Abstract
In this study, LaFeO3 perovskite is synthesized by the flash method, and then a simple method is developed for growing SiO2 on its surface to construct a core/shell nanocomposite. The thickness of the SiO2 shell is controlled by varying the amount of tetra-ethyl orthosilicate (TEOS). Two LaFeO3@SiO2 samples with varying SiO2 thicknesses were synthesized. Herein, the lattice parameters, morphology, magnetic, and adsorption properties of the investigated core/shell nanocomposites are tuned by changing the content of SiO2. Results confirm that when the content of SiO2 increased, the prepared LaFeO3@SiO2 core/shell nanocomposite showed good adsorption performance, and the removal efficiency enhanced from 50 to 60% for LaFeO3 and LaFeO3@SiO2, respectively. It is thought that the enhancement of the adsorption performance is related to the high porosity and amorphous nature of SiO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The past few years have seen a massive research focus on various nanostructures as key materials in every aspect of technology and science [1]. Technologies that depend on nanomaterials offer a wide range of applications, such as energy production [2], environmental [3], and biomedical applications [4]. In specific, semiconductor, metal, and core/shell nanostructures (CSN) are given the most attention; meanwhile, they offer unique properties different from their bulk forms [5]. A size changeover beginning microparticles to nanoparticles imparts extraordinary variations in optical, physiochemical, mechanical, magnetic, and electrical properties [6]. The term “core/shell” was first formulated in science in 1990 during the synthesis of multilayered heterogeneous nanoparticles [7]. Consequently, the term of core/shell nanoparticles (CSN) may be defined as a class of diverse nanostructures stamped together with a boundary among the core (inner layer material) and shell (outer material) [8]. The size-to-thickness ratio of the core or shell may have an effect on their size, shape, and surface. Such as hexagonal, spherical, prismatic, concentric, cube, and tubular shapes of CSNs. In several cases, coatings were found to improve their functionality. Despite the fact that the selection of core and shell components has a synergistic effect [9]. The core/shell properties arise from both the core and shell. Moreover, by changing the core to shell ratio and/or the constituting material, the properties could be changed [10]. Recently, the improvement of core/shell structure including metal cores (e.g., Cu, Zn, Ag, Pd, Pt, and Fe) and metal oxides (as ZnO, TiO2, Cu2O, Fe2O3, and SiO2) as shell materials, has generated massive interest in the region of gas-sensing, drug delivery, catalysis, photocatalysis, and photonic applications [11]. LaFeO3, a perovskite rare-earth-metal-based ferrite, has attracted extensive research interest owing to its unique properties and potential for application improvement in efficient materials [12]. The unique properties of LaFeO3 are related to the existence of the Fe3+ ion in its structure, leading to a wide range of peizo, ferro, and pyroelectrical properties [13]. As everyone is aware, conductive and insulative fillers provide core–shell structures with strong capacitive characteristics, and an insulating shell can lower their carrier concentration [14]. For core/shell structure, the insulating inorganic layers, such as SiO2, Al2O3, (Mn–Zn) Fe2O4, MgO, Fe3O4 and Fe3(PO4)2, etc. [15], and the insulating resin-based organic layers, such as epoxy resin and silicone resin, may generally be divided into two categories. Due to their exceptional durability, insulating inorganic layers thus become more suited for practical application [16]. The most popular adsorbents in water treatment have historically been metal oxides and activated carbon. These compounds have been successful in eliminating a variety of pollutants from water. These materials do have several obvious drawbacks, most of which are connected to environmental issues and potential hazards to human health [17, 18]. Recently, prospective biological applications of silica-coated core–shell magnetite nanoparticles have been researched [19]. SiO2 has unique properties such as high temperature resistance, high strength, a small expansion coefficient and good chemical stability, which enable it to be used in a wide range of applications such as microwave absorption and adsorption [20,21,22]. As a result, we used the flash-combustion method to create LaFeO3 and the ultrasonic-assisted deposition–precipitation method to create LaFeO3@xSiO2 core/shell nanoparticles with different shell concentrations. We investigated the effect of shell and shell concentration on the adsorption of heavy metals such as Cd (II) by studying the removal efficiency of the desired samples.
2 Experimental techniques
2.1 Materials
Lanthanum nitrate [La(NO3)3.6H2O], iron nitrate [Fe(NO3)3.9H2O], glycine [C2H5NO2], and tetraethyle orthosilicate (TEOS) were obtained from LOBA, India. All materials were used without further purification.
2.2 Synthesis LaFeO3
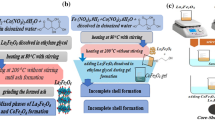
Nanoparticles of LaFeO3 were synthesized by using the flash method, as shown in Fig. 1a. By mixing nitrates of lanthanum (0.1 M) and iron (0.1 M) with glycine as a fuel, a small amount of distilled water was added under strong stirring to get a dissolved mixture. After that, the temperature was adjusted to 250 °C until all the resultant fumes ended. The obtained powder was calcinated at 500 °C for 2 hours at a heating rate of 4 C/min. This process can be expressed by the following equation:
2.3 Synthesis of a LaFeO3@SiO2 core/shell microsphere
The core/shell of LaFeO3@SiO2 was synthesized as shown in Fig. 1b by adding 0.3 g of LaFeO3 nano powder to a mixture consisting of (ethanol, deionized water, and ammonia) and stirring for 1 h. To control the shell thickness, different concentrations of TEOS (e.g., 0.3 and 0.9 ml) were added dropwise to the obtained mixture under vigorous stirring. The final mixtures were stirred continuously for 12 h at room temperature. Then the final powders were collected by centrifugation and dried at room temperature for further characterization and study.
2.4 XRD measurements
XRD investigation was done by using X-ray diffractometer (analytical-x’ pertpro, Cu kα1 radiation, λ = 1.5404 Å, 45 kV, 40 mA, Netherlands). This technique is used to confirm that samples were prepared in pure, single phase without impurities.
2.5 FTIR measurements
A FTIR spectrometer (Perkin-Elmer 2000) with a wavenumber range of 4000–400 cm−1 was used to study the Fourier transformed infrared (FT-IR) spectra.
2.6 Particle morphology
High-resolution transmission electron microscopy (HRTEM, JEOL/JME 2100) was used to study the microstructure and morphology of the prepared samples. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were used to study the surface morphology and chemical structure of the sample using Oxford Inca PentaFETX3-England.
2.7 Magnetic properties
The magnetic properties were investigated by using a vibrating-sample magnetometer (VSM) with a magnetic field up to 20 kOe.
2.8 Batch experiment for removal of heavy metal (Cd (II))
A batch experiment was carried out at different experimental parameters such as pH to study the removal efficiency of the obtained samples, LaFeO3, LaFeO3@0.3 SiO2, and LaFeO3@0.9SiO2. The effect of pH solution was studied by adding (0.1 g) of prepared samples to a standard solution of cadmium nitrate solution (2 ppm). Solution pH was tuned from 2 to 8. After stirring for 1 h, the solutions were collected using a 0.2 µm syringe filter. Atomic absorption spectroscopy (Zeenite 700P, Analytical Jena) was used to determine the final concentration of Cd (II). The removal efficiency was calculated using the following equation [23].
where:
3 Results and discussion
3.1 XRD
Figure 2 displays XRD patterns for the investigated samples, LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2 core/shell nanocomposite. The data were indexed and compared with the ICDD card [00-037-1493]. The obtained data confirmed that the parent sample was obtained in pure single phase and that it has a space group (Pnma) that is related to the orthorhombic structure. There are broad peaks that appear in the XRD pattern after adding silica as a shell to the parent sample (LaFeO3). These broad peaks are characterized by and related to the amorphous nature of silica. By increasing the SiO2 shell thickness around the core sample (LaFeO3) the intensities of XRD peaks decreased when compared with the pure sample LaFeO3.
The lattice parameters (a, b, and c), unit cell volume (V), and theoretical density (ρx) are calculated and listed in Fig. 3 and Table 1 on the basis of orthorhombic symmetry [24]. While, the average crystallite size was calculated using Debye—Sherrer’s formula [25, 26]
where D gives the average crystallite size, λ is the X-ray wavelength, θ represents the Bragg angle, and β is related to the full width at the half maximum intensity of the XRD pattern. We found that, the crystallite size of the investigated samples increased from 28 to 45 nm by increasing the shell thickness owing to increasing the amount of amorphous SiO2 in the sample LaFeO3 nanocomposite, leading to a decrease in crystallinity.
3.2 FTIR study
The presence of a silicate phase is most reliably confirmed using FTIR spectroscopy. FTIR spectra are shown in Fig. 4, the observed band at 560 cm−1 was related to Fe–O–Fe stretches in LaFeO3 [27]. The absorption bands around 1090 cm−1 represented the stretching mode of the Si–O bond, which related to the distinguishing peak of SiO2. The bands that are located around 1430 cm–1 are attributed to the carbonyl group, which is recognized as ambient CO2 that adsorbed on the surface of the prepared samples. Bands at 1620 and 3450 cm−1, were assigned to the bending and stretching vibration modes of the O–H group in water molecules at the samples’ surface.
3.3 HRTEM
Figures 5a–c and 6a, b show the HRTEM photographs, selected area electron diffraction (SAED), and histograms of samples LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2. In Fig. 5a, LaFeO3 has numerous nanoparticles that are stacked on each other. Figure 5b and c confirm the formation of core/shell nanoparticles, in which SiO2 nanoparticles (light color) surround the LaFeO3 surface (black dots) to form irregular agglomerated spherical structures. This could be explained by the high energy surface. It is observed that varying the amount of TEOS from 0.3 to 0.9 ml leads to a change in SiO2 shell thickness, as shown in Fig. 5b and c. By increasing the magnification of TEM micrographs, SAED, as shown in Fig. 6a, b can be obtained, and it consists of bright rings indicating the formation of orthorhombic nanocrystals of high quality. The histogram of the prepared samples was used for calculating the particle size (L) from HRTEM images. The values of (L) were 25, 30, and 40 nm for LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2, respectively, indicating that the investigated samples were synthesized in nanoscale and were comparable with the XRD results.
3.4 FESEM-EDX mapping
Figure 7 illustrates the FESEM image of the LaFeO3@0.3SiO2 sample. The two phases LaFeO3 perovskite and SiO2 are present in the FESEM image. The agglomeration of the sample grains is due to the magnetic properties of the LaFeO3 nanoparticles. The grains of the sample LaFeO3@0.3SiO2 have irregular shape. Figure 8 shows the EDX spectrum of the LaFeO3@0.3SiO2 sample. The EDX analysis illustrates the presence of oxygen, lanthanum, silicon, and iron elements in the sample. The inset table illustrates the weight percentage (wt.%) and the atomic percentage (at.%) of the O, Si, La, and Fe elements. Figure 9 shows the elemental mapping of the sample LaFeO3@0.3SiO2. The oxygen, lanthanum, silicon, and iron elements are distributed in a homogenous distribution.
3.5 Magnetic study
The magnetic properties of LaFeO3@xSiO2 core/shell nanocomposite with different concentrations of TEOS (0, 0.3, and 0.9) are presented in Fig. 10a–c. Crystal structure, porosity, chemical composition, crystallinity, and grain boundaries are affected by these properties. All computed magnetic parameters are listed in Table 2. It is clear from Fig. 10a–c, the saturation magnetization of LaFeO3, LaFeO3@0.3SiO2 and LaFeO3@0.9SiO2 decrease by increasing the quantity of TEOS which related to SiO2 thickness. This decrease in saturation magnetization could be related to the presence of non-magnetic shell layer (SiO2). LaFeO3 has weak magnetic behavior arises from a small canting of Fe3+ spins which leads to weak ferromagnetism [28, 29]. For LaFeO3@0.3SiO2 and LaFeO3@0.9SiO2, the curves have open hysteresis loop at high magnetic field due to the nonferromagnetic thickness layer indicated that these samples are paramagnetic materials [30, 31]. Based on the previous argument the remnant magnetization (Mr) as well as the squarness ratio (Mr/Ms) decreased from 0.118 to 0.056 and 0.196 to 0.118 respectively by increasing the shell content from x = 0 to x = 0.9. The value of the effective magnetic moment (μ) gives by the following equation:
where Mw represents the molecular weight, \({\upmu }_{B}\) gives the Bohr magneton, and NA is the Avogadro number. The value of the effective magnetic moments was 0.025 µB for LaFeO3 and decreased to 0.023 and 0.022 µB for LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2 respectively. This behavior may be due to combinations of non-magnetic phase (SiO2) which leads to deterioration of the magnetic interaction and weaken the spin correlation between the adjacent particles. The coercivity (Hc) has values of 239.24, 291.79, and 317.01 Oe for LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2, respectively. This enhancement of HC value could be due to a shielding phenomenon as the paramagnetic phase (SiO2), protects the weak ferrimagnetic grains of LaFeO3 from the applied field by surrounding their surface. Oppositely, and for the same reason of shielding effect, the exchange bias (HEB) has its highest value for LaFeO3.
Another magnetic parameter that could be calculated from the hysteresis loop is the anisotropy constant, K and it is computed by using Eq. (5) [31]:
The value of the magnetic anisotropy depends on coercivity; its value reveals the dipole’s resistance to go through the annihilation under the effect of the reverse field. From Table 2, it could be observed that the shell effect of SiO2 increases the dipole resistance, consequently increasing the value of magnetic anisotropy as well. The switching field distribution (SFD), which measures the H-M loop rectangularity (Ha) and anisotropy field, could be represented as [28]:
Here, ΔH represents the half width of the dM/dH curve peak and is obtained from Fig. 11a–c.
The calculated value of dM/dH donates the material susceptibility at room temperature. In the other hand, the greater value of dM/dH refers to higher magnetic state stability; in the study, LaFeO3 has the highest magnetic susceptibility at room temperature.
3.6 Adsorption study
Prepared samples of LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2 have been studied for the removal of Cd (II) from wastewater under different pH. The pH value could improve the molecules transformation and have effects on their stability. Figure 12 shows the effect of pH on the Cd (II) uptake. It is clear that for all investigated samples, the adsorption of Cd ions in pH 8 basic media is greater than that observed for the acidic medium. At a basic pH, hydroxide compounds are formed and precipitate [32]. In the acidic medium, the removal efficiency of Cd (II) is small due to the presence of H+ and Cd (II) in the solution and the hydrogen ions competing with the Cd (II) ions on the active sites on the surface of the investigated samples [33,34,35]. The adsorption of Cd ions increased as pH values increased, and the competition between Cd ions and hydrogen ions decreased as the number of hydrogen ions decreased for adsorption on the nanocomposite surface. We also discovered that increasing the TEOS concentration increased removal efficiency, reaching a maximum of 97% for LaFeO3@0.9SiO2. As the concentration of TEOS increased, the thickness of SiO2 increased. The SiO2 layer has many pores, which act as trapping sites for capturing heavy metal. Finally, the ideal value was selected to be 7. At this value, the samples display the maximum adsorption for Cd (II) (50, 55, and 60%) for LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2 respectively. While the basic pH is not preferred, the adsorption is related to the adsorption process as well as the precipitation process. Table 3 shows the removal efficiency of Cd (II) using different samples. The difference in the removal efficiency of the Cd (II) ions from the water is due to the different absorbents and their morphologies.
4 Conclusion
In this work, a simple method was used for preparing a core–shell LaFeO3@SiO2 nanocomposite by using TEOS and LaFeO3 as initial materials. By tuning the TEOS content, the shell thickness, was controlled. We found that the crystallite size was increased from 28 to 45 nm by increasing the shell thickness owing to the increased amorphous nature of SiO2 in the sample LaFeO3 nanocomposite. The particle size (L) of the prepared samples was obtained from the histogram of HRTEM micrographs, its values were 25, 30, and 40 nm for LaFeO3, LaFeO3@ 0.3SiO2 and LaFeO3@0.9SiO2 respectively, indicating that the investigated samples were synthesized in nanoscale and comparable with the XRD results. The coercivity (Hc) has values of 239.24, 291.79, and 317.01 Oe for LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2 respectively. This enhancement of HC value could be due to a shielding phenomenon as the paramagnetic phase (SiO2) protects the weak ferrimagnetic grains of LaFeO3 from the applied field by surrounding their surfaces. Finally, the samples display the maximum adsorption at pH 7 for Cd (II) (50, 55, and 60%) for LaFeO3, LaFeO3@0.3SiO2, and LaFeO3@0.9SiO2, respectively. This work is the first attempt to study how the shell thickness affects the removal efficiency of heavy metals. In future work, more experimental conditions will be studied for increasing the adsorption percentage and improving the surface of core/shell nanocomposite samples.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Z. Wang, H. Jiang, Core-shell FeNi@SiO2 composite with enhanced microwave absorption performance. J. Alloy. Compd. 923, 166468 (2022)
H. Younes, Ru. Li, S.E. Lee, Y.K. Kim, D. Choi, Gradient 3D-printed honeycomb structure polymer coated with a composite consisting of Fe3O4 multi-granular nanoclusters and multi-walled carbon nanotubes for electromagnetic wave absorption. Synth. Met. 275, 116731 (2021)
M.K. Rania Ramadan, Ahmed, Impact of adding vanadium pentoxide to Mn-doped magnetite for technological uses. Appl. Phys. A 128, 1056 (2022). https://doi.org/10.1007/s00339-022-06197-2
A. Aghashiri, S. Hashemian, F.K. Fotooh, Mesoporous LaFeO3 nanoparticles decorated onto activated carbon from agricultural paste (synthesis, characterization and adsorption properties). J. Mol. Struct. 1260, 132822 (2022)
S. Dutta, Br. Gupta, S. Kumar Srivastava, A. Kumar Gupta, Recent advances on the removal of dyes from wastewater using various adsorbents: a critical re- view. Mater. Adv. 2, 4497–4531 (2021)
M.M. Hasan, M.A. Shenashen, M.N. Hasan, H. Znad, M.S. Salman, M.R. Awual, Natural biodegradable polymeric bioadsorbents for efficient cationic dye en- capsulation from wastewater. J. Mol. Liq. 323, 114587 (2021)
N. Saikia, R. Chakravarty, S. Bhattacharjee, R.L. Hota, R.K. Parida, B.N. Parida, Synthesis and characterization of Gd-doped LaFeO3 for device application. Mater. Sci. Semicond. Process. 151, 106969 (2022)
Z. Xiong, L. Huang, J. Peng, Y. Hou, Z. Ding, S. Wang, Spinel-type mixed metal sulfide NiCo2S4 for efficient photocatalytic reduction of CO2 with visible light. ChemCatChem 11, 5513–5518 (2019)
B. Li, F. Wei, B. Su, Z. Guo, Z. Ding, M. Yang, S. Wang, Mesoporous cobalt tungstate nanoparticles for efficient and stable visible-light-driven photocatalytic CO2 reduction. Mater. Today Energy 24, 100943 (2022)
E.E. Ateia, M.K. Abdelamksoud, M.M. Arman, R. Ramadan, A.S. Shafaay, Optimizing the physical properties of cobalt/grapheme nanocomposites for technological applications. Appl. Phys. A 125, 516 (2019). https://doi.org/10.1007/s00339-019-2815-2
Y. Xu, S. Liu, M. Wang, J. Zhang, H. Ding, Y. Song, Y. Zhu, Q. Pan, C. Zhao, H. Deng, Thiourea-assisted one-step fabrication of a novel nitrogen and sulfur co-doped biochar from nanocellulose as metal-free catalyst for efficient activation of peroxymonosulfate. J. Hazard. Mater. 416, 125796 (2021)
C. Yan, L. Fan, Y. Chen, Y. Xiong, Effective adsorption of oxytetracycline from aqueous solution by lanthanum modified magnetic humic acid. Colloids Surf. A Physicochem. Eng. Asp. 602, 125135 (2020)
Y. Yang, S. Zhang, S. Wang, K. Zhang, H. Wang, J. Huang, S. Deng, B. Wang, Y. Wang, G. Yu, Ball milling synthesized MnOx as highly active catalyst for gaseous POPs removal: significance of mechanochemically induced oxygen vacancies. Environ. Sci. Technol. 49, 4473–4480 (2015)
Y.J. Yang, S. Chen, C. Jiang, N. Wang, P. Yang, M. Liu, Y. Cheng, Assembly of flower-like Mn3O4/NiCo-LDH@carbon nanotube nanocomposites on Ni foam for binder-free capacitor electrode. J. Alloy. Compd. 930, 167466 (2023)
K. Sun, S. Feng, Q. Jiang, X. Li, Y. Li, R. Fana, Y. Ana, J. Wang, Intergranular insulating reduced iron powder-carbonyl iron powder/SiO2- Al2O3 soft magnetic composites with high saturation magnetic flux density and low core loss. J. Magn. Magn. Mater. 493, 165705 (2020)
Z. Wang, K. Sun, H. Wu, Y. Qu, J. Tian, L. Ju, R. Fan, Epsilon-near-zero response derived from collective oscillation in the metacomposites with ultralow plasma frequency. Compos. Sci. Technol. 227, 109600 (2022)
R. Ramadan, V. Uskoković, M.M. El-Masry, Triphasic CoFe2O4/ZnFe2O4/CuFe2O4 nanocomposite for water treatment applications. J. Alloy. Compd. 954, 170040 (2023)
R. Ramadan, Study the multiferroic properties of BiFeO3/Ni0.1Fe2.9O4 for heavy metal removal. Appl. Phys. A 129, 125 (2023). https://doi.org/10.1007/s00339-022-06376-1
J. Wang, S. Zheng, Y. Shao, J. Liu, Z. Xu, D. Zhu, Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 349, 293–299 (2010)
M.M. Arman, R. Ramadan, Optical, magnetic, and electrical studies of nanometric Bi1−xNdxFeO3 perovskite. J. Superconduct. Nov. Magn. (2020). https://doi.org/10.1007/s10948-020-05441-1
Y. Liu, Y. Zhang, C. Zhang, B. Huang, Y. Li, W. Lai, X. Wang, X. Liu, Low temperature preparation of highly fluorinated multiwalled carbon nanotubes activated by Fe3O4 to enhance microwave absorbing property. Nanotechnology 29, 365703 (2018)
J. Liu, H. Liang, H. Wu, Hierarchical flower-like Fe3O4/MoS2 composites for selective broadband electromagnetic wave absorption performance. Compos. A 130, 105760 (2020)
X. Su, J. Wang, X. Zhang, S. Huo, W. Chen, W. Dai, B. Zhang, Design of controlledmorphology NiCo2O5 with tunable and excellent microwave absorption performance. Ceram. Int. 46, 7833–7841 (2020)
R. Ramadan, Physical study of cobalt ferrite and its application in purification of water. Appl. Phys. A 125, 825 (2019). https://doi.org/10.1007/s00339-019-3121-8
R. Ramadan, Preparation, characterization and application of Ni-doped magnetite. Appl. Phys. A 125, 586 (2019). https://doi.org/10.1007/s00339-019-2887-z
R. Ramadan, A.M. Ismail, Structural and physical comparison between CS/PVP blend and CS/PVP/Sr-Hexaferrite nanocomposite films. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02684-y
M.M. Armana, S.I. El-Dek, Enhancement in dielectric and optical properties of La1-xCexFeO3 nanoparticles. J. Market. Res. 8(6), 5396–5404 (2019)
K. Yang, J. Ma, X. Qiao, Y. Cui, L. Jia, H. Wang, Hierarchical porous LaFeO3 nanostructure for efficient trace detection of formaldehyde. Sensor Actuat B: Chem. 313, 1208022 (2020)
S. Ingvarssona, G. Xiaoa, S.S.P. Parkinb, W.J. Gallagher, Thickness-dependent magnetic properties of Ni81Fe19; Co90Fe10 and Ni65Fe15Co20 thin films. J. Magn. Magn. Mater. 251, 202–206 (2002)
S. Thirumalairajan, K. Girija, V. Mastelaro, N. Ponpandian, Photocatalytic degradation of organic dyes under visible light irradiation by floral-like LaFeO3 nanostructures comprised of nanosheet petals. New J. Chem. 38, 5480–5490 (2014)
U. Sarac, M.C. Baykul, Y. Uguz, Differences observed in the phase structure, grain size-shape, and coercivity field of electrochemically deposited Ni–Co thin films with different Co contents. J. Supercond. Novel Magn. 28, 3105–3110 (2015)
K.M.S. Khalil, A.H. Mahmoud, M. Khairy, Formation and textural characterization of size-controlled LaFeO3 perovskite nanoparticles for efficient photocatalytic degradation of organic pollutants. Adv. Powder Technol. 33, 103429 (2022)
M.M. Arman, Novel multiferroic nanoparticles Sm1− xHoxFeO3 as a heavy metal Cr6+ ion removal from water. Appl. Phys. A 129(6), 400 (2023)
M.M. Arman, The effect of the rare earth A-site cation on the structure, morphology, physical properties, and application of perovskite AFeO3. Mater. Chem. Phys. 304, 127852 (2023)
E.E. Ateia, D. Gawad, M. Mosry, M.M. Arman, Synthesis and functional properties of La2FeCrO6 based nanostructures. J. Inorg. Organomet. Polym. Mater. (2023). https://doi.org/10.1007/s10904-023-02699-5
S.I. Rania Ramadan, M.M. El-Dek, Arman, Enhancement of Mn-doped magnetite by mesoporous silica for technological application. Appl. Phys. A 126, 900 (2020). https://doi.org/10.1007/s00339-020-04059-3
W. Peng, H. Li, Y. Liu, S. Song, A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 230, 496–504 (2017)
F.T. Alshorifi, S.M. El Dafrawy, A.I. Ahmed, Fe/Co-MOF nanocatalysts: greener chemistry approach for the removal of toxic metals and catalytic applications. ACS Omega 7(27), 23421–23444 (2022)
M.P. Rao, S. Musthafa, J.J. Wu, S. Anandan, Facile synthesis of perovskite LaFeO3 ferroelectric nanostructures for heavy metal ion removal applications. Mater. Chem. Phys. 232, 200–204 (2019)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MMA: Conceptualization, methodology, formal analysis, data curation, investigation, visualization, writing, and review. RR: Conceptualization, methodology, formal analysis, investigation, data curation, visualization, writing, and review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arman, M.M., Ramadan, R. Spherical SiO2 growth on LaFeO3 perovskite to create core–shell structures for Cd(II) adsorption on its surface. J Mater Sci: Mater Electron 34, 1365 (2023). https://doi.org/10.1007/s10854-023-10721-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10721-2