Abstract
In patients with acute circulatory failure, we tested the feasibility of the evaluation of the fluid-responsiveness (FR) by a combined approach with echocardiography and lung ultrasound. We enrolled 113 consecutive patients admitted to the Emergency Department High-Dependency Unit of Careggi University-Hospital from January 2015 to June 2020. We assessed: (1) inferior vena cava collapsibility index (IVCCI); (2) the variation of aortic flow (VTIAo) during the passive leg raising test (PLR); (3) the presence of interstitial syndrome by lung ultrasound. FR was defined as an increase in the VTIAo > 10% during PLR or IVCCI ≥ 40%. FR patients were treated with fluid and those non-FR with diuretics or vasopressors. The therapeutic strategy was reassessed after 12 h. The goal was to maintain the initial strategy. Among 56 FR patients, at lung ultrasound, 15 patients showed basal interstitial syndrome and 4 all-lung involvement. One fluid bolus was given to 51 patients. Among 57 non-FR patients, 26 patients showed interstitial syndrome at lung ultrasound (basal fields in 14, all lungs in 12). We administered diuretics to 21 patients and vasopressors to 4 subjects. We had to change the initial treatment plan in 9% non-FR patients and in 12% FR patients (p = NS). In the first 12 h after the evaluation, non-FR patients received significantly less fluids compared to those FR (1119 ± 410 vs 2010 ± 1254 ml, p < 0.001). The evaluation of the FR based on echocardiography and lung ultrasound was associated with the reduction in fluid administration for non-FR patients compared with those FR.
Similar content being viewed by others
Introduction
Optimal fluid management is one of the cornerstones of hemodynamic management in shock1. The basic physiological target of the administration of fluids is to improve tissue perfusion. In the earliest phase, especially during sepsis, a strong vasodilation leading to a low mean arterial pressure and microcirculatory impairment occurs, eventually associated with high or low cardiac output2. In this phase, the administration of fluids will significantly increase cardiac output in almost all cases, with a possible positive prognostic effect3,4. In the following phases, a condition of fluid responsiveness (FR) persists in less than half of the patients1,5. Therefore, a careful assessment of the potential benefits of fluids administration is highly advised6.
Current guidelines for the management of critical patients1,7 recommend the application of dynamic tests to evaluate fluid responsiveness, instead of static indices previously indicated, but the current practice and the modalities of evaluation of FR in critically ill patients are highly variable8. Moreover, the presence of fluid responsiveness should be balanced with the presence of fluid tolerance, that is the ability of the vasculature and organs to adsorb fluids.
Inferior vena cava collapsibility index (IVCCI) is one of the most popular method to evaluate FR, but its diagnostic accuracy has been questioned9. In non-ventilated patients, passive leg raising (PLR) associated with a non-invasive evaluation of cardiac output or aortic flow variation is actually one of the preferred options10. Lung ultrasound (LU) represents a feasible bedside tool to appreciate early signs of fluids overload in the lungs11, which represent one of the most important sites for the accumulation of extravascular fluid with consequent respiratory deterioration.
In patients with acute circulatory failure, we aimed to test the feasibility of the evaluation of the FR by a combined approach with echocardiography and lung ultrasound and the ability of this approach to improve the management of fluids administration.
Materials and methods
Study design and setting
The study protocol was approved by the “Toscana-Area Vasta-Centro” inter-institutional ethic committee (registration number CEAV 2018-484) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All patients gave informed consent to enter the study.
In this interventional prospective study, we enrolled consecutive patients with acute circulatory failure (ACF) admitted to the Emergency Department High-Dependency Unit of Careggi University-Hospital in Florence from January 2015 to June 2020. All the patients had completed their initial resuscitation.
The ED-HDU (High-Dependency Unit) is a clinical setting where critical patients are managed, with availability of advanced monitoring, non-invasive ventilation and possibility to administer vasoactive drugs, managed by Emergency Physicians; all patients are admitted from the Emergency Department (ED), according to bed availability. Within 48 h from ED admission, the ED-HDU physicians must decide the optimal patients’ disposition, choosing between the ordinary ward, the intensive care unit or another HDU. Because our ED-HDU do not have invasive mechanical ventilators, patients already intubated in the Emergency Room or with a high probability of intubation in the first 24 h are directly admitted into the Intensive Care Unit12.
Study population
We included patients who, after the completion of the early resuscitation, presented at least one of the following signs: (1) Systolic blood pressure < 90 mmHg or the need to use vasopressors, (2) Urine output < 0.5 ml/kg/h, (3) Persistent tachycardia (heart rate > 100 b/min in the absence of hyperthyroidism or fever, persisting more than 30 min) and (4) Mottled skin, as previously described by several authors13,14,15. Exclusion criteria were as follows: subjects aged < 18 years, pregnancy, cardiogenic or hemorrhagic shock, chronic kidney disease requiring dialysis, aortic valve disease (stenosis or regurgitation at least moderate), inadequate acoustic window.
For each patient, basic demographic data and clinical parameters were collected from medical records using a standardized collection template. Laboratory results were collected the day of the test and the day after.
Study protocol
For each patient, we initially performed an echocardiographic examination, with a standardized protocol based on the recommendations of the American and European Societies of Echocardiography16,17. The exam included an assessment of dimensions and systolic function of the left (LV) and right ventricle (RV) and a comprehensive valvular assessment.
The inferior vena cava (IVC) was visualized from the subcostal window and the IVC collapsibility index (IVCCI) was calculated as [(Dmax − Dmin)/Dmax] × 100. An IVCCI ≥ 40% was the threshold to identify fluid-responder patients18,19.
For the passive leg raising, the patients were positioned for 10 min in a semirecumbent position (45°) and the echocardiographic assessment was performed. Then, using an automatic bed elevation technique, the patient’s trunk was lowered from the semirecumbent to the supine position, while the lower limbs were raised to a 45° angle and maintained in this position for 2 min. Patients were adequately sedated during the maneuver and vasoactive drugs, sedatives, and non-invasive ventilation parameters remained constant during the whole procedure20.
Using the apical five-chamber view, at baseline and during the leg raising, the velocity time integral of aortic blood flow (VTIAo) was computed from the area under the envelope of the pulsed-wave Doppler signal, obtained at the level of the aortic annulus. The VTIAo value was averaged over three consecutive measurements in patients with sinus rhythm and over five in those with atrial fibrillation. Patients were considered fluid-responders if the VTIAo increased ≥ 10% during the test. Patients were managed based on the results of PLR; if this test was not feasible, the therapeutic plan was decided based on IVCCI. In the event of persistent hemodynamic instability after the first fluid bolus, a reassessment was performed in order to evaluate the indication to administer further fluid boluses.
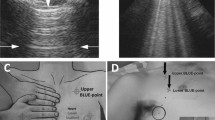
The lungs were examined by using longitudinal and oblique scans on anterolateral and posterior thoracic areas. Anterolateral examination was performed with the patient in the semirecumbent position; whenever possible, dorsal areas were scanned in the sitting position or by turning the patient in the lateral decubitus on both sides in case of forced supine position21.
B-lines were defined as hyperechoic, vertical artifacts arising from the pleural line, reaching the bottom of the screen, and possibly fading or obliterating A-lines. Interstitial syndrome (IS) was defined as signs in both right and left lungs involving 2 or more positive regions. The presence of an interstitial syndrome involving both basal and apical fields was considered a contraindication to the administration of fluids11. The results of the test were immediately available for treating physicians.
The baseline assessment and management of FR and non-FR patients is depicted in Fig. 1. In FR patients, we considered the administration of a fluid bolus, which consisted of 500 ml of crystalloids (Ringer lactates). In non-FR patients, we considered the administration of diuretics or vasopressors The presence of FR was not considered a compulsory indication to administer a fluid bolus20 nor the absence of FR determined the administration of diuretics. The treatment based on the index evaluation was administered within the first hour and thereafter patients were managed according to their clinical condition. The goal was the maintenance of the therapeutic strategy planned after the initial evaluation over the following 12 h. We considered a failure of the initial evaluation the adoption, in the following 12 h, of one of the treatments not included in the arm initially chosen, namely the administration of diuretics in FR patients or the administration of fluids in non-FR patients.
Statistical analysis
Statistical analyses were conducted using IBM SPSS software package (version 27). The calculation of the sample size was initially based on previous papers, which compared the diagnostic accuracy of PLR combined with echocardiography to evaluate FR. Given a sensitivity and specificity of the test around 90%10, confirmed in a preliminary analysis of the first 40 patients of this study group, the requested sample size was 12 patients. In the present study, we did not compare the test with a gold standard, but we assessed the correctness of our results with the following clinical management. As we did not find a study with a similar design for reference, we decided to include around one hundred patients.
Continuous variables were reported as mean ± standard deviation, and comparison between two groups was performed with the Student t-test for unpaired data. Categorical data were presented as absolute numbers and percentages and analyzed using contingency tables. Comparisons between two repeated measures was performed by the t-test for repeated measures.
The sensitivity, specificity, positive and negative predictive value (PPV and NPV) and accuracy of the tests were calculated according to standard formulas; comparison of these parameters between groups was made with Fisher exact test. A two-tails p value < 0.05 was considered significant.
Results
Among 131 patients screened during the study period, 18 presented an exclusion criterion (12 with baseline inadequate acoustic window, 3 patients in dialysis and 3 with aortic valve disease). Therefore, the study population included 113 patients. Patients were included based on the following criteria: (1) 56 patients showed MAP < 65 mmHg, associated in 23 with tachycardia, in 11 with reduced urinary output and in 8 with both; (2) 45 patients showed reduced urinary output, in 14 associated with tachycardia; (3) 12 patients showed tachycardia, in the absence of other causes like fever or hyperthyroidism. At the first assessment, we performed the PLR in 93 patients and in 95 we evaluated IVCCI; 22 patients underwent a second evaluation by the PLR and 47 by IVCCI.
In Table 1, we reported the clinical characteristics of the study population, based on the status of fluid responsiveness. Anamnestic variables, as well as vital signs, were similar regardless of the presence of FR, except for a higher prevalence of CAD among non-FR patients, compared to those FR. At baseline, a similar proportion of FR and non-FR patients received vasoactive medications or non-invasive ventilation. The amount of the initial fluid bolus was similar in FR and non-FR patients, but the latter received a lower dose of maintenance infusion than the FR patients. LV dimensions and systolic function were similar regardless of the state of fluid responsiveness, while TAPSE was significantly lower in non-FR than FR patients (Table 2).
In Fig. 2, we depicted the assessment and the treatment for FR and non-FR patients. Considering FR patients, in 51 the evaluation by the VTIAo variation during PLR was feasible and the treatment planning was based on it. In the remaining 5, the therapeutic decisions were based on IVCCI, which was the only available evaluation. Despite the condition of fluid responsiveness, 19 FR patients (34%) showed interstitial syndrome at lung ultrasound, which involved only basal fields in 15 and all lungs in 4. One fluid bolus was given to 51 patients, including 14 with basal interstitial syndrome and in 2 of them we needed to administer diuretics in following hours for worsening respiratory function; a second fluid bolus was given to 7 patients. In 5 patients, all with basal or all-lung IS, we did not administer a fluid bolus but we avoided diuretics and we could maintain the therapeutic strategy in the following hours. A change in the treatment strategy was necessary in 6 out of 51 patients (12%).
Among 57 non-FR patients, management was based on VTI variation during PLR in 42 patients and on IVCCI in 15. Twenty-six (46%) patients showed interstitial syndrome at lung ultrasound (p = NS vs FR patients), which involved only basal fields in 14 and all lungs in 12. Diuretics were administered to 21 patients and vasopressors were added to treatment in 4 subjects. A change in the treatment plan was made in 5 patients (9%, p = NS compared to FR patients). Therefore, considering both FR and non-FR patients, this approach allowed to establish a correct management of fluid administration in 102 out of 113 patients (90%). In the first 12 h after the evaluation, non-FR patients received significantly less fluids compared to those FR (Table 1).
Creatinine values demonstrated a significant reduction in the twelve hours following the FR assessment (1.8 ± 1.4 mg/dl vs 2. 0 ± 1.6 mg/dl, p = 0.031), while total bilirubin (1.22 ± 1.62 mg/dl vs 1.28 ± 1.93 mg/dl), alanine aminotransferase (102 ± 285 UI/L vs 76 ± 132 UI/L) and N-terminal brain natriuretic peptide (18,794 ± 32,645 ng/L vs 17,143 ± 32,531 ng/L, all p = NS) did not change. In Fig. 3, we compared the results of VTIAo and IVCCI in patients who performed both tests. We pooled the results of the first and the second evaluation to calculate the diagnostic performance of the two tests to evaluate FR and we reported them in Fig. 4. The diagnostic performance of the tests was evaluated based on their ability to identify the correct therapeutic strategy for the following 12 h. The evaluation of VTIAo during PLR showed a significantly better diagnostic accuracy than IVCCI. Both tests correctly identified a higher proportion of patients among non-FR (98% for VTIAo variation during PLR and 82% for IVCCI) than among FR patients (85% and 56%, respectively p = 0.019 and p = 0.002).
Discussion
In a population of non invasive-ventilated patients with acute circulatory failure, vital signs did not discriminate between FR and non-FR patients. The combined approach involving echocardiography and lung ultrasound allowed a feasible and correct evaluation of the FR and the therapeutic plan based on this evaluation was maintained in most patients in the following 12 h. In patients evaluated by the variation of aortic flow during PLR, who accounted for the majority, the initial strategy was maintained more frequently than in those evaluated by the IVCCI. Anyway, as we decided to evaluate a protocol tailored for an HDU, where an invasive monitoring of cardiac output is not feasible, IVCCI represented a feasible alternative in patients who were not candidate to PLR.
Fluids are one of the most administered treatments in critically ill patients, but the awareness that they represent a real drug is not so widespread. In fact, both hypovolemia and hypervolemia are harmful states, with heavy consequence on the outcome of the patients, regardless of the cause of the shock state6. Both inadequate fluid replacement and fluid overload determine tissue hypoperfusion, the first for persistent low cardiac output and the latter for the presence of increased extravascular water, end-organ edema, with final organ dysfunction2. Fluid overload has been shown to be an independent predictor of mortality in the critically ills, including patients with septic shock, acute respiratory distress syndrome, and those undergoing major surgery22,23,24. To assess fluid responsiveness, dynamic tests are recommended over vital signs or static variables25. For non-ventilated patients, fluid challenge or PLR represents possible options as dynamic tests. PLR has the advantage of being completely reversible, as no fluids are administered; its drawbacks are represented by the limited accuracy in patients with high intra-abdominal pressure and the long appraisal curve in the event of using echocardiographic to monitor cardiac output10.
Several authors have demonstrated that a liberal approach to fluid therapy with positive fluid balance is associated with increased mortality rate26,27 and restrictive protocols have been proposed28,29. Douglas et coll.30 recently published the results of the FRESH protocol, which aimed to evaluate whether the resuscitation guided by the assessments of fluid responsiveness based on a dynamic test could improve patients’ outcome. The study was randomized and multicenter, but the study population was finally limited. In patients with septic shock, they demonstrated that fluid and vasopressor resuscitation guided by the use of dynamic tests was safe and determined a reduction in the risk of renal and respiratory failure requiring replacement treatments. These results were reached despite a modest difference in the total amount of fluids administered respectively in the intervention and the usual care arms, without any significant increase in creatinine values in the 72-h study period. Therefore, even a small excess of administered fluids seems to have relevant prognostic consequences.
Our study population included a mixed population of critically ill patients. We showed that a combined approach with the dynamic evaluation of FR associated with the assessment of extravascular lung water allowed us to correctly identify patients, who could benefit from volume replacement or removal. As recommended by the existing literature20,31, the presence or absence of FR was not considered an absolute indication to a fluid bolus or the administration of diuretics, but a guide to decide how to manage fluid administration and avoid the therapeutic measures indicated for the opposite condition. To the best of our knowledge, this is the first study, which assessed the FR with a combined evaluation of a dynamic index and LU. A condition of FR does not imply that the administration of a fluid bolus will improve the clinical status. Even in the presence of a cardiac output increase > 10%, it cannot be taken for granted that the oxygen delivery to peripheral tissues will increase32. Moreover, it has been demonstrated that the condition of FR markedly varies over time, even within few hours, and a careful monitoring is required to avoid fluids overload33. Another important element of novelty is the evaluation of the appropriateness of the therapy after 12 h from the initial evaluation. The treatment plan based on this evaluation was maintained in most patients and we did not observe negative effects on parameters of renal function, as a possible effect of an inadequate administration of fluids.
Among FR patients, a relevant proportion presented interstitial lung syndrome and the contemporary evaluation of both aspects prevented us to administer fluids in those with an extensive lung involvement. In critically ill patients, the presence of the pulmonary interstitial syndrome can be the sign of fluids overload, but can also represent an early indication of altered lung permeability in the context of acute lung injury34,35. This is especially true among septic patients, who represent a relevant proportion of this study population. In fact, despite the presence of FR, these patients were not fluid-tolerant and the risk of increasing extravascular lung water was high. The approach proposed in this study represents an option for serial evaluations at the bedside, as LU can give useful information on fluid tolerance, besides those on FR obtained by VTIAo variation during PLR.
The study has several limitations, first the single center design. We employed echocardiography and the determination of VTIAo variation, to evaluate the response to PLR. This technique requires an adequate training of the operators, thus limiting its applicability. The possibility to use different monitoring methods, like the bioreactance employed in the FRESH study, could improve the diffusion and the feasibility of the dynamic evaluation of FR. Secondly, we did not compare the results obtained with the echocardiographic evaluation of the FR with a reference method. The only possibility was an invasive method to measure the variation of the cardiac output during the maneuver, but this tool is not available in our clinical setting. Therefore, we decided to rely on the coherence of the clinical management with the results of the text in the 12 h following the text itself. In this way we were not able to evaluate the accuracy of the method, but anyway we tested the correctness of our evaluation. We are also aware that the FR status of critical patients may vary, but we considered 12 h an adequate time interval to maintain the initial management plan. On the other side, only a randomized blinded study could allow us to evaluate the impact of this strategy on strong outcomes, like the reduction of in-hospital mortality as well as hospital length of stay. Finally, we are aware that treating physicians were not blinded to the results of the FR evaluation and could have been influenced in their clinical choices by those results. However, the staff was clearly informed about the study design and the treatment strategy was decided on a clinical basis.
Conclusions
In a group of non-ventilated patients, who had already undergone the initial resuscitation, we demonstrated that the evaluation of the FR based on echocardiography and lung ultrasound increased the physician's confidence in reducing the amount of fluids in the patient judged as non-responders, without negative effects on renal function parameters.
Data availability
The datasets used and analyzed during the current study is available from the corresponding author (F. Innocenti, innocenti.fra66@gmail.com) on reasonable request.
References
Cecconi, M. et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 40, 1795–1815 (2014).
Malbrain, M. L. N. G. et al. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 8, 66. https://doi.org/10.1186/s13613-018-0402-x (2018).
Lee, S. J. et al. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: A retrospective cohort study. Chest 146, 908–915. https://doi.org/10.1378/chest.13-2702 (2014).
Cecconi, M. et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit. Care 17, 209. https://doi.org/10.1186/cc11823 (2013).
Malbrain, M. L. et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 46, 361–380. https://doi.org/10.5603/ait.2014.0060 (2014).
Boyd, J. H., Forbes, J., Nakada, T. A., Walley, K. R. & Russell, J. A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 39, 259–265. https://doi.org/10.1097/CCM.0b013e3181feeb15 (2011).
Rhodes, A. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43, 304–377. https://doi.org/10.1007/s00134-017-4683-6 (2017).
Cecconi, M. et al. Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med. 41, 1529–1537 (2015).
Orso, D. et al. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: A systematic review and meta-analysis. J. Intensive Care Med. 35, 354–363. https://doi.org/10.1177/0885066617752308 (2020).
Monnet, X., Marik, P. & Teboul, J. L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 42, 1935–1947. https://doi.org/10.1007/s00134-015-4134-1 (2016).
Lichtenstein, D. A. & Malbrain, M. Lung ultrasound in the critically ill (LUCI): A translational discipline. Anaesthesiol. Intensive Ther. 49, 430–436. https://doi.org/10.5603/AIT.a2017.0063 (2017).
Innocenti, F. et al. Prognostic scores for early stratification of septic patients admitted to an emergency department-high dependency unit. Eur. J. Emerg. Med. 21, 254–259. https://doi.org/10.1097/MEJ.0000000000000075 (2014).
Airapetian, N. et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients?. Crit. Care 19, 400. https://doi.org/10.1186/s13054-015-1100-9 (2015).
Corl, K., Napoli, A. M. & Gardiner, F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emerg. Med. Australas 24, 534–539. https://doi.org/10.1111/j.1742-6723.2012.01596.x (2012).
Wu, J. et al. Evaluation of the fluid responsiveness in patients with septic shock by ultrasound plus the passive leg raising test. J. Surg. Res. 224, 207–214. https://doi.org/10.1016/j.jss.2017.12.014 (2018).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003 (2015).
Rudski, L. G. et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 23, 685–713. https://doi.org/10.1016/j.echo.2010.05.010 (2010).
Barbier, C. et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 30, 1740–1746 (2004).
Muller, L. et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit. Care 16, R188 (2012).
Monnet, X. & Teboul, J. L. Passive leg raising: Five rules, not a drop of fluid!. Crit. Care 19, 18. https://doi.org/10.1186/s13054-014-0708-5 (2015).
Zanobetti, M. et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest 151, 1295–1301. https://doi.org/10.1016/j.chest.2017.02.003 (2017).
Micek, S. T. et al. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit. Care 17, R246 (2013).
Rosenberg, A. L., Dechert, R. E., Park, P. K. & Bartlett, R. H. Review of a large clinical series: Association of cumulative fluid balance on outcome in acute lung injury: A retrospective review of the ARDSnet tidal volume study cohort. J. Intensive Care Med. 24, 35–46. https://doi.org/10.1177/0885066608329850 (2009).
Bednarczyk, J. M. et al. Incorporating dynamic assessment of fluid responsiveness into goal-directed therapy: A systematic review and meta-analysis. Crit. Care Med. 45, 1538–1545. https://doi.org/10.1097/CCM.0000000000002554 (2017).
Evans, L. et al. Executive summary: Surviving sepsis campaign: International guidelines for the management of sepsis and septic shock 2021. Crit. Care Med. 49, 1974–1982. https://doi.org/10.1097/CCM.0000000000005357 (2021).
Tigabu, B. M., Davari, M., Kebriaeezadeh, A. & Mojtahedzadeh, M. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: A systematic review. J. Crit. Care 48, 153–159. https://doi.org/10.1016/j.jcrc.2018.08.018 (2018).
Brotfain, E. et al. Positive fluid balance as a major predictor of clinical outcome of patients with sepsis/septic shock after ICU discharge. Am. J. Emerg. Med. 34, 2122–2126. https://doi.org/10.1016/j.ajem.2016.07.058 (2016).
Hjortrup, P. B. et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: The CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 42, 1695–1705. https://doi.org/10.1007/s00134-016-4500-7 (2016).
Macdonald, S. P. J. et al. REstricted Fluid REsuscitation in Sepsis-associated Hypotension (REFRESH): Study protocol for a pilot randomised controlled trial. Trials 18, 399. https://doi.org/10.1186/s13063-017-2137-7 (2017).
Douglas, I. S. et al. Fluid response evaluation in sepsis hypotension and shock: A randomized clinical trial. Chest 158, 1431–1445. https://doi.org/10.1016/j.chest.2020.04.025 (2020).
Satterwhite, L. & Latham, H. Fluid management in sepsis hypotension and septic shock: Time to transition the conversation from fluid responsive to fluid refractory?. Chest 158, 1319–1320. https://doi.org/10.1016/j.chest.2020.05.524 (2020).
Monnet, X. et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit. Care Med. 41, 1412–1420. https://doi.org/10.1097/CCM.0b013e318275cece (2013).
Hoste, E. A. et al. Four phases of intravenous fluid therapy: A conceptual model. Br. J. Anaesth. 113, 740–747. https://doi.org/10.1093/bja/aeu300 (2014).
Gargani, L. et al. Early detection of acute lung injury uncoupled to hypoxemia in pigs using ultrasound lung comets. Crit. Care Med. 35, 2769–2774. https://doi.org/10.1097/01.CCM.0000287525.03140.3F (2007).
Corradi, F., Brusasco, C. & Pelosi, P. Chest ultrasound in acute respiratory distress syndrome. Curr. Opin. Crit. Care 20, 98–103. https://doi.org/10.1097/MCC.0000000000000042 (2014).
Author information
Authors and Affiliations
Contributions
F.I. and C.S. gave substantial contributions to the conception and design of the work, drafted and revised the manuscript. A.C. and I.T., gave substantial contributions in the acquisition, analysis, or interpretation of data for the work. R.P. revised the results critically for important intellectual content and gave the final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Innocenti, F., Savinelli, C., Coppa, A. et al. Integrated ultrasonographic approach to evaluate fluid responsiveness in critically ill patients. Sci Rep 13, 9159 (2023). https://doi.org/10.1038/s41598-023-36077-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36077-5
- Springer Nature Limited