Abstract
This study investigated the effects of 67 species of macroalgae on methanogenesis and rumen fermentation in vitro. Specimens were analyzed for their effect on ruminal fermentation and microbial community profiles. Incubations were carried out in an automated gas production system for 24-h and macroalgae were tested at 2% (feed dry matter basis) inclusion rate. Methane yield was decreased 99% by Asparagopsis taxiformis (AT) when compared with the control. Colpomenia peregrina also decreased methane yield 14% compared with control; no other species influenced methane yield. Total gas production was decreased 14 and 10% by AT and Sargassum horneri compared with control, respectively. Total volatile fatty acid (VFA) concentration was decreased between 5 and 8% by 3 macroalgae, whereas AT reduced it by 10%. Molar proportion of acetate was decreased 9% by AT, along with an increase in propionate by 14%. Asparagopsis taxiformis also increased butyrate and valerate molar proportions by 7 and 24%, respectively, whereas 3 macroalgae species decreased molar proportion of butyrate 3 to 5%. Vertebrata lanosa increased ammonia concentration, whereas 3 other species decreased it. Inclusion of AT decreased relative abundance of Prevotella, Bacteroidales, Firmicutes and Methanobacteriaceae, whereas Clostridium, Anaerovibrio and Methanobrevibacter were increased. Specific gene activities for Methanosphaera stadtmane and Methanobrevibacter ruminantium were decreased by AT inclusion. In this in vitro study, Asparagopsis taxiformis was most effective in decreasing methane concentration and yield, but also decreased total gas production and VFA concentration which indicates overall inhibition of ruminal fermentation. No other macroalgae were identified as potential mitigants of enteric methane.
Similar content being viewed by others
Introduction
According to the United States Environmental Protection Agency (USEPA), in 2020 agriculture was responsible for 11% of the total greenhouse gas (GHG) emissions in the U.S. (on a CO2 equivalent basis, CO2e) with approximately 30% of these emissions being enteric methane (CH4)1. Within the United States, dairy and beef cattle contributed approximately 169 million metric tons (MMT) of CH4 (43.6 and 125.3 MMT, respectively) on a CO2e basis in 2020 through enteric fermentation1. Enteric methanogenesis is a process by which various end-products of anerobic microbial fermentation in the rumen, mainly CO2 and H2, are metabolized by archaea for energy, creating CH42. Being a downstream product, the quantity of CH4 produced is greatly dependent on the initial fermentation substrate [i.e. overall dry matter intake (DMI) and feed type] and rumen conditions. Beyond feed management and formulation, several diet additives that act as CH4 inhibitors have been identified3,4,5. Among these, macroalgae, specifically Asparagopsis taxiformis (AT), have been identified as a potential candidate to mitigate enteric CH4 emission from livestock.
Asparagopsis taxiformis was shown to almost eliminate enteric CH4 emission in vitro6, whereas in vivo research in both cattle and sheep have reported decreases of 0 to 98%, depending on diet and AT inclusion rates7,8,9,10. While these results are promising, some studies indicated that DMI and milk yield were negatively affected by AT supplementation9,10. Reduced animal performance would decrease the effect of AT on a CH4 intensity (CH4 g/kg milk or meat produced) basis and would likely limit industry adoption of AT.
To the best of our knowledge, AT is not yet cultivated on a commercial scale and wild harvest cannot be sustainable or meet the demand of the global or even U.S. cattle herd of 92 million animals11. It has been estimated that 1% dietary inclusion of AT in U.S. cattle diets alone would require 3 to 3.4 million metric tons of dry AT, which is approximately half of the current global production of all macroalgae12. Asparagopsis taxiformis is a member of the Rhodophyta phylum that is typically found in tropical to warm-temperate waters13. This geographic preference needs to be considered when evaluating the carbon footprint of AT as a feed supplement for ruminants14. Therefore, finding seaweed species that can be grown in aquaculture or sustainably harvested from local waters could provide an advantage of lower CO2e inputs.
The antimethanogenic effect of AT has been attributed to halogenated compounds, specifically bromoform (CHBr3), but seaweeds contain numerous bioactive compounds that may also inhibit methanogenesis15. For example, terrestrial plant compounds with antimicrobial action also found in macroalgae include: halogenated compounds (other than bromoform), alkaloids, phlorotannins, lipids, large polysaccharides, flavonoids and sulfonated glycans16,17. This list is by no means exhaustive but indicates a vast potential for discovery, which to this point has not been fully explored. In this context, a native, high biomass, cultivated seaweed like Saccharina latissima could be a better fit for the U.S. livestock feed market than AT, which currently is not cultivated locally or at large scales. Current or potential ability to be widely cultivated, opportunities to be cultivated within integrated multi-trophic aquaculture or nutrient bioextraction systems, potential bioactive compound content, and opportunities for sustainable wild harvest are all factors that should be considered for viable commercial implementation.
Therefore, the objective of this study was to determine the effect of macroalgae species (Supplementary Table 1) found in coastal waters of the U. S. on CH4 emission, total gas production, microbial profile, and volatile fatty acid and ammonia concentrations in vitro at 2% substrate dry matter (DM) basis inclusion rate, which would be comparable to in vivo feeding rates. We hypothesized that, apart from AT, there may be other macroalgae that could have a sizable CH4 mitigation effect.
Results and discussion
Total gas production and composition
In this series of incubations, total gas production (TGP) was measured continuously as a proxy for fermentation activity and to calculate emission of CH4 and H2. Since methanogenesis in the rumen is a first-order kinetic process18 and large datasets of in vivo work have described CH4 emission as dependent on substrate availability19, TGP and CH4 emission were expressed on a mL/g of DM basis (i.e., emission yield). For most treatments, H2 concentrations measured ranged from not detectable to < 1 mL/g of DM and, therefore, will not be discussed, except for AT.
Asparagopsis taxiformis decreased (P < 0.005) 24-h TGP by an average of 14%, when compared with control (Fig. 1). These results are comparable to the 30% reduction in TGP in vitro reported by Kinley et al. (2016) when AT was administered at 2% organic matter (OM) basis20. Other studies with AT conducted at the same inclusion level also demonstrated a decrease in TGP21,22,23. In the current study, AT decreased (P < 0.001) 24-h CH4 yield by 99% compared with control (Fig. 2), again, in agreement with previous studies20,21,22,23. It is noted that gas and CH4 yields from the current experiment may differ numerically from previous in vitro and in vivo studies with AT due to differences in incubation conditions, type of substrate fermented, inclusion rates, and digesta kinetics, in the case of in vivo experiments9,20,24. At 24-h, H2 emission for AT was drastically increased (P < 0.001; from not detectable to 2.19 mL/g DM. This effect is in agreement with other studies, wherein inhibition of methanogenesis resulted in an increase in H2 emission25,26,27,28,29. Sargassum horneri also decreased (P < 0.001) TGP 10% when compared with control, but only numerically reduced CH4 yield 14%. Comparison of headspace gas samples between S. horneri and control revealed no differences in CH4 concentration, which indicates that this alga did not inhibit methanogenesis, but decreased CH4 emission by inhibiting fermentation and consequently gas production (Supplementary Table 2). Methane emission was also decreased (P = 0.05) 13% by Colpomenia peregrina without negatively effecting TGP. A related species, Colpomenia sinuosa, decreased in vitro CH4 production 50% and TGP 10% when included at 20% (OM basis) compared to a decorticated cottonseed meal control30. Although direct comparison between these in vitro studies is difficult given the differences in inclusion rates and fermentable substrates [total mixed ration (TMR) vs. Flinders grass hay], this brown algal species may have a methane mitigating effect and should be further investigated. Pikea californica, a red seaweed, increased (P = 0.03) gas production 9% when compared with control, without a corresponding increase in CH4 emission (Supplementary Table 2). Observed differences in gas production could be the result of varying contents of fermentable carbohydrates and/or secondary metabolites which, depending on their mode of action, support or inhibit fermentation31,32.
Relative (percent difference, in comparison to set-specific control) effect of macroalgae on 24 h total gas production (mL/g dry matter) in vitro. For set-specific algae identification, see Supplementary Table 1 (ID# within Set). Number of observations used in the statistical analysis: from 26 to 66 (incubation Sets 1 through 15). Mean total gas production for control: 127, 126, 130, 126, 134, 130, 119, 121, 137, 123, 127, 123, 121, 122, and 97 mL/g dry matter, respectively; SEM = 4.1, 2.9, 2.5, 2.4, 5.3, 3.2, 6.2, 3.2, 3.7, 3.3, 4.7, 3.1, 3.5, 4.2, and 3.0, respectively. Means marked with an asterisk differ from the set-specific control (P < 0.05).
Relative (percent difference, in comparison to set-specific control) effect of macroalgae on 24 h methane yield (mL/g dry matter) in vitro. For set-specific algae identification, see Supplementary Table 1 (ID# within Set). Number of observations used in the statistical analysis: from 26 to 66 (incubation Sets 1 through 15). Mean methane yield for control: 7.97, 9.80, 7.61, 6.13, 8.90, 7.99, 7.71, 7.85, 8.48, 9.06, 7.47, 7.51, 8.01, 7.29, and 5.52 mL/g dry matter, respectively; SEM = 0.815, 0.541, 0.514, 0.367, 1.251, 0.666, 0.856, 0.464, 0.464, 0.617, 0.670, 0.560, 0.446, 0.439, and 0.395, respectively. Means marked with an asterisk differ from the set-specific control (P < 0.05).
One study33 examined a combination of algal species administered at 0.5 to 2.0% of OM. The algae were combined by storms washing them ashore. This combination of macroalgae reduced CH4 emission by 12% on average, and up to 16%33. Two of the species in this mix (Chondrus crispus and Fucus vesiculosus) were administered independently (at 0.5% of OM) with no effect on CH4 emission, perhaps indicating a complementary effect of including multiple species not seen at an individual level. Similarly, Chondrus crispus and Fucus vesiculosus had no effect on TGP or CH4 emission in the current study. To our knowledge, there are very few studies that investigated the macroalgae species examined in the current experiment at inclusion rates as low as 2% of substrate DM, making comparisons difficult. In many cases algae were tested at a higher dose (2 to 31% of DM) than in the present study32. Attempting to draw conclusions about CH4 inhibition in lactating cattle at these higher inclusion rates could prove problematic when advanced to in vivo testing stages. Several studies feeding Rhodophyta to lactating dairy cows reported decreases in DMI of 3 to 38%, potentially from an aversion to glutamic acid9,34,35. Additionally, Roque et al., (2021) reported a decrease in DMI when AT was fed to beef cattle at 0.5% of OM8. Therefore, in vitro screening of macroalgae species for enteric CH4 mitigation in cattle should be done at lower levels (≤ 2% DM) that will be practical and least likely to hinder palatability of the diet in vivo.
Volatile fatty acids
Compared to set-specific controls, total volatile fatty acid (VFA) concentration was decreased (P < 0.05) by AT, Fucus evanescens, Ulva intestinalis, and Sarcodiotheca gaudichaudii by 10, 8, 8, and 5%, respectively (Fig. 3). Despite decreasing total VFA concentration, apart from AT, none of these species decreased TGP as would be expected with inhibited fermentation. Additionally, AT decreased (P < 0.001) molar proportion of acetate by 9%, increased (P < 0.001) propionate by 14%, and subsequently decreased (P < 0.001) acetate:propionate ratio by 20% (Fig. 4). The results for AT agree with findings in previous studies at this inclusion level20,21. Interestingly, a similar study found no effect of AT on total VFA concentration but reported similar shifts in acetate and propionate molar proportions22. Similarly, in vivo supplementation of AT (0.5% DM) decreased total VFA and acetate concentrations (11 and 7%, respectively), and increased propionate molar proportion 8% in dairy cows9. In the current study, Laminaria farlowii and Ulva spp. both increased (P < 0.05) molar proportion of propionate by 9%, leading to a corresponding decrease (P ≤ 0.008) in acetate:propionate ratio of 10 and 12%, respectively.
Relative (percent difference, in comparison to set-specific control) effect of macroalgae on total VFA concentration (mM) in vitro. For set-specific algae identification, see Supplementary Table 1 (ID# within Set). Number of observations used in the statistical analysis: from 26 to 66 (incubation Sets 1 through 15). Mean Total VFA production for control: 52.1, 75.2, 63.7, 64.6, 59.3, 62.0, 47.5, 59.5, 65.0, 50.4, 59.1, 49.8, 49.6, 49.0, and 46.9 umol/mL, respectively; SEM = 1.43, 3.87, 2.79, 2.45, 4.19, 2.25, 4.09, 2.09, 3.61, 4.37, 3.92, 2.73, 1.37, 0.97, and 2.53, respectively. Means marked with an asterisk differ from the set-specific control (P < 0.05).
Relative (percent difference, in comparison to set-specific control) effect of macroalgae on acetate:propionate ratio in vitro. For set-specific algae identification, see Supplementary Table 1 (ID# within Set). Number of observations used in the statistical analysis: from 26 to 66 (incubation Sets 1 through 15). Mean acetate:propionate ratio for control: 1.72, 1.82, 2.34, 1.90, 2.06, 1.92, 2.34, 2.63, 2.63, 2.72, 2.38, 2.54, 2.45, 2.61, and 2.25 respectively; SEM = 0.055, 0.085, 0.202, 0.331, 0.427, 0.307, 0.320, 0.085, 0.556, 0.195, 0.943, 1.041, 0.094, and 0.057, respectively. Means marked with an asterisk differ from the set-specific control (P < 0.05).
In the current study, AT increased (P < 0.001) the molar proportion of butyrate by 7% when compared with control. Fucus evanescens decreased (P = 0.04) molar proportion of butyrate by 3%. Isovalerate proportion was increased (P = 0.03) 6 to 9% by Fucus evanescens, Ulva intestinalis, and Sarcodiotheca gaudichaudii. Valerate was only affected by AT, which increased (P < 0.001) its molar proportion by 24%.
Inhibition of methanogenesis leads to an increase of H2 concentration in ruminal fluid and headspace, or the reaction vessel in in vitro systems, as dissolved H2 normally consumed in the reduction of CO2 by archaea will partially accumulate36,37. While not a direct relationship, increased H2 concentrations within the headspace is correlated with higher dissolved H2 concentrations in ruminal fluid38,39,40. Due to the simplicity of sampling headspace gas, the sensitivity required to analyze low (0.1–50 µM) dissolved H2 concentrations, and the low number of studies which report dissolved H2, headspace concentrations were used as a proxy for dissolved H2 concentrations in the current study. With higher dissolved H2 concentrations, production of more H2 through fermentation to acetate, becomes energetically unfavorable which results in increased formation of propionate and in some cases butyrate, which act as H+ sinks37,41. For propionate, this sink comes via the reduction of pyruvate into propionate42. Therefore, inhibiting methane formation will lead to a shift in VFA profile, primarily in the direction of more propionate and butyrate36,37. In agreement with the current study, feeding lactating dairy cows a chemical inhibitor, 3-nitroxypropanol, increased rumen butyrate proportion 21% when CH4 emissions were reduced36. Additionally, valerate has been identified as reductant for archaea and can also function as a H+ sink43. The results from the current study, particularly regarding AT, are compatible with this shunting of H+ to other metabolic pathways when formation of CH4 was decreased in vitro20,24.
Ammonia
Mean ammonia concentration from the present study across all treatments and sets was 7.70 mM (SD ± 2.32), ranging from 1.57 to 14.56 mM (Table 1). Compared with control, ammonia concentration was increased (P < 0.03) 25% by Vertebrata lanosa. Sargassum horneri, Chondracanthus exasperatus, and Sargassum filipendula all decreased (P ≤ 0.04) ammonia concentrations by 16, 19, and 21%, respectively. Aside from Sargassum horneri, these 3 species were analyzed within the same Set (13) where all algae numerically decreased ammonia concentrations, potentially indicating a higher control in Set 13 (9.07 vs. 7.90 mM; Set 13 control and all Sets control, respectively). Obvious commonality in taxonomy is shared between the 2 Sargassum spp., whereas V. lanosa and C. exasperatus are all classified under Rhodophyta.
Increases in ammonia concentration, as in the case of V. lanosa, may simply reflect an increase in crude protein concentration in the seaweed species31. Chemical composition data, however, were not available to confirm this hypothesis. Decreases in ammonia concentration is an indication of enhanced ammonia-N utilization by rumen microbes, which may be limited by carbohydrate availability, or decreased proteolysis39,44. Given the low inclusion level of the macroalgae as substrate, it is unlikely that treatment would affect changes in fermentation (and fermentation end-products) through nutrient availability. In a similar in vitro protocol, alterations of starch source (barley vs. corn) by as much as 75% failed to illicit a significant effect on ammonia concentration in vitro45.
Other possible explanations for decreased ammonia concentration would be interactions with bioactive compounds found in the macroalgae that may inhibit substrate proteolysis16,46. For example, phlorotannins from Ascophyllum nodosum produced a linear decrease in ammonia concentration with increasing dose in vitro39. While in the current study, A. nodosum only numerically decreased ammonia concentration, the principle that bioactive compounds in macroalgae can affect ammonia concentration in vitro is still valid. However, it should be emphasized that in a previous study, phlorotannins were administered as an extract at minimum of 125 µg/mL; almost tenfold the dose used in the current study, assuming similar whole plant concentration of phlorotannin in A. nodosum39. Phlorotannin concentrations within macroalgae were not quantified in the current study.
Microbial profile
In archaeal communities, a total of 157,254 raw reads were generated from 2 incubations. Quality filtering produced a total of 140,395 reads and approximately 11% of sequences were filtered. This produced 4,340 operational taxonomic units (OTU). In bacterial communities, a total of 629,162 raw reads were generated from 2 incubations. Quality filtering produced a total of 410,198 reads and approximately 35% of sequences were filtered. This produced 25,656 amplicon sequences variants.
As the main interest of this study was inhibition of methanogenesis, only AT, chloroform (CHCl3; positive control) and control treatments were pooled, sequenced, and analyzed for differences in total abundance (DNA) amongst bacterial and archaeal OTU. Three archaeal genera were present at greater than 1% abundance: Methanobacteriaceae, Methanobrevibacter and Methanosphaera (Table 2). Methanobrevibacter was by far the most abundant with a relative abundance between 95.0 and 96.5%. Asparagopsis taxiformis increased (P < 0.007) the prevalence of Methanobrevibacter from 95 to 96% abundance, while decreasing (P < 0.001) Methanobacteriaceae from 1.4 to < 0.1% abundance, compared with control. Methanobacteriaceae was also decreased (P < 0.003) by AT compared with CHCl3 (0.53% abundance). Compared with AT and control, CHCl3 decreased (P < 0.01) Methanosphera abundance by 27 and 21%, respectively.
Number of observed archaeal species was different among control, CHCl3, and AT pools (P = 0.004). Lower archaeal species richness was observed within AT samples compared with control and CHCl3 (Supplementary Figs. 1 and 2). Kruskal–Wallis test showed differences (P = 0.005) among pools, however there were no pairwise differences based on Wilcoxon test. Beta diversity PERMANOVA results differed by pool in Weighted UniFrac (P = 0.001); however, pairwise PERMANOVA did not differ.
Analysis of archaeal complementary DNA (cDNA) by RT-qPCR for log copy numbers of gene specific primers Methanosphera stadtmane [mtaB(843)], Methanobrevibacter ruminantium [mcrH(835)], Methanobrevibacter smithii [mtaB(443)], and 16S rRNA revealed several differences between treatments (Supplementary Table 3). Complimentary DNA is a synthetic DNA transcribed from mRNA and can indicate the level of expression for a specific mRNA sequence within a sample [ex. mtaB(843)]47. Gene copy numbers of Methanosphera cDNA based component mtaB(843) was decreased (P ≤ 0.001) by AT and CHCl3 when compared with control (79 and 55%, respectively). Additionally, compared with control mcrH(835) was decreased (P < 0.001) 10% by AT, but was increased (P = 0.002) 5% by CHCl3. No differences were detected in component mtaB(443). Total 16S rRNA was decreased (P = 0.05) 2% by CHCl3. Differences between the methane inhibiting treatments were also observed; as both mtaB(843) and mcrH(835) gene copy numbers were decreased (P ≤ 0.05) by AT compared with CHCl3 (53 and 14%, respectively).
Complementary DNA analysis indicate that although AT increased DNA copy number of Methanobrevibacter, activity level of M. ruminantium was decreased, while M. smithii remained unaffected. In mice models utilizing human strains of M. smithii, non-methanogenic removal of fermentation end products (i.e. CO2) was observed in the form of an incomplete reductive tricarboxylic acid cycle48. The metabolic flexibility of M. smithii in this instance may explain why cDNA expression was unaffected and DNA abundance of the genus was able to increase despite inhibition of methanogenesis. Interestingly, Methanospera DNA abundance was not reduced by AT, but gene activity was severely reduced. A likely explanation for the response difference between DNA and cDNA could be the lack of medium passage that is specific to batch in vitro culture systems. This suggests that, as AT inhibits methanogenesis, accumulation of cDNA is reduced, but the archaeal DNA present at the time of inoculation is retained throughout the incubation. Decreases in gene activity by AT compared with CHCl3 may indicate that while both, CHBr3 and CHCl3 are methane analogs capable of inhibiting methanogenesis, other volatile organic compounds (e.g. dibromochloromethane) within AT are providing additive inhibition of methanogenesis22.
Number of observed bacterial species and Shannon diversity were not different among treatment pools, however Weighted (P = 0.008) and Unweighted (P = 0.02) beta diversity was observed between pools (Supplementary Figs. 3 and 4). No pairwise beta diversity was observed. Bacterial abundance was distributed across 12 phyla with Bacteroidetes and Firmicutes comprising 61.9 and 33.1% of the DNA in control, respectively (Table 3). Additionally, Fibrobacteres accounted for 1.4% of bacteria abundance in control. Asparagopsis taxiformis decreased (P < 0.03) the relative abundance of Bacteroidetes lineages from 61.9 to 57.6% and decreased (P < 0.01) Firmicutes abundance from 33.1 to 30.9%. Fibrobacteres was increased (P < 0.002) by AT from 1.4 to 5.6% compared with control. Spirochaetes was also increased (P < 0.001) by AT from 0.6 to 3.2% compared with control. Similarly, AT decreased (P < 0.006) Bacteroidetes, while increasing (P < 0.001) Fibrobacteres and Spirochaetes compared with CHCl3.
At the genus level, the most abundant bacteria by far were Prevotella which comprised up to 42% of the bacterial abundance (Table 4). Asparagopsis taxiformis decreased (P < 0.001) Prevotella from 40.8 to 35.7% compared with control. Unassigned genera in the family Bacteroidales were the second most abundant and were decreased (P < 0.01) by AT from 11.7 to 8.6% compared with control. For comparison, Pitta et al. (2018) reported similar relative sequence abundance of Bacteroidales (11%) within the ruminal liquid fraction of cows in the same herd as the present study49. Asparagopsis taxiformis increased (P < 0.002) Clostridium and Anaerovibrio abundance from 0.7 to 8.0% and from 1.7 to 3.4% compared with control, respectively. Comparison of AT with CHCl3 had similar results. Correspondingly, AT decreased (P < 0.02) Bacteriodales and Prevotella, while increasing (P ≤ 0.004) Clostridium and Anerovibrio compared with CHCl3.
Spearman correlations
Correlation analysis between species of bacteria and in vitro response variables (gas production and composition, VFA, and ammonia) were conducted on control, CHCl3, and AT pooled samples to determine relationships as affected by the inhibition of methanogenesis (Supplementary Fig. 5 and Supplementary Table 4). Thirty-one significant correlations (r ≥|0.5| and P ≤ 0.05) were observed between Bacteroidetes, Firmicutes, and Spirochaetes genera. Bacteroidales, CF231, Clostridium and Mogibacteriaceae were positively correlated with CH4 emission. A strong, negative correlation (r = − 0.70, P = 0.04) was observed between YRC22 and CH4 emission, along with a very strong positive correlation (r = 0.87, P = 0.002) with H2 emission. Paraprevotellaceae, Clostridiales, Lachnospiraceae, and Treponema also had a very strong positive correlation (r ≥ 0.85, P ≤ 0.004) with H2 emission. These 4 species were numerically, negatively correlated to CH4 emission. Hydrogen emission was negatively correlated (r ≤ − 0.71, P ≤ 0.03) with Prevotella, CF231, and Selenomonas.
Total VFA concentration was positively associated (r = 0.69, P = 0.04) with CF231 only. Proportion of acetate was positively correlated (r ≥ 0.68, P ≤ 0.04) with CF231 and Clostridium, with both genera being negatively correlated (r ≤ − 0.69, P ≤ 0.04) with propionate concentration. Acetate was negatively correlated (r ≤ − 0.67, P ≤ 0.05) with YRC22 and Treponema, again, with the inverse correlation (r ≥ 0.69, P ≤ 0.04) with propionate being observed. Firmicutes (Mogibacteriaceae) was negatively correlated (r = − 0.68, P = 0.04) with butyrate. Finally, valerate was positively correlated (r ≥ 0.69, P ≤ 0.04) with YRC22, Paraprevotellaceae, Lachnospiraceae, and Treponema and negatively correlated (r ≤ − 0.68, P ≤ 0.04) with CF231, Bacteriodales and Mogibacteriaceae.
The positive correlation between H2 and Clostridiales observed in this study aligns with metagenomic and metatranscriptomic data which identified several hydrogenase genes within the order’s genome50. Bacteria possessing these genes provide the majority of the H2 used for hydrogenotrophic methanogenesis. Other genera that represent a large share of hydrogenase activity in ruminants include Clostridia, Bacteroides, Butyrivibrio, Clostridium, Sarcina, and the Christensesnellaceae R-7 group of bacteria50,51. It is unclear why more correlations were not observed between these bacterial groups and H2 yields in the current study. Relative abundance of bacterial DNA is not a reliable indicator of metabolic activity, and metatranscriptomic data for hydrogenase genes is not available.
The correlations between bacterial communities and gas composition reflect the known inverse relationship between CH4 and H2 concentrations, as all identifiable correlations for CH4 and H2 emission were directly opposed; there were no cases of unidirectional correlation of CH4 and H2 with a bacterial genus. Only CF231 and YRC22 had qualifiable correlations with both CH4 and H2 emissions, suggesting that their metabolic activity may be more tightly associated with methanogenesis than the other genera investigated in this study. Interestingly, these two genera were found in higher abundance in rumen microbial communities of low-yield cows compared with high-yield; however, very little is known about their function in rumen physiology52. Another study analyzing cDNA-based bacterial communities in rumen samples from phenotypically high- and low-methane emitting cows observed a negative correlation between YRC22 and propionate53. Direct comparison between those c-DNA-based correlations and DNA-based correlations within the present study cannot be made, but nonetheless suggests some connection between YRC22 and propionate. CF231 had the most observed correlations (7) with response variables in this study. Being positively associated with CH4 emission, total VFA concentration and acetate proportion, CF231 may be preferential to a rumen environment promoting methanogenesis. Conversely, all genera that were positively correlated with H2 had positive correlations with propionate proportion, a known H2 sink. Both patterns agree with H2 metabolism discussed in Janssen (2010)37. While CF231 and YRC22 are genera of interest in both the present study and Mu et al. (2018)52, the authors are unaware of any work describing the specific role and function of these bacteria within the rumen. Future studies should be conducted to detail CF231 and YRC22 activity within the rumen microbiome. More work in vivo needs to be conducted to understand the influence AT may exert on animal production and the rumen microbiome, but it appears that the observed changes in rumen fermentation are driven by the thermodynamics and stoichiometry surrounding H2 metabolism.
Materials and methods
Macroalgae species
Samples for the current study were collected from April 2018 through August 2019 across several coastal US geographies (Supplementary Table 1). Red, brown, and green macroalga were selected based on multiple criteria: current or potential ability to be widely cultivated; opportunities to be cultivated within integrated multi-trophic aquaculture or nutrient bioextraction systems; potential bioactive compound content; opportunities for sustainable wild harvest; and industry interest. Fresh samples were identified, cleaned of epiphytes, rinsed with sterilized seawater and then frozen at − 20 °C for shipment. Upon delivery, samples were lyophilized (HarvestRight, North Salt Lake, Utah), ground in a Wiley mill (1-mm screen, Thomas Scientific, Swedesboro, NJ), and stored in airtight, brown glass vials at 4 °C. Before the in vitro test, samples were ball-ground using a Retsch MM200 mixer mill (Retsch, Inc., Newtown, PA).
Donor cows
Animal used in this study were cared for according to the guidelines of the Pennsylvania State University Institutional Animal Care and Use Committee. The Pennsylvania State University Institutional Animal Care and Use Committee reviewed and approved all procedures involving animals. Animal care and reporting of data within this manuscript adheres to ARRIVE guidelines.
Pairs of 2 ruminally-cannulated (11.0 cm i.d. silicone cannulas; Robyn Williams, Victoria, Australia) Holstein cows were used as rumen inoculum donors. Cows were housed at The Pennsylvania State University’s Dairy Teaching and Research Center tie-stall barn. Due to the large number of incubations conducted over the course of 18 months, a total of 7 cows were used as donors of ruminal fluid in the study. Average lactation number, days in milk, DMI, and milk production of the cows were (mean ± SD): 3.3 ± 0.83 lactations, 238 ± 119 d, 30.5 ± 4.86 kg/d, and 48.6 ± 8.38 kg/d, respectively. Cows had free access to drinking water and diets were fed from a Rissler model 1050 TMR mixer (I.H. Rissler Mfg. LLC, Mohnton, PA). Feeding was once daily at around 0900 h, after the morning milking, and feed was offered ad libitum targeting 10% refusals. Animals were fed a typical TMR containing (%, DM): corn silage (39), alfalfa haylage (12), canola meal (11), ground corn grain (10), roasted whole soybeans (8), cookie meal (7), whole cottonseed (5), sugar (5), grass hay (2), and mineral/Optigen® (Alltech, Nicholasville, KY) mix (2). A composite TMR sample was oven dried at 55 °C for 72 h and submitted to Cumberland Valley Analytical Services Inc. for wet chemistry analyses of CP54, α-NDF55, ADF54, starch56, minerals54, and estimated net energy for lactation (NEL)57. Nutrient composition of the TMR (%, DM basis) was: crude protein, 16.5; NDF, 30.9; ADF, 21.0; starch, 25.5; NEL, 1.61 Mcal/kg DM: Ca, 0.81; and P, 0.43.
Preparation of rumen inoculum
Collections of whole rumen contents took place before feeding and the morning milking at approximately 0500 h. Contents were collected from the ventral sac, reticulum, and caudal and dorsal sections of the feed mat and processed as previously described43. Briefly, contents were filtered through 2 layers of cheesecloth, reserving the filtrate. Strained solids were then combined with McDougal’s buffer58 and vigorously shaken for 30 s to extract loosely-associated microbes. The buffer and filtrate were then combined in equal parts and placed into a prewarmed thermos. Inoculum was transported back to the lab within 20 min of collection. Once at the laboratory, inoculum was transferred into 2 L graduated cylinders and permitted to ferment for 45 min under CO2 at 39 °C; buoyant feed particles were removed by vacuum aspiration and the remaining ruminal inoculum was used for the incubation. Aspiration of these particles improves the uniformity of the inoculum and prevents the addition of unaccounted substrate to the incubation vessels.
In vitro incubation
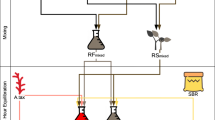
Incubations were conducted with the Ankom RF Gas Production System (Ankom Technology, Macedon, NY) outfitted with 250 mL glass vessels. Incubations were carried out for 24-h in a New Brunswick Innova 44 incubator/shaker (Eppendorf North America, Enfield, CT) at 39 °C and continuous 75 rpm agitation. A total of 30 incubations were completed. Each incubation was replicated and together is referred to as a “Set”. All treatments were run in triplicate within an incubation and all sets contained; TMR only (control), TMR + CHCl3 (positive control), and treatments (TMR + macroalgae) vessels. Prior to inoculation, TMR and, where appropriate, inhibitor or macroalgae (totaling 1.5 g DM) were weighed into the vessels and soaked in 75 mL of McDougall’s buffer for 1.5 to 2.0 h at 39 °C (Fig. 5). Upon conclusion of inoculum processing, each incubation vessel received 75 mL of ruminal inoculum dispensed from a continuously stirred flask, purged with CO2, and kept on a warming plate set at 39 °C. For positive control treatments, 0.5 mL of CHCl3 and buffer solution (0.81 µL/mL) was added. The CHCl3 (99% pure; Sigma Aldrich, St. Louis, MO) was dissolved in McDougall’s buffer and added to the TMR and buffer mixture just before the addition of rumen inoculum. Chloroform concentration was chosen to deliver a CH4 inhibition of around 80%25. Total inoculum volume of all vessels was 150 mL.
Incubation workflow overview. Incubation workflow: (1) Macroalgae (a) and feed substrate are dried, ground, and added to the vessels (c) with the buffer; (2) Rumen inoculum (b) is collected and clarified; (3) Clarified inoculum is added to the vessels; (4) Vessels are sealed and placed into the incubator (d). Incubation layout example (e): Blank (no substrate), Control (feed substrate only), Chloroform (feed substrate + CHCl3), and Macroalgae (feed substrate with 2% DM Macroalgae).
A 2% inclusion level was selected for the algal treatments since AT has been shown to be effective at decreasing CH4 emission at 2% of DM in vitro20,21,22,23. The first 11 sets contained 3 species/set at multiple inclusion levels (< 2% DM basis); but these lower inclusion rates did not produce any measurable effect on CH4 emission and were excluded from the final analysis. Only the 2% inclusion level will be discussed herein. Subsequent sets (12 to 15) only tested algal biomass at 2% inclusion, allowing for 7 species to be screened per set. Initially (incubation sets 1 to 2), AT which was used in an ongoing in vivo study was used as a positive control. Due to a significant decrease in the antimethanogenic activity of AT9, CHCl3 was substituted as a positive control at 10 µmol for incubation sets 3 to 15. All data discussing AT within this manuscript refer to a separate sample of AT (containing 2.44 mg CHBr3/g DM) tested in set 6 that was collected with the rest of the algal samples (Supplementary Table 1).
Sample collection and analysis
Once vessels were inoculated, initial pH was measured (Accumet AR15 pH meter; Fisher Scientific, Waltham, MA), vessels were purged with CO2, sealed, and placed back in the incubator. Vessels were incubated for 24-h and removed from the incubator for headspace gas sampling at 12 and 24-h according to ANKOMRF Gas Production System Operator’s Manual, Appendix C- Head Space Analysis. Vessels were removed from the incubator in batches of 12 to minimize temperature flux during sampling. Gas samples (2 aliquots of 2 mL each) were removed from each vessel and placed into vacuumed 20 mL vials (Agilent Headspace screw-top, Agilent Technologies, Santa Clara, CA). Vials were then pressurized with 22 mL of ultra-high purity (UHP) N2 (999.99 g/kg N2; Praxair Inc., Danbury, CT) for a total volume of 24 mL and were either immediately analyzed for CH4 and H2 or stored at 2 °C and analyzed within 48 h using gas chromatography (GC; Agilent 7980B, Agilent Technologies). Vials were agitated for 1 min (250 rpm) at 40 °C before injection by a PAL RSI 85 autosampler (CTC Analytics, Zwingen, Switzerland) into the GC. For CH4 analysis, samples were injected into a HayeSep Q 80–100 mesh column (1.83 m × 2 mm; Agilent Technologies) at 310 kPa using UHP He (999.99 g/kg He; Praxair Inc.) as a carrier. A deactivated fused silica restrictor (3 m × 320 µm) operating at 58.6 kPa was used leading to flame ionization detector set at 300 °C. Hydrogen analysis was conducted using UHP N2 (999.99 g/kg N2; Praxair Inc.) as a carrier using a HayeSep Q 80–100 mesh column at a flow rate of 5 to 12 mL/min pre- and post-run, respectively. Results were obtained using a thermal conductivity detector at a flow rate of 5 mL/min at 250 °C. Handmade gas standards (35 to 7000 mg/m3 for CH4 and 4 to 900 mg/m3 for H2) were used. Standards were made via serial dilution with UHP N2, using chemically pure CH4 (99.0% purity; Praxair Inc.) and 4.5 grade H2 (99.995% purity; Praxair Inc.). Methane and H2 production were then calculated by multiplying cumulative gas production by the concentrations of the individually analyzed gases.
At incubation endpoint (24-h), vessels were sampled for gas composition as described above. The module assembly recorded temperature and cumulative pressure throughout the experiment. Immediately following gas sampling, final pH was measured for each vessel, which were then placed in an ice bath to cease fermentation and for additional sampling. Liquid aliquots were collected and analyzed for ammonia concentration by colormetric assay59. Liquid samples for VFA analysis were collected according to Yang and Varga (1989)60 and analyzed by gas chromatography (Agilent 7890B; Agilent Technologies) using an 80/100 Chromosorb WAW packed column with ultra-high purity N2 (999.99 g/kg N2; Praxair Inc.) as a carrier61. Results were obtained using a flame ionization detector set at 175 °C. Additional 5 mL liquid samples were collected for bacterial and archaeal abundance analysis and stored frozen at -80 °C.
DNA extraction, 16S rRNA sequencing and bioinformatics
Replicates of control, CHCl3, and AT treatments from Set 6 (6 replicated per treatment) were pooled and genomic DNA was extracted in triplicate from 250 mL of each pooled sample using the repeated bead beating and column (RBB + C) method followed by extraction with a commercial kit (QIAmp Fast DNA Stool Mini Kit; Qiagen Sciences, Germantown, MD) as described in Yu and Morrison (2004)62. Asparagopsis taxiformis was the only macroalgae treatment that was extracted for DNA and 16S rRNA sequencing, as it was the most effective at reducing methane emission. The details related to library preparation and bioinformatics methodology are described in and Kaplan-Shabtai et al. (2021)63. Briefly, all samples were extracted for total genomic DNA, PCR-amplified for the V1-V2 region of the 16S rRNA bacterial gene, and the V6-V8 region of the archaeal 16S rRNA gene with Illumina MiSeqplatform (San Diego, CA). The sequencing reads analyzed for bacterial diversity using the QIIME2 pipeline and archaeal diversity QIIME 1.8.0 pipeline followed by statistical analysis in R (https://www.R-project.org/)64,65. The default parameters were used for all tools used during the analyses unless otherwise specified. For archaeal diversity analysis, the paired-end Illumina reads were joined together using combine_barcodes.py script with a 35 base-pairs overlap. The merged sequences were demultiplexed and quality filtered. Reads were discarded if they did not match the expected sample-specific barcode and 16S primer sequences (forward and reverse primers), or if they contained two or more ambiguous base calls. Reads were also discarded below a Phred quality score of 19.
RT-qPCR analysis
Absolute quantification of ruminal methanogens based on the gene copy numbers was performed by RT-qPCR. Quantification was conducted for genomic DNA samples using a StepOnePlus Real-Time PCR System (Applied Biosystems) with Maxima SYBR Green qPCR Master Mix (2 × ; Thermo Fisher Scientific). The primers for methanogen specific genes and reference gene (16S rRNA) used in this study are listed and summarized in Pitta et al. (2021)66 and the methodology for RT-qPCR conditions was followed as described by Bayer et al., (2014)67. Briefly, the qPCR cycling steps consisted of initial DNA denaturation at 95 °C for 10 min, followed by final denaturation at 95 °C for 15 s, primer annealing at 56 °C for 30 s, and finally PCR extension at 72 °C for 30 s were set up to 40 repeated cycles, along with a melting curve to ensure the specific product amplification. Simultaneously, a tenfold dilution of full-length amplicons of the 16S rRNA reference gene was performed to generate a standard curve. All qPCR assays were performed in triplicate, and a negative (non-template) control was also set with each assay to detect non-specific fluorescence emission68. Analysis of raw data obtained from the assays, qPCR efficiency and gene copy numbers were done using Applied Biosystems StepOne Real-Time PCR Software v. 2.0. The amplification efficiency (98–99%) of the qPCR standard curve (16S copy number) was used to calculate the copy numbers of unknown samples.
Sequence data processing and statistical analysis
The archaeal 16S rRNA reads were analyzed using the QIIME 1.8.0 pipeline65 as described in Pitta et al. (2021)66. The paired-end Illumina reads were joined together using combine_barcodes.py script. The merged sequences were demultiplexed and quality filtered. The operational taxonomic units (OTUs were formed by clustering sequences based on a 97% similarity threshold using the UCLUST algorithm69. Singleton OTU were excluded, and representative sequences for each OTU were aligned with PyNast70. The resultant multiple sequence alignment was used to infer a phylogenetic tree with FastTree71. Taxonomic assignments within the GreenGenes taxonomy72 were generated using the RDP Classifier version 2.273. Alpha diversity was assessed via observed species and Shannon diversity and beta diversity was measured using weighted and unweighted UniFrac distances for both archaeal and bacterial communities.
Data were analyzed within set as a completely randomized design using the MIXED procedure of SAS (version 9.4; SAS Institute, Inc., Cary NC). The model included treatment as a fixed effect. Incubation was considered a random effect. Excluding microbial abundance data, treatment response variables were expressed as the percent difference from control replicate averages within set; the averaged differences were used for the statistical analysis. The measured alpha diversity matrices were compared between the treatment groups using the Wilcoxon/Kruskal–Wallis Rank Sum Test. For beta diversity matrices, a non-parametric permutational multivariate ANOVA test74, implemented in the vegan package for R, was used to test the interactions and main effects. Microbial relative abundance percentages were compared directly, as samples were pooled by treatment (i.e., control, CHCl3, AT) before analysis. Spearman correlation between bacterial genera with a sequence proportion of ≥ 0.01% relative abundance and fermentation parameters were conducted on pooled control, CHCl3, and AT treatments using R. Statistical differences were considered significant at P ≤ 0.05 and trends were declared at 0.05 < P ≤ 0.10. Gas and fermentation response data are presented as LSM. Correlations were declared at P ≤ 0.05 with r values above 0.50 and below -0.50.
Conclusions
Of the 67 species of macroalgae investigated in this in vitro study, A. taxiformis was the only species that had a substantial mitigating effect on CH4 emission. Although Colpomenia peregrina also decreased CH4 emissions, this relatively smaller reduction (< 20%) has yet to be replicated in vivo. The reduction in CH4 by A. taxiformis was accompanied by a decrease in total gas production and total VFA concentration, indicating, at the 2% inclusion rate, a negative effect on overall fermentation. Diminishing the role of CH4 as a H2 sink resulted in a decrease in molar proportion of acetate, and an increase in molar proportions of propionate, butyrate, and valerate. No effect of A. taxiformis on ammonia concentration was observed; however, several other macroalgae decreased ammonia concentrations 16 to 21%. Additionally, Vertebrata lanosa increased ammonia concentration 25%. Asparagopsis taxiformis increased archaeal abundance of Methanobrevibacter while decreasing Methanobacteriaceae, and decreased bacterial abundance of Prevotella while increasing Clostridium. In the conditions of this in vitro study, no other macroalgae approached the efficacy of A. taxiformis in terms of CH4 emission mitigation. By altering the utilization of H2, A. taxiformis influenced the profile and gene expression level of bacterial and archaeal communities, shifting their metabolism to favor the production of VFAs which act as H2 sinks. Two specific bacterial species, CF231 and YRC22, were highly correlated with CH4 and H2 emission when methanogenesis was inhibited by A. taxiformis. Further investigation into the metabolic role of CF231 and YRC22 within an inhibited rumen environment is warranted. No other macroalgae, except for A. taxiformis, demonstrated the potential to be used as a CH4 mitigation tool for livestock in this study.
Data availability
The raw bacterial 16 s rRNA sequencing data are available at NCBI (National Center for Biotechnology Information) BioProject with accession number PRJNA950482.
References
EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks. Accessed April 15, 2022. https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks. (2021).
Hungate, R. E. et al. Formate as an intermediate in the bovine rumen fermentation. J. Bacteriol. 102, 389–397. https://doi.org/10.1128/jb.102.2.389-397 (1970).
Hristov, A. N. et al. Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 91, 5045–5069. https://doi.org/10.2527/jas.2013-6583 (2013).
Beauchemin, K. A. et al. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 14, S2–S16 (2020).
Arndt, C. et al. Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5 °C target by 2030 but not 2050. Proc. Natl. Acad. Sci. 119, e2111294119. https://doi.org/10.1073/pnas.2111294119 (2022).
Li, X. et al. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 58, 681–688. https://doi.org/10.1071/AN15883 (2018).
Kinley, R. D. et al. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 259, 120836. https://doi.org/10.1016/j.jclepro.2020.120836 (2020).
Roque, B. M. et al. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS One 16, e0247820. https://doi.org/10.1371/journal.pone.0247820 (2021).
Stefenoni, H. A. et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 104, 4157–4173. https://doi.org/10.3168/jds.2020-19686 (2021).
Muizelaar, W. et al. Safety and transfer study: Transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 10, 584. https://doi.org/10.3390/foods10030584 (2021).
Matlock, T. (2021) United States Cattle Inventory Down Slightly. https://www.nass.usda.gov/Newsroom/2022/01-31-2022.php Accessed 15 April 2022.
Vijn, S. et al. Key considerations for the use of seaweed to reduce enteric methane emissions from cattle. Front. Vet. Sci. https://doi.org/10.3389/fvets.2020.597430 (2020).
Andreakis, N., Procaccini, G. & Kooistra, W. H. C. F. Asparagopsis taxiformis and Asparagopsis armata (Bonnemaisoniales, Rhodophyta): Genetic and morphological identification of Mediterranean populations. Eur. J. Phycol. 39, 273–283. https://doi.org/10.1080/0967026042000236436 (2004).
Seghetta, M. et al. Seaweed as innovative feedstock for energy and feed—Evaluating the impacts through a Life Cycle Assessment. J. Clean. Prod. 150, 1–15. https://doi.org/10.1016/j.jclepro.2017.02.022 (2017).
Wood, J. M., Kennedy, F. S. & Wolfe, R. S. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B12. Biochemistry 7, 1707–1713. https://doi.org/10.1021/bi00845a013 (1968).
Pérez, M. J., Falqué, E. & Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs https://doi.org/10.3390/md14030052 (2016).
Patra, A. K. & Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 71, 1198–1222. https://doi.org/10.1016/j.phytochem.2010.05.010 (2010).
Waldo, D. R., Smith, L. W. & Cox, E. L. Model of cellulose disappearance from the rumen. J. Dairy Sci. 55, 125–129. https://doi.org/10.3168/jds.S0022-0302(72)85442-0 (1972).
Hristov, A. N. et al. Symposium review: Uncertainties in enteric methane inventories, measurement techniques, and prediction models. J. Dairy Sci. 101, 6655–6674. https://doi.org/10.3168/jds.2017-13536 (2018).
Kinley, R. D. et al. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 56, 282–289. https://doi.org/10.1071/AN15576 (2016).
Machado, L. et al. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 28, 1443–1452. https://doi.org/10.1007/s10811-015-0639-9 (2016).
Machado, L. et al. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J. Appl. Phycol. 28, 3117–3126. https://doi.org/10.1007/s10811-016-0830-7 (2016).
Machado, L. et al. In vitro response of rumen microbiota to the antimethanogenic red macroalga Asparagopsis taxiformis. Microb. Ecol. 75, 811–818. https://doi.org/10.1007/s00248-017-1086-8 (2018).
Machado, L. et al. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces ethane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 56, 282–289. https://doi.org/10.1071/AN15576 (2016).
Bauchop, T. Inhibition of rumen methanogenesis by methane analogues. J. Bacteriol. 94, 171–175 (1967).
Rufener, W. H. & Wolin, M. J. Effect of CCl4 on CH4 and volatile acid production in continuous cultures of rumen organisms and in a sheep rumen. Appl. Microbiol. 16, 1955–1956. https://doi.org/10.1128/aem.16.12.1955-1956 (1968).
Clapperton, J. L. The effect of trichloroacetamide, chloroform and linseed oil given into the rumen of sheep on some of the end-products of rumen digestion. Br. J. Nutr. 32, 155–161. https://doi.org/10.1079/bjn19740065 (1974).
Denman, S. E., Tomkins, N. W. & McSweeney, C. S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 62, 313–322. https://doi.org/10.1111/j.1574-6941.2007.00394.x (2007).
Ungerfeld, E. M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. https://doi.org/10.3389/fmicb.2020.00589 (2020).
Machado, L. et al. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One https://doi.org/10.1371/journal.pone.0085289 (2014).
Maia, M. R. G. et al. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci. Rep. 6, 1–10 (2016).
Abbott, D. W. et al. Seaweed and seaweed bioactives for mitigation of enteric methane: Challenges and opportunities. Animals 10, 1–28. https://doi.org/10.3390/ani10122432 (2020).
Kinley, R. D. & Fredeen, A. H. In vitro evaluation of feeding North Atlantic stormtoss seaweeds on ruminal digestion. J. Appl. Phycol. 27, 2387–2393. https://doi.org/10.1007/s10811-014-0487-z (2015).
Roque, B. M., Salwen, J. K., Kinley, R. & Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 234, 132–138. https://doi.org/10.1016/j.jclepro.2019.06.193 (2019).
Erickson, P. S. et al. Short communication: Kelp taste preferences by dairy calves. J. Dairy Sci. 95, 856–858. https://doi.org/10.3168/jds.2011-4826 (2012).
Melgar, A. et al. Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 103, 410–432. https://doi.org/10.3168/jds.2019-17085 (2020).
Wilhelm, E., Battino, R. & Wilcock, R. J. Low-pressure solubility of gases in liquid water. Chem. Rev. 77, 219–262. https://doi.org/10.1021/cr60306a003 (1977).
Barry, T. N., Thompson, A. & Armstrong, D. G. Rumen fermentation studies on two contrasting diets: 1. Some characteristics of the in vivo fermentation, with special reference to the composition of the gas phase, oxidation/reduction state and volatile fatty acid proportions. J. Agric. Sci. 89, 183–195. https://doi.org/10.1017/S0021859600027362 (1977).
Wang, Y., Xu, Z., Bach, S. J. & McAllister, T. A. Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol. 145, 375–395. https://doi.org/10.1016/j.anifeedsci.2007.03.013 (2008).
Janssen, P. H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 160, 1–22. https://doi.org/10.1016/j.anifeedsci.2010.07.002 (2010).
Johnson, E. D., Wood, A. S., Stone, J. B. & Moran, E. T. Some effects on methane inhibition in ruminants. Can. J. Anim. Sci. 712, 703–712 (1972).
Baldwin, R. L., Wood, W. A. & Emery, R. S. Conversion of glucose-C14 to propionate by the rumen microbiota. J. Bacteriol. 85, 1346–1349 (1963).
Nelson, W. O., Oppermann, R. A. & Brown, R. E. In vitro studies on methanogenic rumen bacteria. II. Fermentation of butyric and valeric acid. J. Dairy Sci. 41, 545–551. https://doi.org/10.3168/jds.S0022-0302(58)90960-3 (1958).
Russell, J. B., Onodera, R. & Hino, T. Ruminal protein fermentation: New perspectives on previous contradictions. Physiol. Asp. Dig. Metab. Ruminants https://doi.org/10.1016/B978-0-12-702290-1.50034-5 (1991).
Hristov, A. N., Ropp, J. K. & Hunt, C. W. Effect of barley and its amylopectin content on ruminal fermentation and bacterial utilization of ammonia-N in vitro. Anim. Feed Sci. Technol. 99, 25–36 (2002).
Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 86, 2010–2037 (2006).
NIH National Human Genome Research Institute. cDNA (copy DNA). Accessed May 11, 2023. https://www.genome.gov/genetics-glossary/Copy-DNA#:~:text=cDNA%20(short%20for%20copy%20DNA,cDNA%20contains%20only%20coding%20sequences (2023).
Samuel, B. S. et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. U. S. A. 104, 10643–10648 (2007).
Pitta, D. W. et al. Alterations in ruminal bacterial populations at induction and recovery from diet-induced milk fat depression in dairy cows. J. Dairy Sci. 101, 295–309 (2018).
Greening, C. et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 13, 2617–2632 (2019).
Leahy, S. C. et al. Electron flow: Key to mitigating ruminant methanogenesis. Trends Microbiol. 30, 209–212 (2022).
Mu, Y. et al. High-production dairy cattle exhibit different rumen and fecal bacterial community and rumen metabolite profile than low-production cattle. MicrobiologyOpen 8, e00673 (2019).
Stepanchenko, N. et al., Microbial composition, rumen fermentation parameters, enteric methane emissions, and lactational performance of phenotypically high- and low-methane emitting dairy cows. J. Dairy Sci. (Preprint). (2023).
AOAC International. Official Methods of Analysis (17th edition), AOAC International (2000).
Van Soest, P. J., Robertson, J. B. & Lewis, B. A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2 (1991).
Hall, M. B. et al. Determination of dietary starch in animal feeds and pet food by an enzymatic-colorimetric method: Collaborative study. J. AOAC Int. 98, 397–409. https://doi.org/10.5740/jaoacint.15-012 (2015).
NRC. Nutrient Requirements for Dairy Cattle. 7th rev. ed. Natl. Acad. Press. (2001).
McDougall, E. I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 43, 99–109. https://doi.org/10.1042/bj0430099 (1948).
Chaney, A. L. & Marbach, E. P. Modified reagents for determination of urea and ammonia. Clin. Chem. 8, 130–132. https://doi.org/10.1093/clinchem/8.2.130 (1962).
Yang, C. M. M. J. & Varga, G. A. Effect of three concentrate feeding frequencies on rumen protozoa, rumen digesta kinetics, and milk yield in dairy cows. J. Dairy Sci. 72, 950–957. https://doi.org/10.3168/jds.S0022-0302(89)79188-8 (1989).
Supelco. Analyzing Fatty Acids by Packed Column Gas Chromatography. Supelco 1–12. (1998)
Yu, Z. & Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36, 808–812. https://doi.org/10.2144/04365st04 (2004).
Kaplan-Shabtai, V. et al. Using structural equation modeling to understand interactions between bacterial and archaeal populations and volatile fatty acid proportions in the rumen. Front. Microbiol. 12, 1457. https://doi.org/10.3389/fmicb.2021.611951 (2021).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 848–857. https://doi.org/10.1038/s41587-019-0190-3 (2019).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. https://doi.org/10.1038/nmeth.f.303 (2010).
Pitta, D. W. et al. Temporal changes in total and metabolically active ruminal methanogens in dairy cows supplemented with 3-nitrooxypropanol. J. Dairy Sci. 104, 8721–8735. https://doi.org/10.3168/jds.2020-19862 (2021).
Bayer, K., Kamke, J. & Hentschel, U. Quantification of bacterial and archaeal symbionts in high and low microbial abundance sponges using real-time PCR. FEMS Microbiol. Ecol. 89, 679–690. https://doi.org/10.1111/1574-6941.12369 (2014).
Boulter, N. et al. A simple, accurate and universal method for quantification of PCR. BMC Biotechnol. 16, 1–14. https://doi.org/10.1186/s12896-016-0256-y (2016).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. https://doi.org/10.1093/bioinformatics/btq461 (2010).
Caporaso, J. G. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267. https://doi.org/10.1093/bioinformatics/btp636 (2010).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS One 5, e9490. https://doi.org/10.1371/journal.pone.0009490 (2010).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618. https://doi.org/10.1038/ismej.2011.139 (2012).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy Appl. Environ. Microbiol. 73, 5261–5267. https://doi.org/10.1128/AEM.00062-07 (2007).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26(32–46), 2001. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x (2001).
Acknowledgements
This work was supported by the USDA National Institute of Food and Agriculture Federal Appropriations under Project PEN 04539 and Accession number 1000803, and USDA National Institute of Food and Agriculture Award 2018-67015-27494. The authors express gratitude to M. Marty-Rivera, S. Augyte and J. Kübler from the University of Connecticut for their efforts in collecting the macroalgae samples.
Author information
Authors and Affiliations
Contributions
This study was designed by D.E.W., H.S. and A.N.H. Macroalgae samples were provided by C.Y. The in vitro experiments were conducted by D.E.W, H.S., S.F.C., C.L., S.E.R., A.M., and M.F. Fermentation sample processing and analyses were carried out by D.E.W. Microbial sequencing and statistical analysis was performed by M.H., K.N., N.I., and D.P. The manuscript was written by D.E.W. and revised by M.H., N.I., D.P., C.Y and A.N.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wasson, D.E., Stefenoni, H., Cueva, S.F. et al. Screening macroalgae for mitigation of enteric methane in vitro. Sci Rep 13, 9835 (2023). https://doi.org/10.1038/s41598-023-36359-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36359-y
- Springer Nature Limited