Abstract
Ho-doped NdFeO3 was synthesized using the citrate method. The X-ray diffraction (XRD) illustrated that Nd0.90Ho0.10FeO3 was crystalline at the nanoscale, with a crystallite size of 39.136 nm. The field emission scanning electron microscope (FESEM) illustrated the porous nature of Nd0.90Ho0.10FeO3, which increases the active sites to absorb the heavy metals on the sample surface. Energy-dispersive X-ray (EDX) data assures the prepared sample has the chemical formula Nd0.90Ho0.10FeO3. The magnetic properties of Nd0.90Ho0.10FeO3 were determined using the magnetization hysteresis loop and Faraday’s method. Many magnetic parameters of the sample have been discussed, such as the coercive field, the exchange bias (Hex), and the switching field distribution (SFD). Ho-doped NdFeO3 has an antiferromagnetic (AFM) character with an effective magnetic moment of 3.903 B.M. The UV–visible light absorbance of Nd0.90Ho0.10FeO3 is due to the transfer of electrons from the oxygen 2p state to the iron 3d state. Nd0.90Ho0.10FeO3 nanoparticles have an optical direct transition with an energy gap Eg = 1.106 eV. Ho-doped NdFeO3 can adsorb many heavy metals (Co2+, Ni2+, Pb2+, Cr6+, and Cd2+) from water. The removal efficiency is high for Pb2+ ions, which equals 72.39%. The Langmuir isotherm mode is the best-fit model for adsorbing the Pb2+ ions from water.
Similar content being viewed by others
Introduction
The orthoferrites are promising materials in many applications due to their chemical stability, magnetic, multiferroic, optical, and dielectric properties1. The general formula of orthoferrites is ABO3, where A is the rare earth elements, i.e., La3+, Nd3+, Sm3+, and Gd3+; B is the transition metal, i.e., Fe3+; and O2- is the oxygen ion. Multiferroic materials such as BiFeO3, NdFeO3, SmFeO3, and LaFeO3 have ferroelectric and magnetic orders2,3,4,5. The applications of ABO3 are spintronics, data storage media, high-frequency devices, water purification, and photocatalysis6,7,8,9,10. The orthoferrites are characterized by low cost, chemical stability, easy fabrication, and many applications11.
NdFeO3 belongs to the ABO3 orthoferrite materials with the space group Pbnm. The Fe3+ ions form the FeO6 octahedron. The magnetic properties of NdFeO3 originate from the Dzyaloshinskii–Moriya exchange interaction of the antiparallel spins between Fe3+–O2-–Fe3+, Nd3+–O2-–Fe3+, and Nd3+–O2-–Nd3+1,12. The optical properties of NdFeO3 originate from the transition of electrons from 2p to 3d orbitals13,14. P. T. H. Duyen et al.15 studied the optical properties of Cd-doped NdFeO3, concluding that increasing the Cd concentration decreased the optical band gap. S. A. Mir et al.16 prepared the Ni-doped NdFeO3 and studied the dielectric properties of the samples.
Numerous photocatalysts, including CuS, TiO2, CuO, BaTiO3, ZnO, and others, are used today in dye degradation17. Their large bandgap energy, quick recombination of photoinduced charge carriers, and low visible light absorption, however, severely limit their practical applicability18. Therefore, it is essential to create photocatalysts with exceptional photocatalytic activity in the visible region and a small bandgap. Perovskite-oxide-based catalysts such as NdFeO3 have lately piqued the interest of researchers due to their good photocatalytic activity, tunable bandgap, high stability, and quick photoinduced electron/hole mobility19,20,21.
The metallic elements that are characterized by their high atomic weight, specific gravity, and toxicity are called heavy metals (HMs), such as lead (Pb2+), chromium (Cr6+), nickel (Ni2+), cadmium (Cd2+), and copper (Co2+). Cadmium, a heavy metal, damages the bones and kidneys22. Cr6+ causes hemorrhage, severe diarrhea, and cancer in the digestive tract23. Increasing the concentration of lead in drinking water causes kidney malfunction, brain tissue damage, and anemia24. There are many techniques used to remove heavy metals from water, such as the precipitation method25, flotation26, and membrane technologies27. The most effective method for removing HMs from water is the adsorption technique, which is characterized by its simplicity and no slugs.
The present paper describes the preparation of the Ho-doped NdFeO3 nanoparticles for the first time using a simple and inexpensive citrate combustion method. The sample was characterized by FESEM, EDX, and elementary mapping. The optical and magnetic properties of Nd0.90Ho0.10FeO3 were studied in detail. The removal of HMs (Co2+, Ni2+, Pb2+, Cr6+, and Cd2+) from water was studied. The Langmuir and Freundlich isotherm models were used to study the adsorption of Pb2+ from water on Nd0.90Ho0.10FeO3.
Experimental work
Materials
Neodymium nitrate, holmium nitrate, and iron nitrate were purchased from Sigma-Aldrich with a purity of 99.9%.
Preparation of the Nd0.90Ho0.10FeO3 sample
The citrate combustion method is characterized by controlling the metal stoichiometry, high purity, low cost, crystallinity, effectiveness, and high yield. Figure 1 shows the flowchart of the synthesis of Nd0.90Ho0.10FeO3 using the citrate combustion method. The (0.9 M) Nd nitrate, (0.1 M) Ho nitrates, (1 M) Fe nitrates, and (2 M) citric acid were dissolved in distilled water. The ammonia solution was used to adjust the pH to 7. The solution was stirred and heated on a magnetic stirrer at 80 °C for one hour, then heated at 270 °C for 3 h until the evolution of fumes stopped. The as-prepared sample was ground using a mortar for one hour. The obtained powder was characterized by XRD to study the crystallinity of the Ho-doped NdFeO3.
Nd0.90Ho0.10FeO3 characterizations and measurements
The crystal structure of Nd0.90Ho0.10FeO3 was studied using XRD (Bruker Advance D8 diffractometer, λ = 1.5406Å) with 2θ in the range of 20°–80°. The XRD data was indexed with the International Centre for Diffraction Data (ICDD) card number 01-089-6644. The morphology of Nd0.90Ho0.10FeO3 was studied using FESEM (model Quanta 250) with EDX and elemental mapping. The magnetic properties of Nd0.90Ho0.10FeO3 were studied by two techniques. The first is measuring the magnetization of the sample using the vibrating sample magnetometer (VSM; 9600-1 LDJ, USA), which uses a magnetic field up to 20 kOe at a temperature of 300 K. The second technique is Faraday’s method, in which a small amount of Nd0.90Ho0.10FeO3 was placed in a glass that was placed at the field gradient to measure the DC magnetic susceptibility with temperature28. The optical properties of Nd0.90Ho0.10FeO3 were studied using a UV–visible spectrophotometer (Jasco (V-630)).
The heavy metals removal from water
The examination of the ability of Nd0.90Ho0.10FeO3 to remove heavy metals such as Co2+, Ni2+, Pb2+, Cr6+, and Cd2+ from water. The removal efficiency is represented by the following steps:
-
1.
The standard solutions (50 ppm) of heavy metals were prepared.
-
2.
10 mL of the standard solutions were added to a beaker with 0.02 g of the sample.
-
3.
The pH value of the solution was adjusted using the ammonia solution or diluted nitric acid.
-
4.
The beakers were stirred on the electric shaker for 1 h at 170 rpm.
-
5.
Take 8 mL of the solutions using a syringe filter.
-
6.
Inductively coupled plasma spectrometry (ICP, Prodigy 7) was used to determine the concentration of the heavy metals.
Results and discussion
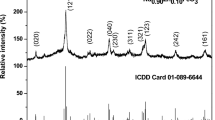
Figure 2 illustrates the XRD of the Nd0.90Ho0.10FeO3 nanoparticles. The most intense peak was observed at 2θ = 32.593º which characterized the (121) plane. The sample has a single phase orthorhombic structure. The average lattice parameters were estimated from all peaks using the following equation29:
The average lattice parameters are reported in Table 1. The crystallite unit cell volume was calculated according to Eq. (2).
The average crystallite size (L) of the sample was estimated from all peaks using the Scherer formula, which is represented by the following equation30:
where λ denotes the X-ray wavelength, β is the full width at half maximum, and θ is the Bragg angle. The value of L is 39.136 nm, which indicates the sample was prepared at the nanoscale.
The tolerance factor relates to the symmetry of the crystal structure and was calculated using Eq. (4).
where rA, rFe, and rO are the ionic radii of the A, Fe, and oxygen ions, respectively. The value of rA was calculated from Eq. (5).
The value of t is one for the ideal cubic structure, while t decreases to one for the orthorhombic structure, where the crystallite size distortion increases. For the investigated sample, the value of t is 0.8875, which indicates the orthorhombic structure of the sample. The theoretical density (Dx) was calculated according to Eq. (6) and reported in Table 1.
where Z is the number of molecules in a unit cell (Z = 4), M refers to the molecular weight of the sample, and N is Avogadro's number. The substitution of Ho3+ ions at the expense of the Nd3+ ion led to an increase in the relative density of NdFeO3. According to M.M. Arman10, the relative density of NdFeO3 is 6.33 g/cm3 and its unit cell volume is 236.9 (Å)3, while Nd0.90Ho0.10FeO3 has a relative density of 7.05 g/cm3 and its unit cell volume is 235.6 (Å)3. This is due to the ionic radius of Ho3+ ions (1.073 Å) being less than that of Nd3+ ions (1.163 Å)31.
Figure 3 illustrates the FESEM image of the Nd0.90Ho0.10FeO3 nanoparticles. The agglomerated particles are a result of the synthesis procedure. The sample has a porous nature, which increases the surface area of Ho-doped NdFeO3. The presence of a lot of active sites of the Nd0.90Ho0.10FeO3 increases the adsorption of HMs on the surface of the Nd0.90Ho0.10FeO3 sample3.
Figure 4 shows the EDX of Ho-doped NdFeO3, which assures the presence of the elements Fe, Ho, Nd, and O in the Nd0.90Ho0.10FeO3. The table inset in Fig. 4 shows the atomic percentage (at%) and weight percentage (wt%) of the elements, which were calculated theoretically from the sample formula and experimentally from the EDX data. The values of at% and wt% of the theoretical and experimental are close to each other, which indicates that the sample was prepared in the same chemical formula, Nd0.90Ho0.10FeO3. The peak observed at 2.11 eV is due to the gold coating of the sample before scanning. In Fig. 4, the carbon ions were observed at 0.27 eV due to the carbon tap where the sample was put inside the FESEM. The slight difference in wt% and at% between experimental and theoretical values is caused by the oxygen deficiency.
Figure 5 shows the mapping of the elements in Ho-doped NdFeO3. Figure 5.a illustrates the homogeneous distribution of the elements in the sample. Figure 5b–e shows the distribution of each element in the sample by distinguished color. The wt% of the elements appearing in the elemental mapping was different from that obtained from EDX analysis due to the maps having been measured for too short a time.
The magnetic properties of Nd0.90Ho0.10FeO3 were studied via the M–H hysteresis loop and Faraday’s method. The magnetic behavior of Nd0.90Ho0.10FeO3 originates from the magnetic coupling between the magnetic ions such as Fe3+, Nd3+, and Ho3+ ions.
Figure 6 illustrates the magnetization hysteresis loop of Nd0.90Ho0.10FeO3, which has AFM behavior with weak ferromagnetic (FM) components32. The values of the saturation magnetization (Ms) and coercive field (Hc) are reported in Table 2. The value of the squareness ratio (SQR) of the sample was calculated from Eq. (7).
The value of SQR was reported in Table 2 and indicates that the type of magnetic interaction is magneto-static interactions33. The exchange bias (Hex) of the sample was calculated from the Eq. (8).
where Hleft and Hright are the intercepts of the MH curve with the negative and positive x-axis, respectively. The presence of a shift in the MH loop around the origin originated from the presence of AFM ordering with (FM) spins in the sample.
The anisotropy constant (K) of Nd0.90Ho0.10FeO3 was calculated from Eq. (9)34:
where Hc is the coercive field of the sample. The value of K was reported in Table 2.
Figure 7 shows the dependence of dM/dH on the magnetic field for Nd0.90Ho0.10FeO3 nanoparticles. The switching field distribution (SFD) and the rectangularity of the H-M loop (Ha) of Nd0.90Ho0.10FeO3 were calculated using the following equations9.
where ∆H represents the half width at the half maximum of the dM/dH peak. Table 2 contains the values of SFD and Ha.
Many researchers have studied the preparation and properties of NdFeO3. M.M. Arman10 prepared the NdFeO3 nanoparticles using the citrate combustion method. The values of Ms and Mr of NdFeO3 are 1.05 emu/g and 0.11 emu/g, respectively. T. Shalini et al.35 studied the structure and magnetic behavior of NdFeO3, which has Ms and Mr equal to 0.521 emu/g and 0.098 emu/g, respectively. In the presence of work, the substitution of Ho3+ ions instead of Nd3+ ions increased the magnetic properties of the NdFeO3 nanoparticles. Where the effective magnetic moment of Nd3+ is 1.14 μB while that of Ho3+ is 10.6 μB36. The presence of Ho3+ in the NdFeO3 increases the magnetic interactions between the magnetic ions such as Ho3+–O2-–Ho3+, Ho3+–O2-–Nd3+, and Ho3+–O2—Fe3+.
Figure 8 shows the dependence of the molar magnetic susceptibility (χM) on T(K). The behavior of χM with temperature assures that the sample has AFM behavior. χM decreases with raising the temperature up to the Neel temperature (TN), after which Nd0.90Ho0.10FeO3 has a paramagnetic behavior. The AFM properties originate from the magnetic interaction between the Fe3+ ions. The relation between χM and the applied field is inversely proportional according to the following equation:
The relation between χM-1 and the temperature is shown in Fig. 9, which applies the Curie–Weiss law. From the slope of the paramagnetic region in Fig. 9, the values of the effective magnetic moment (μeff), the Curie–Weiss constant (θ), and the Curie constant (C) were determined. The value of the μeff was determined from the following equation:
where C denotes the reciprocal of the slope in the paramagnetic part. The values of μeff, θ, and C were reported in Table 3. The value of TN was determined from the differentiation of χM (dχM/dT) and listed in Table 3.
Figure 10 shows the dependence of the absorbance of the UV–visible light by the sample on the wavelength of the incident photons. In the low wavelength region (λ ≤ 460 nm), the absorbance increases rapidly with λ due to increasing the energy of the photons, which allows the electrons to be transferred from the 2p orbital oxygen valance band (V.B.) to the 3d orbital iron conduction band (C.B.). In the high wavelength region (λ > 460 nm), the energy of the photons is low and can’t transfer the electrons from V.B. to C.B.
The optical absorption coefficient (α) is related to the quantity of UV–visible light absorption through the material. The value of α was determined using Eq. (14)37.
where A is the absorbance and l denotes the length of the spacemen. Figure 11 shows the relationship between α and the wavelength. The values of α increased rapidly with increasing λ up to 460 nm, then α decreased slowly with increasing λ. The increasing of α is due to increasing the absorption of photons at low wavelengths and higher frequencies, while the decreasing of α is due to decreasing the absorption of photons at high wavelengths and lower frequencies.
The optical extinction coefficient (k) denotes the losses of electromagnetic energy in Nd0.90Ho0.10FeO3 nanoparticles. k was calculated using the Eq. (15).
Figure 12 studies the dependence of k on wavelength of the photons. The increasing of k with increasing λ of photons is due to the fact that at high λ, the energy of the photons is small and doesn’t absorb in the sample, increasing the energy losses, so k increases.
The Tauc plot was used to determine the optical band gap value (Eg) and the type of optical transition of Nd0.90Ho0.10FeO3. The Tauc equation was represented by Eq. (16)37.
where A and hν are the constant and photon energy, respectively. The (x) value was estimated to be the type of optical transition. For the direct transition, x equals 2, while for the indirect transition, x equals 1. The Tauc plot is represented in Fig. 13, which illustrates that the Nd0.90Ho0.10FeO3 sample has a direct transition with Eg = 1.106 eV. J. S. Prabagar et al.21 prepared NdFeO3 using the citrate sol–gel method with an optical bandgap of 2.48 eV and good photocatalytic activities. While introducing the Ho3+ ions in the NdFeO3 leads to a decrease in Eg to 1.106 eV due to introducing orbitals and states of Ho3+ ions in the NdFeO3 system. The author recommended using Nd0.90Ho0.10FeO3 as a photocatalyst for organic dye degradation in water.
Figure 14 illustrates the dependence of the removal efficiency of the HMs at Ph = 7, which was calculated using Eq. (17).
where \({C}_{f}\) is the final concentration while and \({C}_{i}\) denotes the initial concentration of the HMs. The sample Nd0.90Ho0.10FeO3 has the ability to remove a lot of HMs from water, which indicates that it has a large surface area. The adsorption of the HMs from an aqueous solution depends on many parameters, such as temperature, pH value of the solution, contact time, ionic radii of the HMs, initial concentration of the adsorbent, and molecular weight of the HMs. The highest efficiency of HMs from the water was observed for Pb2+ ions with η = 72.39% due to the fact that Pb2+ ions have a higher molecular weight than the other HMs, leading to easier adsorption.
The effect of the pH value of the solution on the removal efficiency (η) of Pb2+ ions was studied and illustrated in Fig. 15. In the acidic region (pH ≤ 6), there are a lot of H+ ions in the solution, which participate with the Pb2+ ions in the adsorption on the active sites on the surface of Nd0.90Ho0.10FeO3, so η is low. The maximum adsorption of Pb2+ ions was observed at pH = 7, which indicates the optimum pH condition for removal of Pb2+ from aqueous solutions using Ho-doped NdFeO3. The FESEM images of the sample illustrate the porous nature of the surface of the sample, which increases the active sites that adsorb the HMs from the water. In the basic medium (pH = 8), the Pb2+ ions can be precipitated as lead hydroxide, which is not favorable.
The adsorption mechanism of the Pb2+ ions from the water was studied using the adsorption isotherm models. In the present work, the Langmuir and Freundlich isotherm models were used to study the adsorption of Pb2+ on the surface of the sample.
The Langmuir isotherm represents the adsorption of the Pb2+ ions on the surface active sites as a single layer. The assumptions of the Langmuir isotherm are that the HMS is adsorbed on discrete surface active sites, each HM molecule adsorbs on one active site, the sample has a uniform adsorbing surface, and HM molecules don’t interact with each other38. The following equation describes the Langmuir isotherm mode.
where Ce is the equilibrium Pb2+ ion concentration, qm, and KL denote the Langmuir constants. The equilibrium adsorption capacity (qe) was calculated using Eq. (19).
where V and m are the volume of the Pb2+ solution and the adsorbent mass, respectively. Figure 16 shows the fitting of the experimental data with the Langmuir isotherm model.
The Freundlich isotherm describes the mass transportation of the Pb2+ ions from the aqueous solution to the active sites on the porous surface of Nd0.90Ho0.10FeO3. Equation (20) represents the Freundlich isotherm model.
where Kf denotes the Freundlich constant. Figure 17 shows the fitting of the experimental data with the Freundlich isotherm.
From the inset tables in Figs. 16 and 17, the values of R2 are 0.9545 and 0.9399 for the Langmuir and Freundlich isotherm modes, respectively. The Langmuir isotherm mode is the best-fit model for adsorbing the Pb2+ ions from water, and the HMs form monolayer adsorption.
Conclusion
Nd0.90Ho0.10FeO3 was prepared in an orthorhombic structure with a crystallite size of 39.136 nm. FESEM illustrates the agglomerated grains due to the magnetic behavior of the sample. The EDX data shows that the elements Fe, Ho, Nd, and O are present in Ho-doped sample without any impurities. The antiferromagnetic properties of Nd0.90Ho0.10FeO3 originate from the magnetic coupling of Fe3+–O2-–Fe3+, Nd3+–O2-–Fe3+, and Nd3+–O2-–Nd3+. The values of Ms, Mr, Hc, Hex, K, and SDF are 2.42 emu/g, 0.25 emu/g, 200 Oe, -10.86 Oe, 514.14 erg/g, and 2.33, respectively. The sample has a direct optical transition with Eg = 1.106 eV. Nd0.90Ho0.10FeO3 is a good absorber of UV–visible light and can be used for photocatalysis of organic dye degradation in water. The Nd0.90Ho0.10FeO3 nanoparticles have a high efficiency (72.39%) to remove the heavy metal Pb2+ from water. The experimental data is more fitting for the Langmuir isotherm mode.
Data availability
The author declares that all the data supporting the findings of this study are available in the ICDD card number 01-089-6644.
References
Khaled, M. A., Ruvalcaba, J., Córdova, T. F., El Marssi, M. & Bouyanfif, H. Spin-lattice coupling in an epitaxial NdFeO3 thin film. Mater. Lett. 309, 131442 (2022).
Kumar, S. et al. Compositional-driven multiferroic and magnetoelectric properties of NdFeO3-PbTiO3 solid solutions. J. Asian Ceram. Soc. 9(1), 208–220 (2021).
Arman, M. M. Novel multiferroic nanoparticles Sm1-xHoxFeO3 as a heavy metal Cr6+ ion removal from water. Appl. Phys. A 129(6), 400 (2023).
Ahmed, M. A., Selim, M. S. & Arman, M. M. Novel multiferroic La0.95Sb0.05FeO3 orthoferrite. Mater. Chem. Phys. 129(3), 705–712 (2011).
Jia, D. C., Xu, J. H., Ke, H., Wang, W. & Zhou, Y. Structure and multiferroic properties of BiFeO3 powders. J. Eur. Ceram. Soc. 29(14), 3099–3103 (2009).
Cheng, Y., Peng, B., Hu, Z., Zhou, Z. & Liu, M. Recent development and status of magnetoelectric materials and devices. Phys. Lett. A 382(41), 3018–3025 (2018).
Vopson, M. M. Fundamentals of multiferroic materials and their possible applications. Crit. Rev. Solid State Mater. Sci. 40(4), 223–250 (2015).
Yadav, S. K. & Hemalatha, J. Electrospinning and characterization of magnetoelectric NdFeO3–PbZr0.52Ti0.48O3 core-shell nanofibers. Ceram. Int. 48(13), 18415–18424 (2022).
Arman, M. M. & Ramadan, R. Spherical SiO2 growth on LaFeO3 perovskite to create core–shell structures for Cd (II) adsorption on its surface. J. Mater. Sci. Mater. Electron. 34(17), 1365 (2023).
Arman, M. M. The effect of the rare earth A-site cation on the structure, morphology, physical properties, and application of perovskite AFeO3. Mater. Chem. Phys. 304, 127852 (2023).
Bammannavar, B. K. & Naik, L. R. Study of magnetic properties and magnetoelectric effect in (x)Ni0.5Zn0.5Fe2O4+ (1–x) PZT composites. J. Magn. Magn. Mater. 324(6), 944–948 (2012).
Yuan, S. J. et al. Spin switching and magnetization reversal in single-crystal NdFeO3. Phys. Rev. B 87(18), 184405 (2013).
Bharadwaj, P. S. J., Kundu, S., Kollipara, V. S. & Varma, K. B. Structural, optical and magnetic properties of Sm3+ doped yttrium orthoferrite (YFeO3) obtained by sol–gel synthesis route. J. Phys. Condens. Matter. 32(3), 035810 (2019).
Wang, M. & Wang, T. Structural, magnetic and optical properties of Gd and Co co-doped YFeO3 nanopowders. Materials 12(15), 2423 (2019).
Duyen, P. T. H., Diem, C. H. & Tien, N. A. Cd-doped NdFeO3 nanoparticles: Synthesis and optical properties study. J. Mater. Sci. Mater. Electron. 33, 1–10 (2022).
Mir, S. A., Ikram, M. & Asokan, K. Structural, optical and dielectric properties of Ni substituted NdFeO3. Optik 125(23), 6903–6908 (2014).
Shivaraju, H. P., Yashas, S. R. & Harini, R. Application of Mg-doped TiO2 coated buoyant clay hollow-spheres for photodegradation of organic pollutants in wastewater. Mater. Today Proc. 27, 1369–1374 (2020).
Shanmugam, V., Jeyaperumal, K. S., Mariappan, P. & Muppudathi, A. L. Fabrication of novel gC3N4 based MoS2 and Bi2O3 nanorod embedded ternary nanocomposites for superior photocatalytic performance and destruction of bacteria. New J. Chem. 44, 13182–13194 (2020).
Abdi, M., Mahdikhah, V. & Sheibani, S. Visible light photocatalytic performance of La-Fe co-doped SrTiO3 perovskite powder. Opt. Mater. 102, 109803 (2020).
Ismael, M. & Wark, M. Perovskite-type LaFeO3: Photoelectrochemical properties and photocatalytic degradation of organic pollutants under visible light irradiation. Catalysts 9, 342 (2019).
Prabagar, J. S., Tenzin, T., Yadav, S., Kumar, K. M. A. & Shivaraju, H. P. Facile synthesis of NdFeO3 perovskite for photocatalytic degradation of organic dye and antibiotic. Mater. Today Proc. 75, 15–23 (2023).
Godt, J. et al. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 1(1), 1–6 (2006).
Alemu, A., Lemma, B. & Gabbiye, N. Adsorption of chromium (III) from aqueous solution using vesicular basalt rock. Cogent Environ. Sci. 5(1), 1650416 (2019).
Brooks, R. M., Bahadory, M., Tovia, F. & Rostami, H. Removal of lead from contaminated water. Int. J. Soil Sediment Water 3(2), 14 (2010).
Mbamba, C. K., Batstone, D. J., Flores-Alsina, X. & Tait, S. A generalised chemical precipitation modelling approach in wastewater treatment applied to calcite. Water Res. 68, 342–353 (2015).
Da Silva, S. S., Chiavone-Filho, O., de Barros Neto, E. L. & Foletto, E. L. Oil removal from produced water by conjugation of flotation and photo-Fenton processes. J. Environ. Manag. 147, 257–263 (2015).
Neoh, C. H., Noor, Z. Z., Mutamim, N. S. A. & Lim, C. K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 283, 582–594 (2016).
Arman, M. M. Synthesis, characterization, magnetic properties, and applications of La0.85Ce0.15FeO3 perovskite in heavy metal Pb2+ removal. J. Supercond. Nov. Magn. 35(5), 1241–1249 (2022).
Arman, M. M. Preparation, characterization and magnetic properties of Sm0.95Ho0.05FeO3 nanoparticles and their application in the purification of water. Appl. Phys. A 129(1), 38 (2023).
Arman, M. M. & Gamal, A. A. R. Role of Gd3+ and Ho3+ doping on the structure, physical properties and applications of ZnO. Appl. Phys. A 129(5), 331 (2023).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 32(5), 751–767 (1976).
Zhou, J. S., Marshall, L. G., Li, Z. Y., Li, X. & He, J. M. Weak ferromagnetism in perovskite oxides. Phys. Rev. B 102(10), 104420 (2020).
Jiles, D. C. Recent advances and future directions in magnetic materials. Acta Mater. 51(19), 5907–5939 (2003).
Ateia, E. E., Ateia, M. A. & Arman, M. M. Assessing of channel structure and magnetic properties on heavy metal ions removal from water. J. Mater. Sci. Mater. Electron. 33, 1–12 (2022).
Shalini, T., Vijayakumar, P. & Kumar, J. Studies on structural and magnetic properties of NdFeO3 single crystals grown by optical floating zone technique. Bull. Mater. Sci. 43, 1–7 (2020).
Morosan, E. et al. Structure and magnetic properties of the Ho2Ge2O7 pyrogermanate. Phys. Rev. B 77(22), 224423 (2008).
Arman, M. M., Ahmed, M. K. & El-Masry, M. M. Cellulose Acetate polymer spectroscopic study comprised LaFeO3 perovskite and graphene as a UV-to-visible light converter used in several applications. J. Mol. Struct. 1281, 135153 (2023).
Goodfellow, H. D. & Wang, Y. (2nd Eds.). Industrial Ventilation Design Guidebook: Volume 2: Engineering Design and Applications (Academic press, 2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.M. Arman put the idea of the manuscript together, did the experimental work, and then wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arman, M.M. Highly efficient lead removal from water by Nd0.90Ho0.10FeO3 nanoparticles and studying their optical and magnetic properties. Sci Rep 13, 16585 (2023). https://doi.org/10.1038/s41598-023-43734-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43734-2
- Springer Nature Limited