Abstract
Psoriasis is a chronic inflammatory condition associated with genetic and immunological susceptibility. The objective of the study was to evaluate pruritus and sleep quality in correlation (r) to psoriasis severity and to detect their impact on quality of life. Two hundred (200) patients with psoriasis were included. Psoriasis severity was determined using the psoriasis area severity index (PASI), the quality of life (QoL) was assessed by the psoriasis disability index (PDI) questionnaire, and the sleep quality was evaluated by the Pittsburgh sleep quality index (PSQI). Finally, the severity of itching was evaluated using a 12-item pruritus severity scale (PSS). Poor sleep quality was found in 16.0% of patients in this study. Poor sleep was detected among 50.0% of cases with severe psoriasis. PASI scores correlated significantly with sleep quality, duration and sleep disturbances (p < 0.001). The global PSQI and PASI were also significantly correlated (p = 0.004). In conclusion patients complaining of psoriasis exacerbated by pruritus and sleep problems demonstrated lower quality of life in all domains. Sleep disturbances and depressive symptoms impairing quality of life should be taken into consideration when screening patients suffering from psoriasis.
Similar content being viewed by others
Introduction
Psoriasis is a chronic inflammatory skin disease with genetic and immunological background and is exacerbated and triggered by environmental factors1. Sleep disturbances (SDs) are experienced by psoriatic patients and affect their quality of life. A close relationship exists between psoriasis and SDs, the pathophysiological mechanisms of which are not fully understood.
Psoriasis is characterized by activation of T-helper cells (Th-1), antigen presenting cells (APCs) and by recruitment of Th-1 cytokines characterized by expansion and activation of T-helper cells (Th-1), antigen presenting cells, and Th-1 cytokines and interleukins. It had been revealed that increased serum levels of inflammatory cytokines such as tumor necrosis factor alpha and interleukin 6 are responsible for sleep restriction in psoriasis2,3. Moreover; higher concentration of substance P discovered in perilesional skin of psoriatic patients had been related to disturbed sleep cycles and their regulation4. In addition to the above, the lower melatonin levels expressed in serum of psoriatic patients can directly influence sleep habits and potentially be responsible for increased fatigue5.
Pruritus is known to negatively impact sleep in patients with other dermatological conditions including chronic urticaria, chronic pruritus (CP) and atopic dermatitis (AD). Pruritus severity in patients with psoriasis is lower than that in AD or CP but it often intensifies in the evening, thereby potentially interfering with sleep6.
Pruritus defined as an unpleasant sensation that provokes a desire to scratch. The molecular basis of pruritus in psoriasis is still not fully elucidated, albeit a complex interaction between the nervous, neuroendocrine, immune, and vascular systems is suggested. Many mediators were indicated to modulate this sensation in psoriasis, but none has been proven to be a crucial one to date6.
Contradictory findings exist regarding the association of pruritus intensity with sleep disturbance among psoriatic patients. The coexisting relation of SD’s among psoriatic patients is multifactorial and its exact pathophysiologic mechanism remains to be fully elucidated. The purpose of this study was to evaluate pruritus and sleep quality in correlation (r) to psoriasis severity and to detect their impact on quality of life.
Patients and methods
This study was a cross sectional study that included 200 patients diagnosed clinically with psoriasis that were recruited from the outpatient clinics. Twenty three subjects were excluded earlier due to being on topical or systemic medications at the time of data collection. Following the study’s approval by the local ethics committee at the Faculty of Medicine at Al-Azhar University in Damietta (IRB 00012367-21-03-003), all cases included in the study provided their verbal or written informed consent.
Patients were included if they had established psoriasis of more than 6 months and were able to read and write for completing the questionnaire. Exclusion criteria included pregnant or lactating females, other concomitant dermatological or systemic disorders that might cause pruritus such as chronic uremia or cholestasis, and usage of medications that could influence the sensation of itch. Moreover Subjects with cognitive impairment, present psychiatric disorders, active depression, substance use, and smoking as well as those pustular psoriasis and cases with concomitant psoriatic arthritis (PsA) were also excluded.
Every patient was subjected to:
-
(I)
Questioning about:
-
(1)
Age.
-
(2)
Gender.
-
(3)
Smoking and substance use.
-
(4)
Any coexistent chronic diseases (diabetes mellitus, hypertension, heart disease, chronic kidney disease, chronic liver disease, thyroid disease, stroke).
-
(5)
Drugs such as (Opioids, Hypnotics and sedatives, thyroid hormones).
-
(6)
Disease duration.
-
(1)
-
(II)
Dermatological examination:
-
(a)
To exclude dermatological diseases other than psoriasis.
-
(b)
Assessment of psoriasis according to clinical types.
-
(a)
-
(III)
Psoriasis grading with Psoriasis Area Severity Index (PASI) PASI score was used for severity and extent assessment of psoriasis. It ranges from zero (no disease) to 72 (severe disease) (maximal disease severity. Scores below 10 mark mild psoriasis and scores above 20 are regarded as severe while scores falling between 10 and 20 are regarded as moderate psoriasis (6).
-
(IV)
Evaluation of Disease-related Quality of Life of the subjects using Arabic version of Psoriasis Disability Index (PDI) The effect of psoriasis on daily activities, work or school, relationships, leisure, and treatment were assessed using the psoriasis disability index questionnaire (PDI)7. The reliability of the Arabic version of the DPI was validated and was found to be reliable for evaluating the QOL for Egyptian patients with psoriasis and was used in the current study8.
The PDI is made up of 15 simple, disease-related questions. Each question is scored on a scale of 0 to 4, with a maximum score of 45. The lower the score, the lower is the quality of life. The PDI can also be reported as a percentage of a maximum possible score of 45. The overall score indicates how psoriasis has affected the patient over the last four weeks.
-
(V)
Evaluation of sleep quality using arabic translation of Pittsburgh sleep quality index (PSQI) Sleep quality was assessed using the Arabic validated version9 of the PSQI10. Seven components are used; subjective sleep quality, latency of sleep, duration of sleeping, habitual sleep efficiency, disturbances of sleep, whether or not sleep medications are used, and dysfunction of the following day over the last month. Each component is scored from 0 to 3, resulting in a global PSQI score between 0 and 21, with higher scores indicating lower quality of sleep. The PSQI score ≥ 5 was considered as a cut-off for poor sleep quality.
-
(VI)
Assessment of pruritus severity using 12-item pruritus severity scale (PSS) The 12-PSS is a one page assessment tool assessing different aspects of pruritus. Five domains namely: pruritus intensity, pruritus extent, frequency and duration of pruritus, impact of pruritus on daily activities and mood, and assessment of scratching. Participants answered 12 questions referring to their pruritus and the total scoring ranged from 3 (the lowest pruritus intensity) to 22 points (the highest pruritus intensity). According to scores pruritus is classified as mild pruritus (3–6 points) moderate pruritus (7–11 points) or severe pruritus (12–22 points)11.
Sample size calculation
Sample size calculation was based on 90% bad sleep quality among cases with psoriasis retrieved from a previous study by Saçmacı and Gurel12. With a 95.0% Confidence interval and an acceptable margin error of 5.0%, the calculated sample size in the study was determined to be at least 138 participants. Taking into consideration expected dropouts of 20.0%, an approximate sum of 166 participants was determined to be sufficient.
Statistical analysis
The data was anonymized and fed to personal computer software statistical package of social sciences (SPSS), version 16 (SPSS Inc., Chicago, USA). Data had been presented by their mean, median, standard deviation, relative frequency and percentages according to its type and normality of distribution. Groups compared by independent samples “t” test, Chi square test or any equivalent according to type of data. Pearson’s correlation (r) coefficient was calculated to address possible correlation (r) between variables and p value < 0.05 was set as statistically significant.
Study approval statement
This study protocol was reviewed and approved by ethics committee on human research by Al Azhar Damietta faculty of medicine (No. 00012367-21-03-003). All methods were performed in accordance with the relevant guidelines and regulations.
Consent to participate statement
Written informed consents were received from participants upon explanation of the study. Consent for publication were obtained from the participants for publishing the images in the manuscript.
Results
The mean age of the studied cases was 38.9 ± 17.84 years ranging from 6 to 75 years among which half of them were females. One hundred and fifty six cases (78.0%) were diagnosed as plaque type psoriasis, 32 cases (16.0%) suffered from palmo plantar psoriasis and only 12 (6.0%) of cases had scalp psoriasis. The median disease duration was 7.3 ± 6.2 years and ranged from 1 to 30 years.
The median PASI score was 5.6 and ranged from 0.5 to 22.8. Of the studied cases; 76% had mild PASI, 20.0% demonstrated moderate PASI and only 4.0% presented with severe PASI. The pruritus severity scale ranged from 3 to 20 with a median score of 9; with 34.0% of cases presenting with mild pruritus, 32.0% with moderate pruritus and 34.0% with severe pruritus. Psoriasis disability index ranged from 0 to 26 with a median score of 5. Poor sleep quality was found in 16.0% of patients in this study (Tables 1, 2).
Among all sleep quality domains, a statistically significant relation was established between sleep efficiency and the type of psoriasis (p = 0.036). A sleep efficiency score of 0 was detected among 56.2% of cases with palmoplantar psoriasis versus 78.2% of cases with plaque type psoriasis and 83.3% of cases with scalp psoriasis. Scores of 2 and 3 among the efficiency domain was only detectable among cases with plaque type psoriasis when compared to subjects with palmpolantar psoriasis or scalp psoriasis. Sleeping efficiency was significantly lower among subjects with plaque type psoriasis when compared to the other two groups (p = 0.036). The median disease duration among cases with poor sleep was higher than cases with good sleep (10 years vs. 6.5 years; p = 0.039). Longer disease duration was associated with significantly lower quality of sleep in all domains (Tables 3, 4).
Poor sleep was detected among 50.0% of cases with severe psoriasis, 25.0% with moderate psoriasis and 11.8% subjects complaining of mild psoriasis. The scores of subjective sleep quality, sleep duration, habitual sleep efficiency, sleep disturbance and daytime dysfunction had significant correlation (r)s with PASI (p < 0.001). The global PSQI and PASI were also significantly correlated (p = 0.004). Cases with severe pruritus were associated with higher incidence of sleep disturbance in all domains (p < 0.001). The global PSQI and pruritus severity were also significantly correlated (p < 0.001). A median higher psoriasis disability index percent was associated with poor sleep than cases with good sleep (20.5% vs. 4.0%). The lower global PSQI was significantly associated with more psoriasis disability (p < 0.001) (Tables 5, 6, 7, 8).
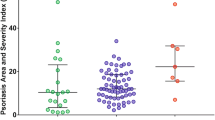
PSS and PDI were significant predictors of poor sleep quality (r = 0.687; r = 0.571; p < 0.001 respectively). According to linear regression analysis; disease duration, PSS and PDI were significant predictors of global sleep score with 64.8% of the score predicted by the 3 variables and the calculated prediction equation (Global score = − 10.09 + 0.067 × disease duration + 0.242 × PSS + 0.150 × PDI) (Tables 9, 10, Fig. 1).
Discussion
The current study showed that 16.0% of psoriatic subjects included reported poor sleep quality. Moreover; we determined a significant impact of psoriasis severity and pruritus on the disability index and quality of sleep.
Different studies investigated the reciprocal effect of sleep impairment and its relation to psoriasis or its severity using different assessment tools such as the PSQI, the Functional Outcomes of Sleep Questionnaire (FOSQ), the General Sleep Disturbance Scale (GSDC) and the general health questionnaire (GHQ-H)12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 (Table 11).
Sleep disturbances (SDs) were found to be higher among patients with psoriatic arthritis (45.1%) when compared to patients with psoriasis (16.0%)29. The current study confirmed previous reports demonstrating that psoriasis severity is associated with increased risk of having sleep disturbances (SDs)20,24,36. Melikoglu found out that in 48 recruited patients of psoriasis PSQI and SDs significantly correlated with PASI scores (p = 0.03)24.
Samaci et al. and Khalaf et al. recruited 60 and 100 patients complaining of psoriasis with a mean PASI score of (10.1 ± 9.7) and (4.97 ± 5.24) respectively. On the contrary to our results, both authors did not find any correlation (r) between either PASI severity or psoriasis duration and the degree of sleep affection12,35. The small percentage of poor sleep quality in the current research could be explained by the fact that mild disease was reported in the majority of our patients. Nevertheless; we detected a significant (p-value = 0.008) positive correlation (r) (r = 0.37) between sleep disturbances and PASI severity. Jaworecka et al. demonstrated that 39.3% (n = 116) of patients reported occasional difficulties in falling asleep, and 22.7% of them (n = 67) had such problems almost every day. Moreover, 20.3% (n = 60) woke up during sleep almost every night, and a further 33.6% (n = 99) reported such problem sporadically33. A study by Nowowiejska et al. reported poor sleep quality, was noticed in 47 patients (78.3%), 24 patients (40.0%) subjectively assessed their sleep quality as fairly bad or bad32.
Pruritus is characterized by uncomfortable burning sensation, numbness or even pain. Psoriatic patients with prutiris experienced less sleep quality and more sleep disturbances and daytime dysfunction than those patients without pruritus. Pruritus intensity correlated with PASI severity and cases with severe pruritus were associated with higher incidence of sleep disturbance in all domains (p < 0.001).
Studies determined that psoriatic patients complaining of pruritus show difficulty in falling asleep and have an increased frequency of sleep fragmentation and nocturnal awakening16,37. Meanwhile it was established that good sleep significantly reduces the intensity of itching related to psoriasis33,38,39.
In the current study patients with higher psoriasis disability index (PDI) had significantly diminished components of all sleep components and daytime dysfunction than those patients with lower score of PDI (p < 0.001). We also observed that total PDI was significantly higher among those subjects with moderate to severe psoriasis when compared to those with mild psoriasis (9.5 vs. 4.0, p < 0.001). Severity of psoriasis had been linked to impaired Quality of life (QoL) in a number of studies22,40.
Among the limitations of the current study are the cross-sectional design and the absence of a healthy control group. Uneven distribution of patients with varying severity of skin lesions represents another limitation as the majority of cases were of mild PASI score. In addition, sleep quality was assessed by means of a questionnaire while a polysomnographic evaluation could have yielded more accurate results.
In conclusion, we demonstrated that psoriatic patients with pruritus and sleep problems had a worse overall quality of life in all domains. Sleep impairment and screening of any depressive and impaired quality of life symptoms shall be considered when treating psoriatic patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- PASI:
-
Psoriasis area severity index
- QoL:
-
Quality of life
- PDI:
-
Psoriasis disability index
- PSQI:
-
Pittsburgh sleep quality index
- PSS:
-
Pruritus severity scale
- Th-1):
-
T-helper cells
- APCs:
-
Antigen presenting cells
References
Aly, D. G., Abdallah, I. Y., Hanafy, N. S., Elsaie, M. L. & Hafiz, N. A. Elevated serum leptin levels in nonobese patients with psoriasis. J. Drugs Dermatol. 12(2), e25–e29 (2013).
Szepietowski, J. C., Reich, A. & Wisnicka, B. Pruritus and psoriasis. Br. J. Dermatol. 151, 1284 (2004).
Halioua, B., Chelli, C., Misery, L., Taieb, J. & Taieb, C. Sleep disorders and psoriasis: An update. Acta Dermatol. Venereol. 102, 00699. https://doi.org/10.2340/actadv.v102.1991 (2022).
Ursavas, A. Upregulating substance P levels to treat obstructive sleep apnea. Expert Opin. Ther. Targets 12, 583–588 (2008).
Bolotna, L. & Sarian, O. Psychopathological disorders as comorbidity in patients with psoriasis (review). Georgian Med. News 301, 143–147 (2020).
Jaworecka, K. et al. Characteristics of pruritus in various clinical variants of psoriasis: Results of the multinational, multicenter, cross-sectional study. Life 11(7), 623 (2021).
Lewis, V. J. & Finlay, A. Y. Two decades experience of the psoriasis disability index. Dermatology 210, 261–268 (2005).
Zedan, H. M., Gaber, H. D., Ibrahim, A. K. & Refaa, E. Z. Reliability and validity of the Arabic version of the psoriasis disability index questionnaire. J. Egypt Womenʼs Dermatol. Soc. 13(3), 143–150 (2016).
Suleiman, K., Hadid, L. A. & Duhni, A. Psychometric testing of the Arabic version of the Pittsburgh sleep quality index (A-PSQI) among coronary artery disease patients in Jordan. J. Nat. Sci. Res. 2(8), 15–20 (2012).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Reich, A., Bozek, A., Janiszewska, K. & Szepietowski, J. C. 12-Item pruritus severity scale: Development and validation of new itch severity questionnaire. Biomed. Res. Int. 2017, 3896423 (2017).
Sacmaci, H. & Gurel, G. Sleep disorders in patients with psoriasis: A cross-sectional study using non-polysomnographical methods. Sleep Breath 23(3), 893–898 (2019).
Sharma, N., Koranne, R. V. & Singh, R. K. Psychiatric morbidity in psoriasis and vitiligo: A comparative study. J. Dermatol. 28, 419–423 (2001).
Delfino, M. Jr., Holt, E. W., Taylor, C. R., Wittenberg, E. & Qureshi, A. A. Willingness-to-pay stated preferences for 8 health-related quality-of-life domains in psoriasis: A pilot study. J. Am. Acad. Dermatol. 59, 439–447 (2008).
Stiens, G. et al. Psychosocial distress in psoriatic outpatients: P50. Br. J. Dermatol. 159, 1419–1420 (2008).
Callis Duffin, K., Wong, B., Horn, E. J. & Krueger, G. G. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J. Am. Acad. Dermatol. 60(4), 604–608 (2009).
Hu, S. W., Holt, E. W., Husni, M. E. & Qureshi, A. A. Willingness-to-pay stated preferences for 8 health-related quality-of-life domains in psoriatic arthritis: A pilot study. Semin. Arthritis Rheum. 39, 384–439 (2010).
Tsai, T. F. et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 63(1), 40–46 (2011).
Ljosaa, T. M., Mork, C., Stubhaug, A., Moum, T. & Wahl, A. K. Skin pain and skin discomfort is associated with quality of life in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 26, 29–35 (2012).
Shutty, B. G. et al. Sleep disturbances in psoriasis. Dermatol. Online J. 19, 1 (2013).
Nyunt, W. W., Low, W. Y., Ismail, R., Sockalingam, S. & Min, A. K. Determinants of health-related quality of life in psoriasis patients in Malaysia. Asia Pac. J. Public Health 27, 662–667 (2015).
Sanchez-Carazo, J. L., López-Estebaranz, J. L. & Guisado, C. Comorbidities and health-related quality of life in Spanish patients with moderate to severe psoriasis: A cross-sectional study (Arizona study). J. Dermatol. 41, 673–678 (2014).
Chiu, H. Y. et al. Concomitant sleep disorders significantly increase the risk of cardiovascular disease in patients with psoriasis. PLoS ONE 11, e0146462 (2016).
Melikoglu, M. Sleep quality and its association with disease severity in psoriasis. Eurasian J. Med. 49, 124–127 (2017).
Henry, A. L., Kyle, S. D., Chisholm, A., Griffiths, C. E. M. & Bundy, C. A cross-sectional survey of the nature and correlates of sleep disturbance in people with psoriasis. Br. J. Dermatol. 177, 1052–1059 (2017).
Wong, I. T. Y., Chandran, V., Li, S. & Gladman, D. D. Sleep disturbance in psoriatic disease: Prevalence and associated factors. J. Rheumatol. 44, 1369–1374 (2017).
Jensen, P., Zachariae, C., Skov, L. & Zachariae, R. Sleep disturbance in psoriasis: A case-controlled study. Br. J. Dermatol. 179, 1376–1384 (2018).
Krajewska-Włodarczyk, M., Owczarczyk-Saczonek, A. & Placek, W. Sleep disorders in patients with psoriatic arthritis and psoriasis. Reumatologia 56(5), 301–306 (2018).
Duvetorp, A. et al. Quality of life and contact with healthcare systems among patients with psoriasis and psoriatic arthritis: Results from the nordic patient survey of psoriasis and psoriatic arthritis (NORPAPP). Arch. Dermatol. Res. 311(5), 351–360 (2019).
Hawro, T. et al. Pruritus and sleep disturbances in patients with psoriasis. Arch. Dermatol. Res. 312(2), 103–111 (2020).
Luca, M., Musumeci, M. L., D’Agata, E. & Micali, G. Depression and sleep quality in psoriatic patients: Impact of psoriasis severity. Int. J. Psychiatry Clin. Pract. 24(1), 102–104 (2020).
Nowowiejska, J. et al. The assessment of risk and predictors of sleep disorders in patients with psoriasis—A questionnaire-based cross-sectional analysis. J. Clin. Med. 10(4), 664 (2021).
Jaworecka, K. et al. The impact of pruritus on the quality of life and sleep disturbances in patients suffering from different clinical variants of psoriasis. J. Clin. Med. 11(19), 5553 (2022).
Sahin, E. et al. Prevalence and factors associated with sleep disturbance in adult patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 36(5), 688–697 (2022).
Khalaf, O. O., El-Komy, M. M., Kattaria, D. B. & El-Mesidy, M. S. Sleep quality among psoriasis patients: Excluding the immunosuppressive therapy effect. Middle East Curr. Psychiatry 30, 5. https://doi.org/10.1186/s43045-023-00305-5 (2023).
Kaaz, K., Szepietowski, J. C. & Matusiak, Ł. Sleep quality among adult patients with chronic dermatoses. Postepy Dermatol. Alergol. 36, 659–666 (2019).
Gowda, S., Goldblum, O. M., McCall, W. V. & Feldman, S. R. Factors affecting sleep quality in patients with psoriasis. J. Am. Acad. Dermatol. 63, 114–123 (2010).
Yosipovitch, G., Goon, A., Wee, J., Chan, Y. H. & Goh, C. L. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br. J. Dermatol. 143, 969–973 (2000).
Amatya, B., Wennersten, G. & Nordlind, K. Patients’ perspective of pruritus in chronic plaque psoriasis: A questionnaire based study. J. Eur. Acad. Dermatol. Venereol. 22, 822–826 (2008).
Dauden, E. et al. Demographic characteristics and health-related quality of life of patients with moderate-to-severe psoriasis: The VACAP study. Actas Dermosifiliogr. 104(9), 80 (2013).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally in production of this work. M.Z., E.E., A.A., D.M. and M.E., designed and performed the research. M.Z., E.E., A.A., D.M. and M.E. performed the work. M.Z., E.E., A.A., D.M. and M.E. analyzed and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaky, M.S., Elgamal, E.E.A., Abd Al Maksoud, A.A. et al. Evaluation of sleep quality and pruritus severity in psoriatic patients and their impact on quality of life: a cross section correlational study. Sci Rep 13, 17541 (2023). https://doi.org/10.1038/s41598-023-44757-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44757-5
- Springer Nature Limited