Abstract
We aimed to derive the Febrile Infants Risk Score at Triage (FIRST) to quantify risk for serious bacterial infections (SBIs), defined as bacteremia, meningitis and urinary tract infections. We performed a prospective observational study on febrile infants < 3 months old at a tertiary hospital in Singapore between 2018 and 2021. We utilized machine learning and logistic regression to derive 2 models: FIRST, based on patient demographics, vital signs and history, and FIRST + , adding laboratory results to the same variables. SBIs were diagnosed in 224/1002 (22.4%) infants. Among 994 children with complete data, age (adjusted odds ratio [aOR] 1.01 95%CI 1.01–1.02, p < 0.001), high temperature (aOR 2.22 95%CI 1.69–2.91, p < 0.001), male sex (aOR 2.62 95%CI 1.86–3.70, p < 0.001) and fever of ≥ 2 days (aOR 1.79 95%CI 1.18–2.74, p = 0.007) were independently associated with SBIs. For FIRST + , abnormal urine leukocyte esterase (aOR 16.46 95%CI 10.00–27.11, p < 0.001) and procalcitonin (aOR 1.05 95%CI 1.01–1.09, p = 0.009) were further identified. A FIRST + threshold of ≥ 15% predicted risk had a sensitivity of 81.8% (95%CI 70.5–91.0%) and specificity of 65.6% (95%CI 57.8–72.7%). In the testing dataset, FIRST + had an area under receiver operating characteristic curve of 0.87 (95%CI 0.81–0.94). These scores can potentially guide triage and prioritization of febrile infants.
Similar content being viewed by others
Introduction
Young infants with fever are at risk of bacteremia, meningitis and urinary tract infections (UTIs), collectively named serious bacterial infections (SBIs)1. The fear of missing SBIs has led to low physician thresholds to perform invasive investigations (including blood, urine and cerebrospinal fluid [CSF] cultures), resulting in a large number of unnecessary hospitalizations and rising healthcare costs2. Widespread empirical antibiotic use has also contributed to global antibiotic resistance3. Clinical prediction rules have thus far focused on identifying infants at low risk of SBIs who do not require extensive tests1,4,5. These clinical prediction rules have potential to reduce the number of invasive procedures6,7, but do not provide comprehensive guidance on which young febrile infant should be prioritized to receive urgent antibiotics. Such guidance is needed to reduce recognition delays and shorten time-to-antibiotics for infants who require urgent interventions8. Moreover, generalizability of these prediction rules has been questioned, with variable diagnostic performance in different populations9,10.
More recently, data-driven techniques including machine learning methods have been employed to derive and validate models to predict which young febrile infants are at risk of SBIs and invasive bacterial infections (IBIs)—namely meningitis and bacteremia11,12. These machine learning algorithms use commonly available triage information including age and temperature, and laboratory tests such as abnormal urinalysis, white blood cell, absolute neutrophil count (ANC), and procalcitonin to build scores that predict for the presence of SBI or IBI. Implementation of these algorithms could potentially reduce unnecessary lumbar punctures by approximately 70%11. However, these models are computationally complex and are not easily interpreted by clinicians11.
The AutoScore machine learning-based method, previously described as a combination of machine learning and logistic regression, automates the development of parsimonious and transparent risk models13. As compared to other machine learning methods, Autoscore has the potential to develop point-based scores that are interpretable by clinicians and can be translated into clinical practice. One example was the development and assessment of a Score for Emergency Risk Prediction (SERP) to estimate mortality after emergency admissions14.
We aimed to derive interpretable risk scores based on routinely available patient information and clinical data, to quantify risk of SBIs among infants < 3 months presenting with fever.
Methods
Study design and setting
We performed a prospective observational study for febrile infants < 3 months old presenting to a tertiary pediatric hospital in Singapore between December 2018 and December 2021. Our hospital is one of two pediatric hospitals in the country, with an annual ED attendance of about 150,000 children. Infants < 3 months old are routinely hospitalized in our institution. Neonates (defined as < 28 days old) receive the entire septic workup (blood, urine and CSF cultures) and proceed on to receive empirical antibiotics, while infants between 28 and 90 days’ old have variable investigations depending on the temperature trend and clinical assessment of the child. Regardless of the extent of investigations, infants < 3 months are monitored in the hospital until they are fever-free for 24 h, before discharge. We defined fever as an axilla or rectal temperature of 38 °C and above. Between December 2018 to December 2020, infants were recruited as part of a heart rate variability (HRV) study (NCT04103151). We subsequently obtained ethics approval to collect data from all febrile infants who presented between January 2021 and December 2021. We obtained approval from the SingHealth Institutional Review Board E in Singapore (2017/2680) with waiver of informed consent. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Data variables
We recorded patient demographics including age, birth gestation, and sex. We collected routine triage information including vital signs (i.e., temperature, heart rate, respiratory rate, and oxygen saturations). Our department uses the severity index score (SIS), a composite measure of respiratory effort, activity, color, play and temperature, to assess the acuity of a child at triage15. We obtained data on presence of comorbidities, duration of fever, and maternal Group B streptococcus (GBS) status. Laboratory investigations included hemoglobin, total white blood cell count, ANC, platelets, C-Reactive Protein (CRP), and procalcitonin. Fluid from urine and cerebrospinal fluid (CSF) were sent for analysis. Urine was tested for leukocyte esterase (graded as negative, 1 + , 2 + or 3 +) and nitrite (positive or negative). CSF was analyzed for cells and clarity. We obtained culture results from blood, urine and CSF. We recorded if the infant received fluid bolus resuscitation or inotropic support, intravenous antibiotics, or ventilator (both invasive and non-invasive) support. We also documented the need for high acuity care, defined as High Dependency (HD) and Intensive Care Unit (ICU) care.
Outcome variables
SBI was defined as bacteremia, meningitis or UTI1. Bacteremia and meningitis were defined as pure growth of a pathogen in blood and CSF, respectively. When the bacteria grown was considered likely to be a contaminant (e.g., coagulase-negative staphylococcus), the case was not considered as SBI. UTIs were defined as growth of a single pathogen (a) > 100,000 colony-forming units (CFU/ml) in a clean catch specimen, or (b) ≥ 50,000 CFU/ml in a catheterized specimen, or (c) 10,000–50,000 CFU/ml in a catheterized specimen with an abnormal urinalysis (positive for leucocyte esterase or nitrite)8. We also recorded the duration of hospital stay. The study team members who recorded the outcome of SBI were not blinded to the clinical variables listed above.
Statistical analysis
Data management
We described categorical variables using frequencies and percentages. Continuous variables were described using mean (and standard deviation, SD) or median (and interquartile range, IQR), depending on normality. Data were analyzed using R software, v 4.2.1 (R Foundation for Statistical Computing).
Because we wanted a practical tool to drive decision-making, we derived our score in 2 stages based on information that would be available to the ED physician. FIRST represents the initial triage phase and included patient demographics, vital signs and history-taking. FIRST + represents a more advanced phase after consultation and included laboratory investigations, such as urine and blood test results. Laboratory results would routinely require a turnaround time of up to 2 h, before becoming available to the ED physician. In our hospital, procalcitonin is part of the routine laboratory workup for hospitalized young infants with fever. For the group that physicians chose not to perform this blood test, we assigned them as ‘clinically not indicated’, rather than exclude them, because this reflected a group that yielded diagnostic information.
AutoScore machine learning-based method
To derive FIRST, we utilized the AutoScore technique. The AutoScore machine learning-based method has been described as a combination of machine learning and logistic regression, and automates the development of parsimonious risk models13. AutoScore consists of the following modules: variable transformation, variable ranking, score derivation and model selection, score fine-tuning, and model evaluation. The variable transformation module converts all continuous variables into categorical ones based on prespecified cutoffs. We transformed continuous variables to categorical variables with five categories based on 4 prespecified cutoffs, which included the 5% quantile, the 20% quantile, the 80% quantile, and the 95% quantile. The variable ranking module uses random forest to rank the variables based on their contribution to the outcome prediction. We used the ‘Parsimony Plot’ to show the predictive contribution of each variable. The score derivation module constructed a logistic regression model using the transformed variables, starting with the highest ranked variable and then adding on one variable at a time, following the order of their ranking. The study team selected the variables to be included in the final clinical score based on their domain knowledge and each variables’ contribution to the outcome prediction. We defined a significant correlation between two variables as having an absolute Pearson’s correlation coefficient of ≥ 0.2. Since such correlations would hinder accurate model fitting and prediction from subsequent logistic regression, only one variable from each significantly correlated variable group was selected into the subsequent analysis. The selected variables were used to build a multivariable logistic regression model, which was then converted to a clinical score. Study team members fine-tuned the resulting clinical score based on their domain knowledge for more practical and interpretable cutoff values. The final variables selected into the scoring models were fine-tuned according to clinical discretion and ease of use. Specifically, age was divided into only three groups: < 21 days, 21 to less than 28 days, and ≥ 28 days. Temperature was divided into four groups: < 38.5 °C, 38.5 °C to less than 39 °C, 39 °C to less than 40 °C, and ≥ 40 °C. Finally, the model evaluation module examined the out-of-sample prediction performance of the finalized clinical score. We presented the models’ performance on both the training and testing data, using area under receiver operating characteristic (ROC) curve, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), as well as the model calibration results.
For sample size estimation, at a target sensitivity of 85%, marginal error of 0.05, and an event rate of 0.2 (based on published pilot data8), we aimed for a total of 980 febrile infants16. We did not perform multiple imputation for missing values. We only encountered missing values for laboratory values and detailed the number with complete data in the Results section.
Training and testing data
We divided our dataset into the training set and testing set. The training set was used for variable selection and score derivation, while the testing set was only used for model evaluation to examine the clinical score’s real-world performance on previously unseen data. The two sets were divided using the randomized stratified sampling, where the proportion of patients with SBI was the same in both sets. In this study, we used 80% and 20% of the available data for the training and testing set, respectively.
We followed the TRIPOD checklist for prediction model development17.
Ethics approval
We obtained approval from the SingHealth Institutional Review Board E in Singapore (2017/2680) with waiver of informed consent. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Results
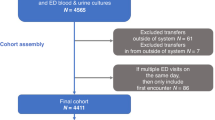
We analyzed 1002 children in total, with an SBI rate of 22.4% (224/1002) (Fig. 1). The median age was 30 days (IQR 10–60) and there were 574 (57.3%) males. 741/1002 (73.9%) infants underwent urine cultures, 678/1002 (67.7%) blood cultures, and 477/1002 (47.6%) CSF cultures. Among infants with SBIs, the most common infection was UTIs (199/224, 88.8%), followed by bacteremia (22/224, 9.8%) and meningitis (3/224, 1.3%). Among the 22 infants with bacteremia, 11 (50.0%) had more than one source of SBI. The most common pathogens for UTIs (including co-infections) were Escherichia coli (153/199, 76.9%), Klebsiella pneumoniae (30/199, 15.1%) and Enterococcus faecalis (12/199, 6.0%). Among the 22 infants with bacteremia, 7 (31.8%) had GBS, 7 (31.8%) had Escherichia coli, 2 (9.0%) had Klebsiella pneumoniae. There were 2 patients (9.0%) who grew Staphylococcus aureus in both blood and urine cultures.
Infants with SBIs were older than those without SBIs (median age 46 days, IQR 21–69 vs. 28 days IQR 8–55, p < 0.001), and were more likely to be male (166/224, 74.1% vs. 408/778, 52.4%, p < 0.001) (Table 1). At triage, the presenting temperature and heart rate were significantly higher among infants with SBIs (38.7 °C SD 0.7 vs. 38.4 °C SD 0.5, p < 0.001 and 169 bpm SD 22 vs. 160 bpm SD 20, p < 0.001). Laboratory markers of inflammation (total white blood cell count, ANC, CRP, procalcitonin) were significantly higher among those with SBIs compared to those without (Table 1). There was no significant difference in the proportion of infants who received a fluid bolus between groups. However, infants with SBIs did experience a longer hospital stay compared to those without (median 4.0 days, IQR 3.0–5.0 vs. median 3.0 days IQR 2.0–4.0, p < 0.001). 6/224 (2.7%) of infants with SBIs and 14 /778 (1.8%) without SBIs required high acuity (HD and ICU) care (p = 0.407). One child required 10 days of non-invasive ventilation and inotropic support. This 31-day old infant had GBS bacteremia and meningitis, and had a prolonged hospital stay of 58 days. Data divided into training and testing sets are presented in Supplementary Tables 1 and 2.
Based on 994 children with complete data, we built FIRST and FIRST + scores using the AutoScore pipeline. After variable transformation and correlation elimination (as per Methods), the selected candidate variables as well as the importance ranking for each variable are described in Supplementary Figs. 1 and 2. Candidate variables were then used for score derivation, which provided cross-validated parsimony plots shown in Supplementary Figs. 3 and 4. The correlation analyses for FIRST and FIRST + are found in Supplementary Figs. 5 and 6.
Based on the performance of each variable in the parsimony plots and clinical discretion of variable usability, we built the multivariable logistic regression model for SBIs in our cohort (Supplementary Table 3). In FIRST, age (aOR 1.01 95%CI 1.01–1.02, p < 0.001), high temperature (aOR 2.22 95%CI 1.69–2.91, p < 0.001), male sex (aOR 2.62 95%CI 1.86–3.70, p < 0.001) and fever of 2 or more days (aOR 1.79 95%CI 1.18–2.74, p = 0.007) were independently associated with SBIs. In FIRST + , all triage variables remained significant except for fever of 2 or more days. In addition, abnormal urine leukocyte esterase (aOR 16.46 95%CI 10.00–27.11, p < 0.001) and procalcitonin (aOR 1.05 95%CI 1.01–1.09, p = 0.009) were independently associated with SBIs (Supplementary Table 3). The calibration results and conversion plots for FIRST and FIRST + are detailed in Supplementary Figs. 7–10, while the decision curve analysis can be found in Supplementary Fig. 11.
The final risk scores selected for FIRST and FIRST + are presented in Table 2. Age, temperature, sex and day of fever were selected for FIRST. We found a U-shaped risk relationship for age. There was increased risk < 21 days old and ≥ 28 days, when compared to infants 21 to < 28 days old. Temperature had a linear relationship with likelihood of SBI, with increased risk scores assigned as the temperature increased. Children with 2 days or more of fever were more likely to have SBI. Urine leukocyte esterase and procalcitonin were further selected for FIRST + . The greater the abnormality in urine leukocyte esterase and procalcitonin, the higher the likelihood of SBI (Table 2).
Taking various thresholds, we present the proportion of patients who would test positive, and the corresponding performance of FIRST and FIRST + (Table 3). For example, at a FIRST threshold of ≥ 15% predicted risk (FIRST cut-off score ≥ 30), the model had a sensitivity of 93.2% (95%CI 84.1–100%), NPV of 94.0% (95%CI 86.3–100%), corresponding specificity of 29.9% (95%CI 22.7–37%) and would classify 75% of patients as high risk for SBI. When laboratory investigations are available, a FIRST + threshold of ≥ 15% predicted risk (FIRST + cut-off score ≥ 36) had a sensitivity of 81.8% (95%CI 70.5–91.0%), NPV of 92.7% (95%CI 88.2–96.5%), corresponding specificity of 65.6% (95%CI 57.8–72.7%) and classify 45% as high risk for SBI. The FIRST and FIRST + scoring models performed with a ROC of 0.71 (95%CI 0.62–0.79) and 0.87 (95%CI 0.81–0.94) on the testing set, respectively (Fig. 2).
Area under receiver operating characteristic (ROC) curves of febrile infants risk score at triage (FIRST and FIRST +). *FIRST = Febrile Infants Risk Score at Triage. FIRST consists of Age, Temperature, Male Sex, Fever for 2 or more days FIRST + consists of Age, Temperature, Male Sex, Abnormal urine leukocyte esterase and procalcitonin.
Discussion
We studied 1002 febrile infants and reported an SBI rate of 22.4%, largely attributed to UTIs. Based on the Autoscore methodology, we derived FIRST, a triage predictive model that included age, temperature, sex and day of fever. We went on to derive and test FIRST + , based on availability of investigation results, and found that urine leukocyte esterase and procalcitonin were independently associated with SBIs. Adding on laboratory results improved the performance from a ROC of 0.71 (95%CI 0.62 – 0.79) (FIRST) to 0.87 (95%CI 0.81 – 0.94) (FIRST +) on the testing set.
The strength of our study is in the derivation and testing of an interpretable risk score. A previous supervised learning model for risk stratification of febrile infants acknowledged that it lacked parameter cutoffs and was computationally complex11. While machine learning models have promising performance compared to traditional scoring systems12,18, these have been difficult to translate to clinical practice because of the lack of recommended thresholds for action. In contrast, we assigned risk scores that quantified risk for SBI at each predictive risk threshold. Although our risk score requires refining and external validation, it can potentially guide clinical practice. Existing published clinical prediction rules have variable performance in different populations. A prior external validation of the PECARN rule in our population reported a sensitivity of 88.9%, specificity of 28.9%, and a ROC of 0.59 (0.42–0.76)19. These studies focus on identifying a group at low risk of SBI1, while our aim is to derive a tool that predicts for SBI, thereby serving as an adjunct to help clinicians prioritize which febrile infant requires urgent further investigations and management.
We reported the sensitivity and specificity at various thresholds (Table 3) to demonstrate how FIRST and FIRST + can aid clinicians to make informed decisions on disposition (hospitalization versus discharge), invasive investigations (including blood and CSF cultures), intervention (early antibiotics versus watchful waiting). These thresholds may vary based on physician practices and resource availability in different health services settings. For example, at the ED triage, most clinicians would favor a low threshold (one with a high sensitivity and NPV) to expedite care for the infant. A FIRST predictive threshold of 10% (score ≥ 21) with a sensitivity of 95.5% (95%CI 88.6–100%) and an NPV of 95.1% (86.8–100%) can prompt early consultation and close monitoring in the ED. Once these infants are examined and have initial laboratory investigations, clinicians may be willing to consider a higher threshold for action (FIRST +), one that has a higher specificity and will result in fewer infants subjected to more invasive tests. A major motivation would be to reduce unnecessary invasive blood and CSF tests. In this case, a FIRST + threshold of 15% (score ≥ 36) would have a higher specificity of 65.6% (95%CI 57.8–72.7%). In this case, 45% of infants would then be subject to further invasive investigations and empirical antibiotics.
We reported a higher prevalence of SBIs (22.4%) than another large multicenter cohort by the Pediatric Emergency Care Applied Research Network (PECARN) (9.3%)1. In the PECARN study, infants with clinical sepsis were excluded. However, clinician suspicion in this young infant population is notoriously inaccurate, hence we did not exclude these infants20. Also, our center is a pediatric tertiary institution (only one of two in the country) that receives walk-ins, as well as referrals from primary care and other hospitals. If the febrile infant was otherwise well with a known source of fever (e.g., respiratory symptoms likely secondary to an upper respiratory tract infection), the infant may have been managed in the primary care setting and not referred to our institution. Our findings must be interpreted in the context of this higher-than-expected SBI prevalence.
We found that the higher the temperature at triage, the more likely the febrile infant had an SBI, corresponding to higher risk scores. This is consistent with a retrospective cohort reported in our local population21. A multi-center study of 540 febrile infants similarly concluded that infants with IBIs had a higher median temperature compared to those without IBIs (38.8 °C vs. 38.4 °C)22. In our study, we only included infants who were febrile at triage. We did not account for infants who had fever at home but were afebrile at ED triage. It has been reported that infants who were afebrile at presentation to the ED but had fever at home had a significant risk of meningitis and other SBIs23. The presence and height of fever prior to ED attendance deserves further study.
The most common type of SBI in our study cohort was UTI, which is consistent with that of other study populations1. The diagnosis of UTI in a young febrile child can be challenging due to non-specific clinical presentation and the challenges associated with collecting a clean urine specimen24. In our study, we found that the presence of leukocyte esterase was independently associated with the presence of UTI. Besides leukocyte esterase, clinicians should also take into consideration the presence of nitrite in the urine dipstick, and urine white cell count when urinalysis results are available25. The large majority of febrile infants with UTIs were males (151/199, 81.2%), accounting for the male predominance in the SBI cohort (166/224,74.1%), overall. We did not have data on circumcision rates in this study cohort, and recognize that if circumcision rates were low, that may have contributed to the high UTI prevalence among male infants.
Procalcitonin has been widely reported to be useful in this population and has been included in risk stratification algorithms1,4. Procalcitonin has been reported as an early marker of infection, and elevated levels between 12 and 36 h of fever suggest the presence of an IBI in hospitalized febrile neonates 26. Our data-driven risk scores provided threshold cutoffs at 0.05, 0.36 and 4.38 (Table 2). Adding procalcitonin to a febrile infant clinical pathway resulted in decreased lumbar puncture for infants with at low risk of SBI26. However, more infants were assigned high risk and underwent laboratory investigations, resulting in no net change in overall resource utilization. This study highlights the need to study the implications of routine procalcitonin testing7,27.
We recognize the limitations to this study. Being a single-center study, the patient population is likely to differ from those of other centers, necessitating external validation and refinement of our risk score. We did not have culture results for all febrile infants (specifically infants 28–90 days old) because the decision on the extent of investigations was determined by the attending pediatrician. However, all febrile infants were hospitalized and monitored until 24 h afebrile before discharge, making a missed SBI less likely. Axillary temperature is the standard practice in our center, which is potentially confounded by over-wrapping and less accurate than rectal temperature. However, axillary temperature measurement is less invasive than rectal measurement, and the triage nurses are taught to take the axillary temperature with a single layer of clothing only. Our study dates included that of the COVID-19 pandemic when the presence of SARS-CoV-2 as well as the institution of national lockdowns could potentially have affected the prevalence of SBIs. The sensitivity of our model appears lower than some previously reported machine learning models11,12. However, in the FIRST methodology, all continuous variables are converted to categorical variables based on pre-specified cutoffs. This process of conversion likely reduced the prediction power of the continuous variables, accounting for a less ideal performance as compared to random forest models that applied continuous variables directly. Subsequent validation of this risk score must keep close surveillance on missed cases of SBI, quality of care and impact on health services28. Febrile infant populations vary depending on SBI prevalence, accessibility to care and healthcare-seeking behaviors. Some febrile infants may present within hours of the onset of fever, while others may present after the first day of fever. Therefore, risk scores need to be fine-tuned based on the unique characteristics of the local population.
Conclusion
We derived and internally validated clinical risk scores (FIRST and FIRST +) for febrile infants that quantify the risk of SBIs using routinely available clinical predictors. If externally validated, this risk score has potential to guide triage and prioritization of febrile infants.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- ANC:
-
Absolute neutrophil count
- CRP:
-
C-reactive protein
- CSF:
-
Cerebrospinal fluid
- ED:
-
Emergency department
- GBS:
-
Group B streptococcus
- HD:
-
High dependency
- IBI:
-
Invasive bacterial infection
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristics
- SBIs:
-
Serious bacterial infection
- UTI:
-
Urinary tract infection
References
Kuppermann, N. et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 173, 342–351 (2019).
Yao, S. H. W., Ong, G. Y. K., Maconochie, I. K., Lee, K. P. & Chong, S. L. Analysis of emergency department prediction tools in evaluating febrile young infants at risk for serious infections. Emerg. Med. J. 36, 729–735 (2019).
Cantey, J. B., Lopez-Medina, E., Nguyen, S., Doern, C. & Garcia, C. Empiric antibiotics for serious bacterial infection in young infants: Opportunities for stewardship. Pediatr. Emerg. Care 31, 568–571 (2015).
Mintegi, S. et al. Accuracy of a sequential approach to identify young febrile infants at low risk for invasive bacterial infection. Emerg. Med. J. 31, e19-24 (2013).
Aronson, P. L. et al. A prediction model to identify febrile infants ≤60 days at low risk of invasive bacterial infection. Pediatr. 144, e20183604 (2019).
Geanacopoulos, A. T. et al. Declines in the number of lumbar punctures performed at United States children’s hospitals, 2009–2019. J. Pediatr. 231, 87-93.e1 (2021).
Coyle, C., Brock, G., Wallihan, R. & Leonard, J. C. Cost analysis of emergency department criteria for evaluation of febrile infants ages 29 to 90 days. J. Pediatr. 231, 94-101.e2 (2021).
Yang, J. et al. Delays in time-to-antibiotics for young febrile infants with serious bacterial infections: A prospective single-center study. Front. Pediatr. 10, 873043 (2022).
Bhaskar, V., Batra, P. & Mahajan, P. Identifying serious bacterial infections in febrile young infants. Indian Pediatr. 58, 15–19 (2021).
Velasco, R., Gomez, B., Benito, J. & Mintegi, S. Accuracy of PECARN rule for predicting serious bacterial infection in infants with fever without a source. Arch. Dis. Child. 106, 143–148 (2021).
Ramgopal, S., Horvat, C. M., Yanamala, N. & Alpern, E. R. Machine learning to predict serious bacterial infections in young febrile infants. Pediatrics 146, e20194096 (2020).
Chiu, I.-M., Cheng, C.-Y., Zeng, W.-H., Huang, Y.-H. & Lin, C.-H.R. Using machine learning to predict invasive bacterial infections in young febrile infants visiting the emergency department. J. Clin. Med. 10, 1875 (2021).
Xie, F., Chakraborty, B., Ong, M. E. H., Goldstein, B. A. & Liu, N. AutoScore: A machine learning-based automatic clinical score generator and its application to mortality prediction using electronic health records. JMIR Med. informatics 8, e21798 (2020).
Xie, F. et al. Development and assessment of an interpretable machine learning triage tool for estimating mortality after emergency admissions. JAMA Netw. open 4, e2118467 (2021).
Nelson, K. G. An index of severity for acute pediatric illness. Am. J. Pub. Health 70, 804–807 (1980).
Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 48, 193–204 (2014).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for iniividual prognosis or diagnosis (TRIPOD): The TRIPOD statemnet. Ann. Intern. Med. 162, 600 (2015).
Lee, B., Chung, H. J., Kang, H. M., Kim, D. K. & Kwak, Y. H. Development and validation of machine learning-driven prediction model for serious bacterial infection among febrile children in emergency departments. PLoS ONE 17, e0265500 (2022).
Sutiman, N., Khoo, Z. X., Ong, G. Y. K., Piragasam, R. & Chong, S. L. Validation and comparison of the PECARN rule, step-by-step approach and the lab-score for predicting serious and invasive bacterial infections in young febrile infants. Ann. Acad. Med. Singap. 51, 595–604 (2022).
Nigrovic, L. E. et al. The yale observation scale score and the risk of serious bacterial infections in febrile infants. Pediatrics 140, e20170695 (2017).
Victoria, T. S. R., Yong-Kwang, O. G., Pin, L. K., Sashikumar, G. & Shu-Ling, C. Pyrexia in a young infant—is height of fever associated with serious bacterial infection?. BMC Pediatr. 22, 188 (2022).
Michelson, K. A. et al. Height of fever and invasive bacterial infection. Arch. Dis. Child. 106, 594–596 (2021).
Orfanos, I., Fernandez, J. S., Elfving, K., Alfvén, T. & Eklund, E. A. Paediatric emergency departments should manage young febrile and afebrile infants the same if they have a fever before presenting. Acta Paediatr. 111, 2004–2009 (2022).
Ramsay, J. A. et al. Urinary tract infections in children: Building a causal model-based decision support tool for diagnosis with domain knowledge and prospective data. BMC Med. Res. Methodol. 22, 218 (2022).
Boon, H. A., Struyf, T., Bullens, D., Van den Bruel, A. & Verbakel, J. Y. Diagnostic value of biomarkers for paediatric urinary tract infections in primary care: Systematic review and meta-analysis. BMC Fam. Pract. 22, 193 (2021).
Romain, A.-S. et al. Procalcitonin at 12–36 hours of fever for prediction of invasive bacterial infections in hospitalized febrile neonates. Front. Pediatr. 10, 968207 (2022).
Widmer, K. et al. Use of Procalcitonin in a febrile infant clinical pathway and impact on infants aged 29 to 60 days. Hosp. Pediatr. 11, 223–230 (2021).
Foster, L. Z. et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr. Qual. Saf. 5, e252 (2020).
Funding
This work was supported by the National Medical Research Council, Singapore (NMRC) NMRC/MOH-CNIG18May 0005 (PI: S-LC). The funders had no role in the study design, data collection, analysis and interpretation of the data, writing of the report, and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by S-LC, CN, RP, ZXK (Khoo), ZXK (Koh), DG and NL. Data collection was performed by S-LC and RP. Data analysis were performed by S-LC, CN, GY-KO, JHL, MEHO and NL. The first draft of the manuscript was written by S-LC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chong, SL., Niu, C., Ong, G.YK. et al. Febrile infants risk score at triage (FIRST) for the early identification of serious bacterial infections. Sci Rep 13, 15845 (2023). https://doi.org/10.1038/s41598-023-42854-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42854-z
- Springer Nature Limited