splicing and cross-talk with light signaling. Plant Cell Physiol. 59, 1104–1110 (2018).
Timm, S. & Hagemann, M. Photorespiration—How is it regulated and how does it regulate overall plant metabolism?. J. Exp. Bot. 71, 3955–3965 (2020).
Yang, Z. et al. Systematic identification and analysis of light-responsive circular RNA and co-expression networks in lettuce (Lactuca sativa). G3 Genes Genom. Genet. 10, 2397–2410 (2020).
Boddy, L. Pathogens of autotrophs. In The Fungi 245–292 (Academic Press, 2016).
Mieslerova, B., Lebeda, A., Petrželová, I. & Korbelova, P. Incidence of lettuce downy mildew (Bremia lactucae) and powdery mildew (Golovinomyces cichoracearum) in natural populations of prickly lettuce (Lactuca serriola). Plant Protect. Sci. 49, S24–S32 (2013).
Fan, J. & Doerner, P. Genetic and molecular basis of nonhost disease resistance: Complex, yes; silver bullet, no. Curr. Opinion Plant Biol. 15, 400–406 (2012).
Lebeda, A. & Petrželová, I. Variation and distribution of virulence phenotypes of Bremia lactucae in natural populations of Lactuca serriola. Plant Pathol. 53, 316–324 (2004).
Lebeda, A., Sedlářová, M., Petřivalský, M. & Prokopová, J. Diversity of defence mechanisms in plant–oomycete interactions: A case study of Lactuca spp. and Bremia lactucae. Eur. J. Plant Pathol. 122, 71–89 (2008).
Carbone, F. et al. Identification of miRNAs involved in fruit ripening by deep sequencing of Olea europaea L. transcriptome. PLoS One 14, e0221460. https://doi.org/10.1371/journal.pone.0221460 (2019).
Samad, A. F. A. MicroRNA and transcription factor: Key players in plant regulatory network. Front. Plant Sci. 8, 565 (2017).
Spanudakis, E. The role of microRNAs in the control of flowering time. J. Exp. Bot. 2, 365–380 (2014).
Terzi, L. & Simpson, G. Regulation of flowering time by RNA processing. Nuclear Pre-mRNA Process. Plants 25, 201–218 (2008).
Lu, Y. et al. Suppression of glycolate oxidase causes glyoxylate accumulation that inhibits photosynthesis through deactivating Rubisco in rice. Physiologia Plantarum 150, 463–476 (2014).
Noctor, G., Veljovic-Jovanovic, S., Driscoll, S., Novitskaya, L. & Foyer, C. H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration?. Ann. Bot. 89, 841–850 (2002).
Møller, I. M., Jensen, P. E. & Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481 (2007).
Soldatenko, A. et al. The economy of vegetable growing: The state and the present. Russ. Veg. 5, 63–68 (2018).
Koralewski, T. E. & Krutovsky, K. V. Evolution of exon–intron structure and alternative splicing. PLoS One 6, e18055. https://doi.org/10.1371/journal.pone.0018055 (2011).
Chen, R. & Jeong, S.-S. Functional prediction: Identification of protein orthologs and paralogs. Protein Sci. 9, 2344–2353 (2000).
Tatusov, R. L., Koonin, E. V. & Lipman, D. J. A genomic perspective on protein families. Science 278, 631–637 (1997).
Zhang, Y., Gao, P. & Yuan, J. S. Plant protein-protein interaction network and interactome. Curr. Genom. 11, 40–46 (2010).
Engqvist, M. K., Eßer, C., Maier, A., Lercher, M. J. & Maurino, V. G. Mitochondrial 2-hydroxyglutarate metabolism. Mitochondrion 19, 275–281 (2014).
Engqvist, M. K. et al. GLYCOLATE OXIDASE3, a glycolate oxidase homolog of yeast L-lactate cytochrome c oxidoreductase, supports L-lactate oxidation in roots of Arabidopsis. Plant Physiol. 169, 1042–1061 (2015).
Bondarenko, V. S. & Gelfand, M. S. Evolution of the exon–intron structure in ciliate genomes. PLoS One 11, e0161476. https://doi.org/10.1371/journal.pone.0161476 (2016).
Panchy, N., Lehti-Shiu, M. & Shiu, S.-H. Evolution of gene duplication in plants. Plant Physiol. 171, 2294–2316 (2016).
Moore, R. C. & Purugganan, M. D. The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Boil. 8, 122–128 (2005).
Taylor, J. S. & Raes, J. Duplication and divergence: The evolution of new genes and old ideas. Annu. Rev. Genet. 38, 615–643 (2004).
Xie, T. et al. Genome-wide analysis of the lateral organ boundaries domain gene family in Brassica napus. Genes 11, 280 (2020).
Hurst, L. D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. TRENDS Genet. 9, 486–487 (2002).
Yang, Z. & Bielawski, J. P. Statistical methods for detecting molecular adaptation. Trends Ecol. Evolut. 15, 496–503 (2000).
Morgan, C. C., Loughran, N. B., Walsh, T. A., Harrison, A. J. & O’Connell, M. J. Positive selection neighboring functionally essential sites and disease-implicated regions of mammalian reproductive proteins. BMC Evolut. Boil. 10, 39 (2010).
Zhang, Y., Gao, P. & Yuan, J. S. Plant protein–protein interaction network and interactome. Curr. Genom. 11, 40–46 (2010).
McMillan, D. G. et al. Protein–protein interaction regulates the direction of catalysis and electron transfer in a redox enzyme complex. J. Am. Chem. Soc. 135, 10550–10556 (2013).
Roberts, M. R. Does GABA act as a signal in plants? Hints from molecular studies: Hints from molecular studies. Plant Signal. Behav. 2, 408–409 (2007).
Maurino, V. G., & Engqvist, M. K. 2-Hydroxy acids in plant metabolism. The Arabidopsis book/American Society of Plant Biologists 13 (2015).
Tolbert, N., Oeser, A., Kisaki, T., Hageman, R. & Yamazaki, R. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J. Biol. Chem. 243, 5179–5184 (1968).
Vishwakarma, A., Tetali, S. D., Selinski, J., Scheibe, R. & Padmasree, K. Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway in Arabidopsis thaliana. Ann. Bot. 116, 555–569 (2015).
Arnao, M. B. & Hernández-Ruiz, J. Melatonin: plant growth regulator and/or biostimulator during stress?. Trends Plant Sci. 19, 789–797 (2014).
Hasan, M. K. et al. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 6, 601 (2015).
Moya-Garzon, M. D. et al. New salicylic acid derivatives, double inhibitors of glycolate oxidase and lactate dehydrogenase, as effective agents decreasing oxalate production. Eur. J. Med. Chem. 237, 114396 (2022).
Cohen, Y., Rubin, A. E. & Kilfin, G. Mechanisms of induced resistance in lettuce against Bremia lactucae by DL-β-amino-butyric acid (BABA). Eur. J. Plant Pathol. 126, 553–573 (2010).
Xia, K., Pan, X., Chen, H., Xu, X. & Zhang, M. Rice miR168a-5p regulates seed length, nitrogen allocation and salt tolerance by targeting OsOFP3, OsNPF2. 4 and OsAGO1a, respectively. J. Plant Physiol. 280, 153905 (2023).
Zhou, J. et al. CRISPR-Cas9 mediated OsMIR168a knockout reveals its pleiotropy in rice. Plant Biotechnol. J. 20, 310–322 (2022).
Vaucheret, H. AGO1 homeostasis involves differential production of 21-nt and 22-nt miR168 species by MIR168a and MIR168b. PLoS One 4, e6442 (2009).
Rhoades, M. W. et al. Prediction of plant microRNA targets. Cell 110, 513–520 (2002).
Xie, Z., Kasschau, K. D. & Carrington, J. C. Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr. Biol. 13, 784–789 (2003).
Xie, Z. et al. Expression of Arabidopsis MIRNA genes. Plant Physiol. 138, 2145–2154 (2005).
Vaucheret, H., Mallory, A. C. & Bartel, D. P. AGO1 homeostasis entails coexpression of MIR168 and AGO1 and preferential stabilization of miR168 by AGO1. Mol. Cell 22, 129–136 (2006).
Schmid, M. et al. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37, 501–506 (2005).
Winter, D. et al. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2, e718 (2007).
Pick, T. R. et al. PLGG1, a plastidic glycolate glycerate transporter, is required for photorespiration and defines a unique class of metabolite transporters. Proc. Natl. Acad. Sci. 110, 3185–3190 (2013).
Cai, X. et al. Expression analysis of oxalate metabolic pathway genes reveals oxalate regulation patterns in spinach. Molecules 23, 1286 (2018).
Collins, K. et al. SpinachBase: A central portal for spinach genomics. Database 2019, 72 (2019).
Goodstein, D. et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186 (2012).
Goodstein, D., et al. Phytozome Comparative Plant Genomics Portal (2014).
Marchler-Bauer, A. et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 43, D222–D226 (2015).
Lu, S. et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 48, D265–D268. https://doi.org/10.1093/nar/gkz991 (2020).
Gasteiger, E., et al. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook 571–607. Springer (2005).
Bernhofer, M. et al. Nlsdb—Major update for database of nuclear localization signals and nuclear export signals. Nucleic Acids Res. 46, 503–508 (2018).
Nair, R., Carter, P. & Rost, B. NLSdb: Database of nuclear localization signals. Nucleic Acids Res. 31, 397–399 (2003).
Cokol, M., Nair, R. & Rost, B. Finding nuclear localization signals. EMBO Rep. 1, 411–415 (2000).
Horton, P., Park, K.-J., Obayashi, T., & Nakai, K. Protein subcellular localization prediction with WoLF PSORT. Paper presented at the Proceedings of the 4th Asia-Pacific Bioinformatics Conference (2006).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Thompson, J. D., Gibson, T. J. & Higgins, D. G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protocol. Bioinform. 1, 2–3 (2003).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Boil. Evolut. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Boil. Evolut. 38, 3022–3027 (2021).
Guo, A.-Y., Zhu, Q.-H., Chen, X. & Luo, J.-C. GSDS: A gene structure display server. Yi Chuan Hereditas 29, 1023–1026 (2007).
Hu, B. et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 31, 1296–1297. https://doi.org/10.1093/bioinformatics/btu817 (2015).
Bailey, T. L. et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009).
Bailey, T. L., Johnson, J., Grant, C. E. & Noble, W. S. The MEME suite. Nucleic Acids Res. 43, W39–W49 (2015).
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202 (2020).
Brown, G. R. et al. Gene: a gene-centered information resource at NCBI. Nucleic Acids Res. 43, D36–D42 (2015).
Johnson, M. et al. NCBI BLAST: A better web interface. Nucleic Acids Res. 36, W5–W9 (2008).
Wang, Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49–e49 (2012).
Wang, Y., Li, J. & Paterson, A. H. MCScanX-transposed: Detecting transposed gene duplications based on multiple colinearity scans. Bioinformatics 29, 1458–1460. https://doi.org/10.1093/bioinformatics/btt150 (2013).
Wittkopp, P. J. & Kalay, G. Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 13, 59–69 (2012).
Biłas, R., Szafran, K., Hnatuszko-Konka, K. & Kononowicz, A. K. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. 127, 269–287 (2016).
Bülow, L., & Hehl, R. Bioinformatic identification of conserved cis-sequences in coregulated genes. In Plant Synthetic Promoters 233–245. Springer (2016).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327 (2002).
Chen, C., Chen, H., He, Y., & Xia, R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 289660 (2018).
Heng, H., Guoqiang, H., Jin, S., Fengli, Z. & Dabing, Z. Bioinformatics analysis for piezo in rice. Reprod. Breed. 1, 108–113 (2021).
Tong, M. et al. Identification and functional analysis of the CorA/MGT/MRS2-type magnesium transporter in banana. PLoS One 15, e0239058 (2020).
Szklarczyk, D. et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 (2010).
Szklarczyk, D. et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Yu, X. et al. Comparative analysis of Italian Lettuce (Lactuca sativa L. var. ramose) transcriptome profiles reveals the molecular mechanism on exogenous melatonin preventing cadmium toxicity. Genes 13, 955 (2022).
Luo, C. et al. LsAP2 regulates leaf morphology by inhibiting CIN-like TCP transcription factors and repressing LsKAN2 in lettuce. Horticult. Res. 8, 24 (2021).
Dai, X., Zhuang, Z. & Zhao, P. X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 46, W49–W54 (2018).
Guo, Z. et al. PmiREN: A comprehensive encyclopedia of plant miRNAs. Nucleic Acids Res. 48, D1114–D1121 (2020).
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project Number (RSP2023R165), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
M. conducted research under the supervision of M.S. and wrote the initial draft of the manuscript. S.S., Q.A., M.S.H., U.H., D.A., and M.A.S. provided resources for research work and made final editing in the manuscript. All authors reviewed and approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mariyam, Shafiq, M., Sadiq, S. et al. Identification and characterization of Glycolate oxidase gene family in garden lettuce (Lactuca sativa cv. ‘Salinas’) and its response under various biotic, abiotic, and developmental stresses. Sci Rep 13, 19686 (2023). https://doi.org/10.1038/s41598-023-47180-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47180-y
- Springer Nature Limited