Abstract
Systemic lupus erythematosus (SLE) is a complicated chronic autoimmune disorder. Several genetic and environmental factors were suggested to be implicated in its pathogenesis. The main objective of this study was to examine how exposure to selected environmental factors was associated with SLE risk to support the development of disease preventive strategies. A case–control study was conducted at the Rheumatology outpatient clinic of Alexandria Main University Hospital, in Alexandria, Egypt. The study sample consisted of 29 female SLE patients, and 27 healthy female controls, who matched the cases on age and parity. Data were collected by a structured interviewing questionnaire. Blood levels of lead, cadmium, and zinc of all participants were assessed by flame atomic absorption spectrometry. The multivariate stepwise logistic regression model revealed that five factors showed significant association with SLE, namely living near agricultural areas, passive smoking, blood lead levels ≥ 0.075 mg/L, and exposure to sunlight (odds ratio (OR) 58.556, 95% confidence interval (CI) 1.897–1807.759, OR 24.116, 95% CI 1.763–329.799, OR 18.981, 95% CI 1.228–293.364, OR 9.549, 95% CI 1.299–70.224, respectively). Whereas walking or doing exercise were significantly protective factors (P = 0.006). The findings of this study add to the evidence that SLE can be environmentally induced. Preventive measures should be taken to address the environmental risk factors of SLE.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a chronic rheumatic autoimmune disorder that could be manifested by many symptoms. It is a multi-system disease that may involve nearly any organ resulting in serious organ complications and even death1,2. It predominantly affects women in the child-bearing ages3. SLE was suggested to be a condition of multifactorial etiology; including genetics, hormones, and environmental exposures.
It is currently known that different environmental factors could trigger SLE onset and flares in genetically susceptible individuals4,5. Owing to the increasing prevalence and overall SLE burden, efforts have been made to recognize these genetic and non-genetic factors6.
The role of the environment was more prominent by the fact that SLE concordance among identical monozygotic twins is below 25%7. In addition, the contribution of environmental factors to SLE risk has been evaluated to constitute 56%8.
Smoking, silica dust, UV radiation, infections, stress, air pollution, pesticides, and heavy metals are the major environmental risk factors having some evidence for association with SLE9.
In recent years, heavy metal pollution has become a significant health issue; continuous exposure to low levels of these toxic trace elements may result in bioaccumulation and produce a wide variety of biological effects on human beings10.
Lead and cadmium are known to pose serious risks to human health. Toxicity of these agents is evidenced by being identified in the top 10 environmental hazards by the Agency for Toxic Substances and Disease Registry11.
Environmental sources of lead include inhalation of airborne dusts containing lead and ingestion through food or water contaminated by lead. Old deteriorating household paints, and lead use in some traditional medicines and cosmetics can also be a source of lead exposure12. In addition, active and passive smoking were found to be associated with increased blood lead levels13.
Cadmium is present in cigarette smoke, air, food, and water. It can enter human bodies through inhalation, ingestion and dermal contact14.
Experimental studies of lead and cadmium exposure in rodent models proposed that metals may play a causative role in SLE15,16. There is relatively little data pertaining to lead or cadmium exposure with the risk of SLE in humans; exposure to stained or leaded glass as a hobby was found to be more common among SLE cases than controls17.
Trace elements such as zinc play a crucial role in growth and development of all organisms. Zinc is the second most abundant trace metal in the human body after iron, but zinc cannot be stored and has to be taken up daily via food to guarantee sufficient supply. However, it was stated that zinc excess as well as zinc deficiency may result in severe disturbances in immune cell numbers and activities leading to immune dysfunction18.
A study by Sahebari et al.19 found that serum Zn values were lower in SLE patients than healthy age and sex-matched controls. On the other hand, zinc-deficient diets retarded autoantibody production and enhanced survival in mice20. In their review, Constantin et al.21 advised to restrict consumption of some minerals such as zinc and sodium.
As the data about the relation between environmental risk factors and SLE in Egypt are lacking and the incidence is increasing, the present study was proposed to determine the association between some environmental exposures with SLE risk; in order to assess the extent to which SLE is environmentally induced.
Results
Estimation of SLE risk in relation to some socio-demographic characteristics
Analysis of data regarding the socio-demographic characteristics revealed that patients and controls were similar in terms of demographic background, no statistical significant difference between cases and controls except for the education level, the occupation, and the residence near agricultural areas (Tables 1, 2).
Regarding the educational level, there was a statistically significant difference between cases and controls (P < 0.05). The percentage of illiterate cases was (31%) and constituted more than double their percentage in the control group (14.8%). Also, the percentage of cases with higher education was only (3.4%), which is very low compared to controls (14.8%). The findings showed that females with SLE who were in the primary or preparatory educational level were significantly more likely to be at risk of SLE (OR 14.67, 95% CI 1.16–185.23) compared to controls.
With regard to occupation, there was a statistically significant difference between cases and controls (P < 0.05), the majority of cases were housewives (86.2%) compared to (33.3%) in the controls group.
Estimation of SLE risk in relation to some lifestyle factors
Lack of physical activity, exposure to domestic animals, or to sun light showed significant results (Table 3). As regards walking and physical activity, a high proportion of SLE patients (89.7%) do not like to walk or perform any physical activity compared to (40.7%) in the control group.
Sedentary lifestyle increased SLE risk by 12.61 folds (OR 12.61, 95% CI 3.05–52.17). Dealing with domestic animals was another risk factor that was tested in the current study; the results showed that 58.6% of cases were exposed to animals more than controls (29.6%). This may be because 48.3% of cases live in rural areas compared to 7.4% of controls. There was a statistically significant increase in SLE risk when dealing with farm animals (sheep, chicken) (OR 3.36, 95% CI 1.11–10.19).
Results about UV radiation exposure and SLE risk showed a statistically significant increase of SLE risk (OR 13.714, 95% CI 3.768–49.920).
Estimation of SLE risk in relation to some indoor environmental risk factors
The risk values for the association between some indoor environmental exposures and SLE were statistically not significant except for the type of drinking water, the filled tube cooker as the fuel used at home, and passive smoking (Table 4); 62.1% of SLE patients use tap water for drinking compared to 22.2% of controls, meanwhile, the majority of controls (74.1%) use filtered water compared to 37.9% of cases. The use of filtered water for drinking was assumed to have protective effects against SLE.
A statistically significant difference (X2 = 16.021, P = 0.001) between cases and controls with regard to fuel used at home, where 79.3% of cases reported the use of gas cylinders compared to 25.9% of controls. The use of gas cylinders constituted 10.95 times more risk to SLE than the use of natural gas pipes (OR 10.95, 95% CI 3.16–38.01).
Regarding passive smoking at home, which was assessed by the number of active smokers who were residing in the same house, (62.1%) of cases were exposed to Environmental Tobacco Smoke (ETS) versus (29.6%) of controls, there was a statistically significant difference between cases and controls with regard to exposure to ETS (OR 3.886, 95% CI 1.273–11.861).
Moreover, SLE risk increased with the increase in the number of cigarettes smoked per day (the risk was 2.96 and 10.36 times for light and heavy passive smoking (OR 2.96, 95% CI 0.9–9.75; OR 10.36, 95% CI 1.1–97.69 respectively). A significant trend for risk was noticed with increased passive smoking (X2 for trend = 5.87, P = 0.015) concluding that ETS may be an important risk factor for SLE.
Estimation of SLE risk in relation to a family history of any auto-immune disease
The study found a statistically significant difference between cases and controls regarding the family history of any auto immune disease as illustrated in (Table 5), 33.3% of controls had surprisingly a family history of an auto immune disease including SLE compared to 3.4% of cases.
Estimation of SLE risk in relation to hormonal factors
The findings of the present study showed the prevalence of menstrual disorders and hormonal disturbances among SLE patients as demonstrated in Table 5. Early menopause was present in (31%) of SLE patients compared to (0%) of controls, irregular menstrual cycle was prevalent in (40%) of SLE patients compared to (8%) of controls, (37.9%) of cases used hormonal therapy compared to (11.1%) of controls, and (31%) of cases had problems in uterus versus (7.4%) of controls.
There was a statistically significant increase in SLE risk with early menopause, menstrual irregularities, use of hormonal therapy, and presence of problems in uterus (OR 31.26, 95% CI 1.7–575.54; OR 7.67, 95% CI 1.3–45.29; OR 4.889, 95% CI 1.19–20.13; OR 5.625, 95% CI 1.09–29.03 respectively).
Estimation of SLE risk in relation to blood levels of lead, cadmium and zinc
As shown in (Table 6), the range of values of blood lead levels (Pb) in the cases group was very extensive from minimum 0.0237 mg/L to maximum 0.6951 mg/L and it is higher than the range of values in the controls group. The median concentration of blood lead in the cases group (0.115 ± 0.165) was significantly higher than in the controls group (0.067 ± 0.077). There were significantly higher blood lead levels in the cases group compared to the controls group (U = 210, P = 0.003).
After categorization of the levels of blood lead in the sample, the SLE risk associated with blood lead levels ≥ 0.09 mg/L was higher and statistically significant (OR 7.71, 95% CI 1.85–32.21) in comparison with subjects having blood lead levels < 0.05 mg/L. A statistically significant trend was computed (Chi-square trend for odds = 7.77, P value = 0.005) showing that the more the increase in blood lead level, the more the chance of SLE occurrence.
The median levels of blood cadmium (Cd) in cases group (0.059 ± 0.102 mg/L) were significantly higher than in the controls group (0.017 ± 0.042 mg/L) as U = 216, P = 0.004.
As presented in (Table 6), the level of blood cadmium in the sample was categorized into groups, females having blood cadmium levels from 0.03 to less than 0.07 and ≥ 0.07 mg/L blood had 6.68 and 4.45 times more risk to develop SLE in comparison with females having blood cadmium levels < 0.03 mg/L blood and the risks for these upper two categories were statistically significant (95% CI 1.58–28.29; 1.18–16.8 respectively). The observed increased trend was found to be statistically significant (X2 trend for odds = 4.98, P = 0.026).
Regarding blood zinc levels (Zn), the median concentration of zinc in the controls group (2.275 ± 0.707 mg/L blood) was lower than the median concentration of zinc in the cases group (2.660 ± 0.970 mg/L blood), but the median blood zinc values in the cases and controls groups did not differ significantly (U = 290.5, P > 0.05).
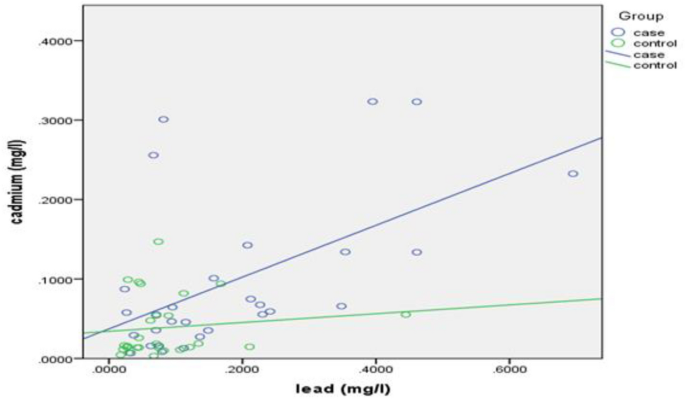
Pearson’s correlation coefficient was calculated to demonstrate the relationship between lead and cadmium blood levels with each other and yielded a result of r = 0.548, P = 0.002, portraying a positive, moderate, linear, and significant correlation between lead and cadmium blood levels for the cases group (Fig. 1), whereas a non-significant correlation was observed in the controls group (r = 0.123, P = 0.542).
Analysis of risk factors affecting SLE risk by stepwise logistic regression
A multivariate stepwise logistic regression model was built in order to determine which of these predictors really contribute to predicting SLE risk, and exclude those who do not.
Negelkerke R Square suggests that the model explains 78.4% of the variance in the outcome. The accuracy of the model was 87.5%, depicting that the model can correctly classify 87.5% of the cases. The sensitivity and specificity of the model were calculated, and they were 86.2%, and 88.9%, respectively.
As illustrated in (Table 7), the final model revealed that only five factors showed significant association with SLE. The risk was highest for subjects living near agricultural areas with an OR of 58.556 (95% CI 1.897–1807.759), followed by subjects exposed to passive smoking with an OR of 24.116 (95% CI 1.763–329.799), then subjects having blood lead levels ≥ 0.075 mg/L who were 18.981 times (95% CI 1.228–293.364) more likely to have SLE than those having blood lead levels < 0.075 mg/L, followed by subjects exposed to the sunlight who were at increased risk of SLE by 9.549 (95% CI 1.299–70.224). Whereas walking or doing exercise were significantly protective against SLE (P = 0.006); the table demonstrated a negative B coefficient (− 5.246) which indicates that a decrease in the walking and exercise is associated with a greater likelihood of SLE risk.
Discussion
The current study purpose was to gain insights into the etiology of SLE and some possible risk factors.
The statistically significant difference between cases and controls regarding the education level assumes that lower education level—which is an indicator of socioeconomic status—may constitute a risk for SLE. That is similar to other studies that showed the association between lower education level and SLE onset and flare22,23.
The distribution of the study sample according to the residence area revealed that 48.3% of cases reported living near agricultural areas compared to 7.4% of controls, with 11.67 times more SLE risk (OR 11.67, 95% CI 2.32–58.6) compared to those not living near these areas. This may be attributed to some environmental exposures such as exposure to sunlight and pesticides used in agriculture, in addition to lower socioeconomic status, lower educational level, and poverty. This finding is in accordance with Pons-Estel et al. (2012) who concluded that rural residence was associated with high levels of disease activity at diagnosis and with renal disease occurrence in a Latin American multi-ethnic cohort (OR 1.65, 95% CI 1.06–2.57; OR 1.77, 95% CI 1.00–3.11)24.
Our data showed an increase of SLE risk due to sedentary lifestyle which is in accordance with a cross sectional study that showed that a high proportion of SLE patients were physically inactive with a long daily sedentary time25.
The statistically significant increase in SLE risk when dealing with animals or birds was in the same line with a case control study in Southern Sweden that reported a statistically significant difference between cases and controls regarding close contact with sheep26. This was not in the same line with the results of a recent study which supported the idea that exposures related to childhood farm residence and livestock farming may decrease susceptibility to developing SLE and that contact with livestock may confer protection against SLE27.
Current study findings support the role of sun exposure as a trigger for SLE, adding evidence to experimental and human studies that have shown that it can trigger disease onset and induce disease flares in SLE patients26,28,29.
ETS as a risk factor for SLE was not enormously previously discussed except for a cross sectional study of Brazilian SLE patients that assessed the association between smoking and SLE and confirmed that never smokers confer a 22% relative SLE risk reduction compared to ever smokers (including second hand smokers)30.
In addition, data from a cohort of SLE patients and controls suggested that secondhand smoke during childhood may be a risk factor for SLE (OR 1.81, 95% CI 1.13–2.89)31. Whereas, a US prospective cohort study concluded that Early-life exposure to cigarette smoke due to mothers’ or fathers’ smoking did not increase the risk of adult-onset SLE (RR 0.9, 95% CI 0.6–1.4; RR 1.0, 95% CI 0.8–1.3 respectively)32.
Our data revealed that the highest proportion of the study patients (96.6%) had negative family history of SLE. This supports the great contribution of other risk factors rather than the genetic factors in the development of SLE; suggesting the influence of environmental triggers on disease expression8,17. This finding was in line with a study that reported that autoimmune diseases in family members have not been associated with SLE33. On the other hand, a previous case control study reported the prevalence of auto-immune disease in first degree relatives in cases more than controls (53% vs 39%) and stated that a family history of any auto-immune disease was associated with increased risk of SLE (OR 6.8, 95% CI 1.4–32)26. This finding is also in contrast with other previous studies that stated that family history of an auto immune disease in first degree relatives (parents or siblings) was associated with increased SLE risk34,35.
The results of the current study regarding SLE risk in relation to hormonal factors was in concordance with a cohort study that revealed that menstrual irregularity was associated with an increased SLE36. In the same line comes a cross sectional study of 61 SLE patients, in which 49.2% of the patients had menstrual irregularities, of which 60% had sustained amenorrhoea (premature menopause) compared to the control group (16.7%)37. Moreover, a cross sectional study (N = 87) showed menstrual alterations in 37.9% of SLE patients and amenorrhea in 11.5% of patients which was higher than the general population38. Additionally, an increased SLE risk was reported in the NHS (Nurses’ Health Study) with the use of estrogen replacement therapy39. Moreover, a population‐based nested case control study found that the current use of COCs (Combined Oral Contraceptives) was associated with an increased SLE risk (RR 1.54, 95% CI 1.15–2.07)40.
Other studies contradicted the findings of the current study and stated that the use of HRT (Hormone Replacement Therapy) in postmenopausal SLE women did not appear to increase the rate of lupus flares and appeared to be well tolerated and safe in postmenopausal SLE patients41,42.
Data from experimental studies suggested that heavy metals may enhance systemic autoimmunity or accelerate disease progression in experimental models of lupus and that co-exposure to certain heavy metals may increase the risk associated with other exposures5.
Detailed studies on SLE onset and flares with reference to lead, cadmium, and zinc are scanty. Therefore, the current study was conducted to evaluate the role of lead, cadmium, and zinc in SLE. All blood samples were found to have lead, cadmium, and zinc concentrations.
It was observed that the median blood lead levels in the cases group as well as the controls group in this study were higher than the CDC permissible range of less than 5 µg/dL of lead in children and adults (0.05 mg/L) and this is indicative of the extent of environmental lead pollution43. It was also noticed that the median blood cadmium levels for both cases and controls in this study were higher than the WHO permissible range of 0.03–0.12 µg/dL of Cd44. This suggests more protection measures to be taken into consideration in order to avoid the toxic effects of lead and cadmium.
It was observed that the median blood zinc levels were not consistent with reference ranges of zinc in blood (70–120 µg/dL)45, requiring further consideration of zinc levels to avoid overdose and toxicity.
In contrast to the current study is a recent similar case control study that reported that SLE diagnosis was associated with lower serum Zn (P = 0.003), and Pb (P = 0.020)46. Other studies reported similar results47,48. However, some studies did not observe a significant difference in serum Zn concentrations between SLE patients and healthy controls which is similar to the finding of the present study49,50.
A case control study reported a positive correlation between lead and cadmium blood levels for the exposed group (ρ = 0.39, P = 0.023) which was consistent with the finding of the current study, whereas blood zinc levels correlated negatively with both lead (ρ = − 0.41, P = 0.015) and cadmium blood levels (ρ = − 0.44, P = 0.009). In addition, no correlations between the studied metals were found in the control group and the study suggested that zinc insufficiency is more likely to occur in cases of combined exposure to cadmium and lead, because of competition between similar ions for receptors involved in absorption, transport, storage or function, and this would explain the negative correlation in the controls group between zinc blood levels with either lead and cadmium levels51.
Limitations of the study
-
The potential of case control studies for recall bias and misclassification error; as most exposure information were based on self-reported history so some inaccuracies can be expected. The design of the study also limited the ability to ascribe causal relationships to the associations detected and to control for all potential confounders.
-
Absence of genetic information for participants, so the potential effects of genetic heterogeneity on the association between risk factors and SLE risk could not be determined.
-
Human population is rarely exposed to a single agent over time, and there may be a significant delay between exposure and the onset of the disease.
-
The small sample size may make it difficult to determine if a particular outcome is a true finding and in some cases no difference between the study groups is reported.
Conclusion and recommendations
To date, our knowledge about the etiology of SLE is still unclear and limited; genetic and environmental interactions were suggested.
From the present case control study, it is concluded that the risk portion attributed to unsafe and unhealthy environment was found to be quite significant, showing how the environment can play an important role in SLE occurrence.
Exposure to potential environmental risk factors specifically heavy metals should not be under-estimated. Hence, there is an urgent need for interventions to reduce environmental risk factors exposure in order to achieve substantial public health gains.
Increasing the awareness of patients about the environmental pollutants and the ways to protect their health is necessary. As well as the awareness of health care providers about environmental risk factors, so they can advise the patients about the ways of reduction of exposure to environmental hazards. Educational awareness programs to patients and their family should be carried out through media and non-governmental organizations (NGOs) to raise their knowledge about environmental hazards and how to minimize the sources of their exposure and their consequent negative impacts.
Additional experimental and epidemiological studies are required to determine the causative role of several environmental exposures, to confirm data from case control studies.
Methods
This case control study was conducted at the Rheumatology outpatient clinic of Alexandria Main University Hospital. The Inclusion criteria were female patients diagnosed with idiopathic SLE according to SLICC criteria52. The controls included healthy females who accompanied the SLE patients who came from remote rural areas in their visit to the Rheumatology outpatient clinic. The cases and controls were matched for age and parity.
Exclusion criteria included (1) male SLE patients “they were excluded mainly to avoid gender bias that may affect the results due to the hormonal effect. Besides, the disease affects mainly females”; (2) drug-induced Lupus; (3) overlap syndrome as lupus and rheumatoid arthritis; (4) any other rheumatic diseases; (5) coexisting morbidity not related to SLE, e.g. diabetes, hypertension; (6) cancer; (7) dementia or psychosis; (8) intake of nutritional supplements in the 6 months prior to the blood collection.
Sample size
Based on a previous case control study, the mean of serum Zinc among systemic lupus erythematosus (SLE) was 700.61 ± 135.91 and among controls was 860.45 ± 123.74, using an alpha error of 0.05 and power 98%48. The minimum required sample size was estimated to be 46 adults, 23 for each group, which was increased to 56 adults, 29 cases and 27 controls. The sample size was calculated using G. Power software.
Data collection methods and tools
-
1.
A pre-designed pre-coded structured interviewing questionnaire was used to collect data from all participants (cases and controls). It included personal and socio-demographic data, data about occupation, lifestyle factors, the medical history, the smoking history including exposure to passive smoking, and some possible indoor environmental risk factors aiming to ascertain exposure to some environmental risk factors suspected to affect SLE risk. Regarding the evaluation of poor water quality, the participants were asked about the availability and quality of drinking water, any problems concerning drinking water (clarity, taste, smell), the type of drinking water pipes (with or without lead) by asking them whether they are old or newly installed, and the drinking water source (filtered, bottled, or tap water). Whereas for sun exposure, they were asked about the daily exposure to the sun, duration of exposure, wearing of protective clothes, and use of sunscreen, as well as whether there is an occupational sun exposure. Concerning the evaluation of sedentary lifestyle, they were questioned whether they perform any physical activity and the duration per week, whether they prefer walking or taking the car, and the duration of watching TV.
Questions of the questionnaire were taken from similar previously validated Arabic and English research questionnaires26,53. In addition, it was assessed by an expert at the faculty of medicine, Alexandria University. The English version of the questionnaire underwent a forward and back translation by native speakers whom are experts in public health.
-
2.
Laboratory investigation
A blood sample (5 mL) was drawn from each participant to measure blood levels of lead, cadmium, and zinc. The samples were transferred into heparinized collection tubes. Cadmium, lead, and zinc were extracted from the blood samples using the conventional wet acid digestion method using concentrated nitric acid (HNO3). A blank using deionized water instead of blood was done for each batch of analysis for comparison44,54. The digested samples were filtered and were subjected to elemental analysis using flame atomic absorption spectrophotometer (Shimadzu model AA-6650) at the central laboratory of the High Institute of Public Health (HIPH), Alexandria University.
Statistical design
SPSS version 21 was used for data entry and analysis. Qualitative variables were described through number and percentages of cases. For quantitative continuous variables, tests of normality were done. Mean and standard deviation (mean ± SD) were calculated if the variable follows normal distribution, and median and interquartile range (median ± IQR) if it does not follow normal distribution. “Pearson’s Chi-square test (X2)” was used to calculate significant differences between cases and controls for the categorical data. If the assumptions were violated, “Fisher’s exact test” (if 2*2 table) or “Monte Carlo test” (if m*n table) were used.
Differences between the means of the two groups were examined using independent t test for the continuous, normally distributed variables. The Mann–Whitney–Wilcoxon non-parametric test (U), for the continuous, non-normally distributed variables. Odds ratio (OR) was calculated to measure SLE risk. Increasing trends in SLE risk concerning some risk factors were tested using “Chi-square for trend”. Pearson’s correlation coefficient was used to test the association between quantitative variables.
A multivariate stepwise logistic regression analysis was used to see if there were significant associations between specific exposures and SLE and to adjust for some potential confounders. Negelkerke R2 was calculated to tell the amount of variation in SLE risk which is explained by the model.
Ethical approval
Approval of the Ethics Committee of the High Institute of Public Health for conducting the research was obtained. Approval for conducting the study at the Rheumatology Outpatient Clinic of Alexandria Main University Hospital was obtained from the hospital outpatient clinics director after a formal written request for that. The study was performed in accordance with the ethical standards in the Declaration of Helsinki. A written informed consent was taken from all study participants after explanation of the purpose and benefits of the research. Anonymity and confidentiality were ensured. All methods were performed in accordance with the relevant guidelines and regulations.
Data availability
The datasets used and/or analyzed during the current study are not publicly due to privacy and ethical concerns but are available from the corresponding author on reasonable request.
References
Ferenkeh-Koroma, A. Systemic lupus erythematosus: Nurse and patient education. Nurs. Stand. 26(39), 49–57. https://doi.org/10.7748/ns2012.05.26.39.49.c9134 (2012).
Zucchi, D. et al. Systemic lupus erythematosus: One year in review 2023. Clin. Exp. Rheumatol. 41(5), 997–1008. https://doi.org/10.55563/clinexprheumatol/4uc7e8 (2023) (Epub 2023 May 3).
Lai, Y. et al. Different pregnancy outcomes in patients with systemic lupus erythematosus treated with belimumab. Lupus 32(1), 149–154. https://doi.org/10.1177/09612033221141805 (2023) (Epub 2022 Dec 5).
Woo, J. M. P., Parks, C. G., Jacobsen, S., Costen-bader, K. H. & Bernatsky, S. The role of environmental exposures and gene–environment interactions in the etiology of systemic lupus erythematous. J. Intern. Med. 291, 755–778 (2022).
Barbhaiya, M. & Costenbader, K. H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 28(5), 497–505. https://doi.org/10.1097/BOR.0000000000000318 (2016).
Gergianak, I. et al. Epidemiology and burden of systemic lupus erythematosus in a Southern European population: Data from the community-based lupus registry of Crete, Greece. Ann. Rheum. Dis. 76, 1992–2000. https://doi.org/10.1136/annrheumdis-2017-211206 (2017).
Bhaskar, L. V. K. S. & Nagaraju, G. P. Clinical and immunogenetic aspects of systemic lupus erythematosus. Crit. Rev. Immunol. 39(5), 343–360. https://doi.org/10.1615/CritRevImmunol.2020033247 (2019).
Leffers, H. C. B., Lange, T., Collins, C., Ulff-Moller, C. J. & Jacobsen, S. The study of interactions between genome and exposome in the development of systemic lupus erythematosus. Autoimmun. Rev. 18(4), 382–392. https://doi.org/10.1016/j.autrev.2018.11.005 (2019).
Gulati, G. & Brunner, H. I. Environmental triggers in systemic lupus erythematosus. Semin. Arthritis Rheum. 47(5), 710–717. https://doi.org/10.1016/j.semarthrit.2017.10.001 (2018).
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B. & Beeregowda, K. N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7(2), 60–72. https://doi.org/10.2478/intox-2014-0009 (2014).
ATSDR [internet]. ATSDR’s Substance Priority List. 2022. https://www.atsdr.cdc.gov/spl/index.html.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metal toxicity and the environment. Exp. Suppl. 101, 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6 (2012).
Mannino, D. M., Homa, D. M., Matte, T. & Hernandez-Avila, M. Active and passive smoking and blood lead levels in US adults: Data from the Third National Health and Nutrition Examination Survey. Nicotine Tob. Res. 7(4), 557–564. https://doi.org/10.1080/14622200500185264 (2005).
Virani, S. et al. DNA methylation is differentially associated with environmental cadmium exposure based on sex and smoking status. Chemosphere 145, 284–290. https://doi.org/10.1016/j.chemosphere.2015.10.123 (2016).
Hudson, C. A., Cao, L., Kasten-Jolly, J., Kirkwood, J. N. & Lawrence, D. A. Susceptibility of lupus-prone NZM mouse strains to lead exacerbation of systemic lupus erythematosus symptoms. J. Toxicol. Environ. Health Part A 66(10), 895–918. https://doi.org/10.1080/15287390306456 (2003).
Leffel, E. K., Wolf, C., Poklis, A. & White, K. L. Drinking water exposure to cadmium, an environmental contaminant, results in the exacerbation of autoimmune disease in the murine model. Toxicology 188(2–3), 233–250. https://doi.org/10.1016/s0300-483x(03)00092-1 (2003).
Kamen, D. L. Environmental influences on systemic lupus erythematosus expression. Rheum. Dis. Clin. N. Am. 40(3), 401–412. https://doi.org/10.1016/j.rdc.2014.05.003 (2014).
Maywald, M., Wessels, I. & Rink, L. Zinc signals and immunity. Int. J. Mol. Sci. 18(10), 2222. https://doi.org/10.3390/ijms18102222 (2017).
Sahebari, M. et al. Association between serum trace element concentrations and the disease activity of systemic lupus erythematosus. Lupus 23(8), 793–801. https://doi.org/10.1177/0961203314530792 (2014).
Beach, R. S., Gershwin, M. E. & Hurley, L. S. Nutritional factors and autoimmunity. II. Prolongation of survival in zinc-deprived/W mice. J. Immunol. 128(1), 308–313 (1982).
Constantin, M. M. et al. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis. Exp. Ther. Med. 17(2), 1085–1090. https://doi.org/10.3892/etm.2018.6986 (2019).
Ward, M. M. Education level and mortality in systemic lupus erythematosus (SLE): Evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheumatol. 51(4), 616–624. https://doi.org/10.1002/art.20526 (2004).
Zhang, L. et al. Lack of patient education is risk factor of disease flare in patients with systemic lupus erythematosus in China. BMC health Serv. Res. 19(1), 378. https://doi.org/10.1186/s12913-019-4206-y (2019).
Pons-Estel, G. J. et al. The impact of rural residency on the expression and outcome of systemic lupus erythematosus: Data from a multiethnic Latin American cohort. Lupus 21(13), 1397–1404. https://doi.org/10.1177/0961203312458465 (2012).
Margiotta, D. P. E. et al. Physical activity and sedentary behavior in patients with Systemic Lupus Erythematosus. PLoS One 13(3), e0193728. https://doi.org/10.1371/journal.pone.0193728 (2018).
Bengtsson, A. A., Rylander, L., Hagmar, L., Nived, O. & Sturfelt, G. Risk factors for developing systemic lupus erythematosus: A case–control study in southern Sweden. J. Rheumatol. (Oxf). 41(5), 563–571. https://doi.org/10.1093/rheumatology/41.5.563 (2002).
Parks, C., Long, S., Beane-Freeman, L., Jonathan, H. & Dale, S. Systemic lupus erythematosus and Sjögren’s syndrome in the agricultural health study: Lower risk associated with childhood farm residence and raising livestock [abstract]. Arthritis Rheumatol. 71, 10 (2019).
Mak, A. & Tay, S. H. Environmental factors, toxicants and systemic lupus erythematosus. Int. J. Mol. Sci. 15(9), 16043–16056. https://doi.org/10.3390/ijms150916043 (2014).
Cooper, G. S. et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: Silica, sunlight, solvents. Rheumatology (Oxford) 49(11), 2172–2180. https://doi.org/10.1093/rheumatology/keq214 (2010).
Montes, R. A. et al. Smoking and its association with morbidity in systemic lupus erythematosus evaluated by the systemic lupus international collaborating clinics/American College of Rheumatology Damage Index: Preliminary Data and Systematic Review. Arthritis Rheumatol. 68(2), 441–448. https://doi.org/10.1002/art.39427 (2016).
Minkin, S. J., Slan, S. N., Gilkeson, G. S. & Kamen, D. L. Smoking and secondhand smoke among patients with systemic lupus erythematosus and controls: Associations with disease and disease damage. Arthritis Res. Ther. 16(Suppl 1), A40. https://doi.org/10.1186/ar4656 (2014).
Simard, J. F., Costenbader, K. H., Liang, M. H., Karlson, E. W. & Mittleman, M. A. Early-life exposure to cigarette smoke and adult-onset SLE. Lupus 18(5), 431–435. https://doi.org/10.1177/0961203308098186 (2009).
Grimaldi-Bensouda, L. et al. The risk of systemic lupus erythematosus associated with vaccines: An international case–control study. Arthritis Rheumatol. 66(6), 1559–1567. https://doi.org/10.1002/art.38429 (2014).
Cooper, G. S., Dooley, M. A., Treadwell, E. L., StClair, E. W. & Gilkeson, G. S. Hormonal and reproductive risk factors for development of systemic lupus erythematosus: Results of a population-based, case–control study. Arthritis Rheumatol. 46(7), 1830–1839. https://doi.org/10.1002/art.10365 (2002).
Kuo, C. F. et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern. Med. 175(9), 1518–1526. https://doi.org/10.1001/jamainternmed.2015.3528 (2015).
Costenbader, K. H., Feskanich, D., Stampfer, M. J. & Karlson, E. W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheumatol. 56(4), 1251–1262. https://doi.org/10.1002/art.22510 (2007).
Fatnoon, N. N., Azarisman, S. M. & Zainal, D. Prevalence and risk factors for menstrual disorders among systemic lupus erythematosus patients. Singap. Med. J. 49(5), 413–418 (2008).
Nonato, D. R. et al. Menstrual disturbances in systemic lupus erythematosus patients using immunossuppressants. Rev. Bras. Reumatol. 50(5), 501–515 (2010).
Sanchez-Guerrero, J., Liang, M. H., Karlson, E. W., Hunter, D. J. & Colditz, G. A. Postmenopausal estrogen therapy and the risk for developing systemic lupus erythematosus. Ann. Intern. Med. 122(6), 430–433. https://doi.org/10.7326/0003-4819-122-6-199503150-00005 (1995).
Bernier, M. O., Mikaeloff, Y., Hudson, M. & Suissa, S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheumatol. 61(4), 476–481. https://doi.org/10.1002/art.24398 (2009).
Buyon, J. P. et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: A randomized trial. Ann. Intern. Med. 142(12 Pt 1), 953–962. https://doi.org/10.7326/0003-4819-142-12_part_1-200506210-00004 (2005).
Mok, C. C. et al. Safety of hormonal replacement therapy in postmenopausal patients with systemic lupus erythematosus. Scand. J. Rheumatol. 27(5), 342–346. https://doi.org/10.1080/03009749850154357 (1998).
ATSDR. Lead Toxicity. What Are U.S. Standards for Lead Levels? [2019-b]. https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=8.
Alli, L. A. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip. Toxicol. 8(3), 146–150. https://doi.org/10.1515/intox-2015-0022 (2015).
Mashhadi, M. A., Bakhshipour, A., Zakeri, Z. & Ansari- Moghadam, A. Reference range for zinc level in young healthy population in Southeast of Iran. Health Scope 6, 1. https://doi.org/10.17795/jhealthscope-18181 (2016).
Pedro, E. M. et al. Trace elements associated with systemic lupus erythematosus and insulin resistance. Biol. Trace Elem. Res. 191(1), 34–44. https://doi.org/10.1007/s12011-018-1592-7 (2019).
Yilmaz, A., Sari, R. A., Gundogdu, M., Kose, N. & Dag, E. Trace elements and some extracellular antioxidant proteins levels in serum of patients with systemic lupus erythematosus. Clin. Rheumatol. 24(4), 331–335. https://doi.org/10.1007/s10067-004-1028-y (2005).
Toth, C. N. et al. Elemental analysis of whole and protein separated blood serum of patients with systemic lupus erythematosus and Sjogren’s syndrome. Biol. Trace Elem. Res. 179(1), 14–22. https://doi.org/10.1007/s12011-017-0945-y (2017).
Almroth, G., Westberg, N. G. & Sandstrom, B. M. Normal zinc and selenium levels in patients with systemic lupus erythematosus. J. Rheumatol. 12(3), 633–634 (1985).
Nossent, J., Lester, S., Rischmueller, M. & Zalewski, P. No zinc deficiency but a putative immunosuppressive role for labile Zn in patients with systemic autoimmune disease. Curr. Rheumatol. Rev. 13(1), 59–64. https://doi.org/10.2174/1573397111666151026223501 (2017).
Gidikova, P. L. Blood lead, cadmium and zinc correlations in elderly rural residents. Folia Med. (Plovdiv). 61(1), 113–119. https://doi.org/10.2478/folmed-2018-0051 (2019).
Petri, M. et al. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64(8), 2677–2686. https://doi.org/10.1002/art.34473 (2012).
Hussein, M. F. Some Environmental Risk Factors of Autism Spectrum Disorder (Unpublished Doctoral Dissertation) (High Institute of Public Health, 2017).
Lemos, V. A. & de Carvalho, A. L. Determination of cadmium and lead in human biological samples by spectrometric techniques: A review. Environ. Monit. Assess. 171(1–4), 255–265. https://doi.org/10.1007/s10661-009-1276-z (2010).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization and design of the work: R.R., M.H., M.A., and A.A.; methodology: R.R., M.H., and M.A.; selection of cases and controls: A.A.; data analysis: R.R., and M.H.; data curation: R.R., and M.H.; writing—original draft preparation, R.R.; writing—review and editing: R.R., M.H., M.A., and A.A.; visualization: R.R. All authors read and approved the final submitted revised manuscript and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Refai, R.H., Hussein, M.F., Abdou, M.H. et al. Environmental risk factors of systemic lupus erythematosus: a case–control study. Sci Rep 13, 10219 (2023). https://doi.org/10.1038/s41598-023-36901-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36901-y
- Springer Nature Limited