Abstract
The association between nonalcoholic fatty liver disease (NAFLD) and sarcopenia is known. We aimed to determine the association between skeletal muscle mass changes and NAFLD status. This retrospective single-center study analyzed patients who underwent health screening twice between November 2009 and December 2017, with a temporal gap of 6 ± 0.5 years. The degree of sarcopenia was assessed using appendicular skeletal muscle mass (ASM) adjusted for weight and body mass index (BMI). Changes in hepatic steatosis and fibrosis status were evaluated using noninvasive serum markers. Patients with a decrease in ASM/BMI (n = 353) had increased hepatic steatosis index (HSI) and fatty liver index (FLI) scores during 6 years (p < 0.05). The baseline sarcopenia group had a greater elevation in NAFLD fibrosis score (NFS) over 6 years than those without baseline sarcopenia. ASM changes over 6 years showed a negative correlation with variations in HSI (β = − 0.96 in ASM/Weight and -28.93 in ASM/BMI) and FLI (β = − 5.44 in ASM/Weight and − 167.12 in ASM/BMI). Subgroup analyses showed similar results according to sex and age. Sarcopenia may worsen steatosis and vice versa. Skeletal muscle status can be used to predict the course of NAFLD and establish individualized treatment strategies.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD), characterized by hepatic fat accumulation in the absence of excessive alcohol consumption, is the most common cause of chronic liver disease worldwide1,2. NAFLD is a spectrum including isolated hepatic steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and ultimately cirrhosis3. NAFLD is associated with increased liver-related mortality, including complications of portal hypertension, liver failure, and hepatocellular carcinoma4,5,6. Furthermore, NAFLD is associated with cardiovascular disease and malignancy-related mortality7. Additionally, the most important histologic feature—which determines long-term prognosis—is fibrosis stage rather than hepatic inflammation8,9. Therefore, improving hepatic fibrosis is considered an important surrogate endpoint of NAFLD patients10.
A previous study has shown that NAFLD is closely associated with insulin resistance11. Since skeletal muscle is the major site for glucose disposal by stimulation of insulin, low skeletal muscle mass promotes insulin resistance independent of obesity, leading to hepatic steatosis12,13. Moreover, NAFLD is a chronic low grade inflammatory state with increased circulating inflammatory cytokines, which might cause muscle depletion12. Recent studies have shown that, in patients with NAFLD, sarcopenia is significantly associated with steatohepatitis and fibrosis, independent of obesity and insulin resistance14. However, because these were cross-sectional studies, it is difficult to establish the causal relationship between sarcopenia and NAFLD. Although a large cohort study showed that skeletal muscle mass change was associated with the development of NAFLD or resolution of pre-existing NAFLD15, there is a lack of studies showing the association between changes in muscle mass and intrahepatic fibrosis.
Therefore, we aimed to investigate whether changes in muscle mass affect changes in hepatic steatosis and fibrosis, measured by noninvasive serum markers in NAFLD patients.
Methods
Study population
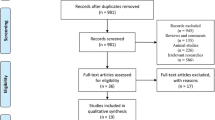
This was a retrospective study conducted in St. Vincent`s Hospital, the Catholic University of Korea, which reviewed the medical records of health screening between November 2009 and December 2017. Those who had received health screening more than twice in our center, who were diagnosed with fatty liver through abdominal ultrasound (US) were included. The ACUSON Sequoia 512 (Siemens Medical Solution, Mountain View, CA, USA) or EPIQ 5 US system (Philips Ultrasound, Bothell, WA, USA) were used for abdominal US, and the examination was performed by experienced radiologists.
There were 59,886 health screening cases during the study period. Patients without fatty liver on US were excluded (n = 39,667). Additionally, cases with hepatitis B surface antigen or undetermined hepatitis B surface antigen (n = 894), hepatitis C antibody (n = 84), liver cirrhosis (n = 99), insufficient medical records (n = 7755), a history of concomitant malignancy (n = 338), and excessive alcohol consumption (≥ 30 g/day for men, ≥ 20 g/day for women, n = 1078) were excluded.
Finally, the data of 606 patients were analyzed by limiting the subjects to those who received health screening only twice at an interval of 5.5–6.5 years. The enrollment process for the study population is shown in Fig. 1. The participants’ demographics, height, weight, comorbid diseases, social history, and results of various blood tests performed during screening were investigated. This study complies with the Declaration of Helsinki. Due to the retrospective design, we did not obtain informed consent for each participant. Ethical approval including the informed consent waiver, and monitoring of details of the research process was performed by the institutional review board of St. Vincent`s Hospital (VC17RESI0213).
Muscle mass parameters and definition of sarcopenia
The appendicular skeletal muscle mass (ASM) was obtained by adding the skeletal muscle mass of each limb measured by simultaneous multifrequency impedance measurements (Inbody770, Inbody Co., Korea). Sarcopenia was evaluated using the following two indices normalizing ASM with body weight and body mass index (BMI): ASM/weight and ASM/BMI. The definitions of sarcopenia were as follows: (1) ASM/weight < 2 standard deviation below the mean in healthy young adults, according to nationwide health examinations of the Korean population (sex-specific, < 29.0% in males or < 22.9% in females)16, (2) ASM/BMI < 0.789 m2 in males, or < 0.512 m2 in females, respectively17.
Concomitant metabolic disorders
Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or a history of antihypertensive treatment. Those with a fasting serum glucose level ≥ 126 mg/dL or a history of hypoglycemic agent use were defined as having diabetes mellitus18. Metabolic syndrome was diagnosed when three or more of the following requirements were met: (1) waist circumference: male, > 90 cm; female, > 80 cm; (2) triglyceride ≥ 150 mg/dL or ongoing drug treatment for hypertriglyceridemia; (3) high-density lipoprotein (HDL) cholesterol: male, < 40 mg/dL; female, < 50 mg/dL or ongoing drug treatment for reduced HDL cholesterol; (4) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or ongoing antihypertensive medications; (5) fasting serum glucose ≥ 100 mg/dL or ongoing drug treatment for elevated blood glucose19,20.
Measuring hepatic steatosis and fibrosis
Based on the screening results, the following four noninvasive serum markers at two separate visits were calculated: NAFLD fibrosis score (NFS), Fibrosis-4 index for liver fibrosis (FIB4), hepatic steatosis index (HSI), and fatty liver index (FLI)21,22,23,24. Among them, NFS and FIB4 are scales used to predict the presence or extent of hepatic fibrosis, whereas HSI and FLI are scales evaluating the degree of hepatic steatosis. At the second visit (after 6 years), we calculated the variance (delta, Δ) of the four indices from the first visit and attempted to find correlations with sarcopenia.
Statistical analysis
Data are expressed as means with standard deviations or numbers with percentages for continuous and categorical variables, respectively. Differences between the two groups were analyzed using t-tests and chi-square tests. Regression analyses were performed for correlations between muscle mass parameters and liver-related indices (adjusting for age, sex, concomitant diabetes, hypertension, and metabolic syndrome were adjusted). All data analyses were performed using the R Studio software for data analysis (ver. 1.0.153; R Foundation for Statistical Computing, Vienna, Austria). p values < 0.05 were considered statistically significant.
Results
Baseline characteristics of the study population
Data from 606 subjects were analyzed. The median age of the subjects at the first visit was 47 years, and the male sex was dominant (67.8%). Among the study population, 67 subjects (11.1%) had diabetes, 204 (33.7%) had hypertension, and 247 (40.8%) had metabolic syndrome at the first visit. Sixty-three participants (10.4%) met the criteria for sarcopenia based on ASM/weight or ASM/BMI. The group with sarcopenia (meeting criteria according to either ASM/weight or ASM/BMI) at the first visit showed a significantly higher BMI (p < 0.05) and higher proportion of hypertension and metabolic syndrome than the others. Laboratory findings showed that white blood cells (WBC) count, hemoglobin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and triglycerides were significantly higher (p < 0.05) in the sarcopenia group than in the non-sarcopenic group. The baseline characteristics of the study population and details of the differences according to the presence of sarcopenia are described in Table 1.
State of sarcopenia and NAFLD indices at the baseline and 6 years later
The ASM/weight and ASM/BMI of the study population did not change significantly over 6 years. However, the proportion of sarcopenia showed a 6-year increasing trend from the baseline. Among the liver-related indices, NFS and FIB4 decreased significantly over 6 years (p < 0.05). Furthermore, HSI increased after 6 years, while FLI showed a statistically insignificant decreasing pattern (Table 2).
Based on the changes in ASM/BMI, we divided all patients into two groups: those who showed a decrease in muscle mass over 6 years and those who did not. Approximately 58% of the subjects showed a decrease in the ASM/BMI over 6 years. When comparing clinical factors at the time of the first visit, the prevalence of metabolic syndrome was lower in the group with decreased ASM/BMI than the group with no decrease in ASM/BMI. Additionally, over the 6 years, the HSI and FLI showed a significant increase in the group with decreased ASM/BMI (p < 0.05). Regarding the laboratory findings, WBC count, hemoglobin, fasting glucose, total protein, albumin, AST, ALT, and γ-glutamyl transferase (GGT) levels showed a greater increase in the group with decreased ASM/BMI. The total cholesterol and triglyceride levels decreased in both groups; however, the variation was smaller in the group with decreased ASM/BMI. Furthermore, low-density lipoprotein (LDL) cholesterol levels increased over 6 years in the group with decreased ASM/BMI (Table 3). We performed the same analysis for the subgroup of 42 patients with sarcopenia by ASM/BMI during their baseline visit. Similar tendencies were seen throughout the population, but the statistical significance of the two groups could not be confirmed (Supplement Table S1).
Changes in NAFLD indices according to sarcopenia status at baseline
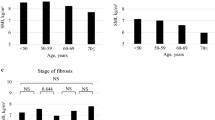
Details regarding the changes in the four liver-related indices, according to the baseline sarcopenia status, are provided in Table 4. Based on both ASM/weight and ASM/BMI, the progression of NFS was greater in the sarcopenia group than in the non-sarcopenia group, indicating that the degree of hepatic fibrosis progression may become more severe in patients with sarcopenia than in those without. There were no significant differences in the changes in FIB4, HSI, and FLI. In addition, we conducted subgroup analyses according to sex and age (age > 50 years or < 50 years). The ΔNFS was significant in the male group (p < 0.05); however, no differences were observed among the four indices in the female group (Supplementary Table S2). In the subgroup analysis of the age groups, results similar to the entire study population were confirmed (Supplement Table S3).
Prediction of the progression of steatosis and fibrosis using the change in muscle mass
We performed regression analyses to find out the relationship between changes in skeletal muscle mass and NAFLD indices. The increase in muscle mass showed a negative correlation with the changes in FLI and HSI over 6 years (Table 5). As muscle mass increased over the 6-year period, the degree of hepatic steatosis—reflected in FLI and HSI—also showed significant improvement (p < 0.05). In contrast, the hepatic fibrosis indicators NFS and FIB4 did not show any significant correlations with changes in muscle mass over 6 years.
We performed a subgroup regression analysis according to sex, age, and the presence of diabetes (Supplement Tables S4, S5 and S6, respectively). Regardless of sex, age, or concomitant diabetes, in the subgroup analysis, HSI and FLI showed a negative correlation with increasing ASM/weight and ASM/BMI, similar to the total study population. Furthermore, in this subgroup analysis, NFS and FIB4—which represent hepatic fibrosis—showed the same tendencies as the study population.
Discussion
In this study, we investigated the relationship between the extent of skeletal muscle mass changes and hepatic fibrosis or steatosis in NAFLD patients. We found that patients with NAFLD and sarcopenia at the first visit had a higher BMI, as well as a higher prevalence of hypertension or metabolic syndrome. Additionally, patients with increased HSI and FLI showed decreased skeletal muscle mass over the 6-year period, as well as worsened serum glucose, lipid profile, and liver function. Whereas those with increased muscle mass over the 6-year period showed an improvement in hepatic steatosis, which was reflected in the FLI and HSI variances.
Our study used data from repetitive measurements in the same NAFLD patient group over a 6-year period; therefore, we were able to investigate quantitative changes in skeletal muscle mass, sarcopenia status, hepatic fibrosis, and steatosis over time. Meanwhile, abdominal US used for the diagnosis of NAFLD was performed by experienced experts in the referral hospital, which guaranteed the high quality of examination. Another strength of this study is that, in the process of evaluating hepatic steatosis or fibrosis, two different indices were used in an effort to reduce measurement method bias.
Our study results show that the exacerbation of sarcopenia and NAFLD are closely associated. Moreover, NAFLD and sarcopenia share many pathophysiological mechanisms, such as insulin resistance, chronic inflammatory conditions, and cell damage caused by oxidative stimulation25. Several previous studies have focused on the association between sarcopenia and chronic liver disease, especially NAFLD26,27,28. Of note in our study, the reduction in muscle mass exacerbated hepatic steatosis. Patients with sarcopenia at baseline had a higher rate of hepatic fibrosis progression, which was reflected in the change in NFS over 6 years. Similar results have been found in other studies14,15,16. A Korean longitudinal study similar to ours, with a 7-year observation period, indicated that an increase in skeletal muscle mass had a positive effect in slowing down the progression of NAFLD15. There are several explanations regarding how skeletal muscle gain is involved in reducing fat accumulation in the liver and slowing NAFLD progression. First, elevated basal metabolic rate due to increased skeletal muscle mass leads to higher caloric consumption, which is involved in weight loss and fatty liver improvement29. Second, skeletal muscle is one of the key players in glucose metabolism. The skeletal muscle is a target organ of insulin, and increased muscle mass may be related to improved glucose metabolism capacity. In addition, a study on a skeletal muscle-specific, conditional transgenic mice reported that increased muscle mass prevented insulin resistance, which is one of the key mechanisms of NAFLD30.
Myokines from skeletal muscle also play important roles in the sarcopenia-NAFLD relationship. They control the hepatic metabolism of glucose and fatty acids and have anti-inflammatory effects against the proinflammatory activation of adipocytes31,32. The association between the change in skeletal muscle mass and NAFLD progression can also be explained in terms of malnutrition, which is a known cause of NAFLD33. Since sarcopenia is often accompanied by malnutrition, increased skeletal muscle mass in these patients may suggest an improvement in nutritional status and accompanying resolution of the cause of NAFLD exacerbation34. Considering the above-mentioned, treatment for sarcopenia may be a potential therapeutic intervention for NAFLD35. Therefore, investigating the effect of sarcopenia-targeted therapy on NAFLD status through subsequent research may be helpful in expanding NAFLD treatment strategies in the future.
In the absence of approved pharmacological treatment, lifestyle modification including exercise is central to the treatment of NAFLD. Exercise can reduce intrahepatic fat content and serum aminotransferase level even without significant weight loss36,37. Exercise showed a beneficial effect on functional capacity in patients with NAFLD38. Additionally, exercise also reduces muscle loss and prevents progression of sarcopenia39,40. Therefore, exercise can play an important role as a therapeutic intervention in patients with NAFLD and patients with sarcopenia. However, more studies are needed to determine the optimal exercise type, intensity, and duration to improve sarcopenia and NAFLD.
Consensus guidelines on sarcopenia suggested various methods to assess the skeletal muscle mass41,42. While magnetic resonance imaging, computed tomography scans, and dual-energy x-ray absorptiometry (DEXA) are regarded as reliable reference techniques for the ASM measurement, their accessibility in clinical settings is limited, and both CT and DXA can entail exposure to radiation43. Alternatively, the impedance measurement has advantages in terms of being relatively inexpensive, rapid, and portable without carrying the risk of radiation exposure. In several studies comparing the accuracy of lean soft tissue mass measurement with DEXA, impedance measurements showed satisfactory performance against DEXA43,44. Considering the appropriate diagnostic performance and good accessibility, impedance measurements are expected to play a crucial role in the clinical field of sarcopenia.
In studies on sarcopenia, height, body weight, and BMI have been mainly used to adjust ASM measurements. According to the AWGS2019 guideline, BMI-adjusted muscle mass was recommended for the evaluation of sarcopenia in clinical and research fields42, but there is still a lack of studies that compare weight-adjusted ASM and BMI-adjusted ASM with long-term data. In a study that investigated sarcopenia and hepatic fibrosis, there was no apparent difference between ASM/Weight and ASM/BMI. This was consistent with our results16. At present, we suggest that both ASM/Weight and ASM/BMI can be applicable in clinical and research settings for sarcopenia in patients with NAFLD.
As muscle mass typically declines with aging, it is expected that the proportion of patients with sarcopenia in the same group will increase over time. In our data, patterns of increasing sarcopenia rates were confirmed with a time interval of 6 years as expected. However, the data did not show statistical significance (Table 2). Considering that relatively healthy adults were set as the study population, we also concluded that the effect of aging alone on the sarcopenic state of the study group was insignificant.
Hepatic fibrosis is one of the most important factors determining the long-term prognosis of NAFLD. This study aimed to determine the potential impact of sarcopenia on the progression of hepatic fibrosis. Although the increase in NFS over 6 years was higher in patients with baseline sarcopenia, no significant association between sarcopenia and changes in FIB-4 was shown in our analyses. Since a patient’s age is included in the formula for NFS and FIB4, these two indices tend to increase over time as the patient ages24. However, contrary to our expectations, the values of the study population were lower after 6 years, and the correlation with the change in skeletal muscle mass was not significant, even after adjusting for age, sex, and metabolic syndrome.
These results can be attributed to several factors. First, the potential factors related to hepatic fibrosis may not have been sufficiently controlled in this study. Since patients who visited at least twice for health screening were targeted, the health-related interests and efforts of the study subjects may be higher than those of the general population. Therefore, the variables included in the calculation of fibrosis indices, other than age, may be improved over 6 years, making NFS and FIB4 better than predicted. Another possible reason for our unexpected results is that the 6-year time interval between the two observation points may not be sufficient for observing significant changes in hepatic fibrosis. According to a meta-analysis study targeting liver biopsy-based studies, the time required for the progression from baseline stage 0 fibrosis to hepatic fibrosis stage 1 was approximately 14.3 years in NAFLD patients, and 7.1 years in patients with NASH45. Since this study was conducted on asymptomatic patients visiting for health screening, the majority of the study group may have NAFLD rather than NASH. Therefore, it may take more than 6 years to identify the progression of fibrosis.
This study had several limitations. First, for the evaluation of hepatic steatosis, HSI and FLI—which are noninvasive serum markers—were chosen. Although these markers are widely used in clinical fields due to their convenience, with validated diagnostic performances in previous studies, their limitations are that they are less accurate and unable to quantitatively evaluate hepatic histologic changes, compared to US or magnetic resonance imaging46,47. Additionally, NFS and FIB4, which represent hepatic fibrosis, are known to have a lower accuracy than biopsy- or imaging-based methods48,49. In future studies investigating the impact of sarcopenia on liver diseases, we recommend a test method that can more directly evaluate hepatic steatosis or fibrosis.
Second, since we only collected data at two time points (with a 6-year interval), continuous follow-up for skeletal muscle mass and each liver-related index was not possible. Therefore, the identification of the changes between the two time points is limited. Third, lifestyle-related factors such as alcohol consumption, cigarette smoking, or exercise may have influenced the sarcopenia status of the study population. Regarding alcohol consumption, we defined the study population as patients with non-alcoholic fatty liver disease. As patients with alcohol consumption above a certain level were excluded, the possibility that the drinking habit had an effect on the sarcopenia status of the study group may be minimal. Unfortunately, due to the nature of the retrospective study, information on smoking history and exercise-related factors could not be obtained. In future related studies, it will be necessary to investigate and analyze detailed lifestyle variables.
Fourth, although the patient’s history of diabetes was included as a variable in our study, we were unable to suggest a parameter that could quantify insulin resistance, such as the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). Considering well-known associations between insulin resistance & NAFLD and sarcopenia, objective indicators reflecting insulin resistance may be required for future studies of NAFLD. Measurement of serum insulin levels for NAFLD patients as a daily practice may also be considered noteworthy.
Lastly, since the patients included were relatively healthy, there is an overall lack of patients with advanced fibrosis, to show a significant conclusion in temporal change of hepatic fibrosis, even in non-invasive setting. Furthermore, insufficient sample size can be one of the reasons for inconsistent relationships between hepatic fibrosis and sarcopenia in our data; therefore, future researches including patients with more advanced fibrosis will be needed.
In conclusion, skeletal muscle loss can accelerate the exacerbation of hepatic steatosis in patients with NAFLD, and vice versa. It is worth noting that improvements in sarcopenia may be a therapeutic target in the treatment of NAFLD. In future studies, it may be necessary to concentrate on the effects of therapeutic interventions for sarcopenia on NAFLD status, with a prospective design.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690. https://doi.org/10.1038/nrgastro.2013.171 (2013).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84. https://doi.org/10.1002/hep.28431 (2016).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55, 2005–2023. https://doi.org/10.1002/hep.25762 (2012).
Sayiner, M., Koenig, A., Henry, L. & Younossi, Z. M. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin. Liver Dis. 20, 205–214. https://doi.org/10.1016/j.cld.2015.10.001 (2016).
Younossi, Z. & Henry, L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology 150, 1778–1785. https://doi.org/10.1053/j.gastro.2016.03.005 (2016).
Sohn, W. et al. Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin. Mol. Hepatol. 27, 157–174. https://doi.org/10.3350/cmh.2020.0176 (2021).
Ekstedt, M. et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44, 865–873. https://doi.org/10.1002/hep.21327 (2006).
Hagstrom, H. et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273. https://doi.org/10.1016/j.jhep.2017.07.027 (2017).
Taylor, R. S. et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 158, 1611-1625.e1612. https://doi.org/10.1053/j.gastro.2020.01.043 (2020).
Cheung, A. et al. Defining improvement in nonalcoholic steatohepatitis for treatment trial endpoints: Recommendations from the Liver forum. Hepatology 70, 1841–1855. https://doi.org/10.1002/hep.30672 (2019).
Brunt, E. M. et al. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 1, 15080. https://doi.org/10.1038/nrdp.2015.80 (2015).
Bhanji, R. A., Narayanan, P., Allen, A. M., Malhi, H. & Watt, K. D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 66, 2055–2065. https://doi.org/10.1002/hep.29420 (2017).
Srikanthan, P., Hevener, A. L. & Karlamangla, A. S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 5, e10805. https://doi.org/10.1371/journal.pone.0010805 (2010).
Lee, Y. H. et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 63, 776–786. https://doi.org/10.1002/hep.28376 (2016).
Kim, G. et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A 7-year longitudinal study. Hepatology 68, 1755–1768. https://doi.org/10.1002/hep.30049 (2018).
Koo, B. K. et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 66, 123–131. https://doi.org/10.1016/j.jhep.2016.08.019 (2017).
Studenski, S. A. et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69, 547–558. https://doi.org/10.1093/gerona/glu010 (2014).
Song, D. S., Chang, U. I., Kang, S. G., Song, S. W. & Yang, J. M. Noninvasive serum fibrosis markers are associated with coronary artery calcification in patients with nonalcoholic fatty liver disease. Gut Liver 13, 658–668. https://doi.org/10.5009/gnl18439 (2019).
Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 (2002).
Huang, P. L. A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2, 231–237. https://doi.org/10.1242/dmm.001180 (2009).
Angulo, P. et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854. https://doi.org/10.1002/hep.21496 (2007).
Bedogni, G. et al. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33. https://doi.org/10.1186/1471-230X-6-33 (2006).
Lee, J. H. et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42, 503–508. https://doi.org/10.1016/j.dld.2009.08.002 (2010).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751. https://doi.org/10.1038/ajg.2016.453 (2017).
Kim, B. J. et al. Lower hand grip strength in older adults with non-alcoholic fatty liver disease: A nationwide population-based study. Aging (Albany, NY). 11, 4547–4560. https://doi.org/10.18632/aging.102068 (2019).
Ooi, P. H. et al. Sarcopenia in chronic liver disease: Impact on outcomes. Liver Transpl. 25, 1422–1438. https://doi.org/10.1002/lt.25591 (2019).
Wijarnpreecha, K., Panjawatanan, P., Thongprayoon, C., Jaruvongvanich, V. & Ungprasert, P. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J. Gastroenterol. 24, 12–17. https://doi.org/10.4103/sjg.SJG_237_17 (2018).
Hong, H. C. et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 59, 1772–1778. https://doi.org/10.1002/hep.26716 (2014).
Evans, P. L., McMillin, S. L., Weyrauch, L. A. & Witczak, C. A. Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nutrients 11, 2432. https://doi.org/10.3390/nu11102432 (2019).
Izumiya, Y. et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 7, 159–172. https://doi.org/10.1016/j.cmet.2007.11.003 (2008).
Yang, Y. J. & Kim, D. J. An overview of the molecular mechanisms contributing to musculoskeletal disorders in chronic liver disease: Osteoporosis, sarcopenia, and osteoporotic sarcopenia. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22052604 (2021).
Balakrishnan, R. & Thurmond, D. C. Mechanisms by which skeletal muscle myokines ameliorate insulin resistance. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23094636 (2022).
Bellentani, S. & Marino, M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann. Hepatol. 8, S4–S8. https://doi.org/10.1016/S1665-2681(19)31820-4 (2009).
Sieber, C. C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 31, 793–798. https://doi.org/10.1007/s40520-019-01170-1 (2019).
Dasarathy, S. & Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 65, 1232–1244. https://doi.org/10.1016/j.jhep.2016.07.040 (2016).
Nam, H. et al. Effect of exercise-based interventions in nonalcoholic fatty liver disease: A systematic review with meta-analysis. Dig. Liver Dis. https://doi.org/10.1016/j.dld.2022.12.013 (2023).
Babu, A. F. et al. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: A systematic review and meta-analysis. Nutrients https://doi.org/10.3390/nu13093135 (2021).
Gonzalez, A. et al. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Transl. Myol. https://doi.org/10.4081/ejtm.2021.9630 (2021).
Weinheimer, E. M., Sands, L. P. & Campbell, W. W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 68, 375–388. https://doi.org/10.1111/j.1753-4887.2010.00298.x (2010).
Joo, S. K. & Kim, W. Interaction between sarcopenia and nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 29, S68-s78. https://doi.org/10.3350/cmh.2022.0358 (2023).
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Chen, L. K. et al. Asian Working Group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300-307.e302. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Ballesteros-Pomar, M. D. et al. Bioelectrical impedance analysis as an alternative to dual-energy X-ray absorptiometry in the assessment of fat mass and appendicular lean mass in patients with obesity. Nutrition 93, 111442. https://doi.org/10.1016/j.nut.2021.111442 (2022).
Kim, M. & Kim, H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur. J. Clin. Nutr. 67, 395–400. https://doi.org/10.1038/ejcn.2013.9 (2013).
Singh, S. et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 13, 643-654.e641–649. https://doi.org/10.1016/j.cgh.2014.04.014 (2015). (quiz e639–640).
Jung, T. Y., Kim, M. S., Hong, H. P., Kang, K. A. & Jun, D. W. Comparative assessment and external validation of hepatic steatosis formulae in a community-based setting. J. Clin. Med. 9, 2851. https://doi.org/10.3390/jcm9092851 (2020).
European Association for the Study of the Liver. Electronic address, e. e. e., Clinical Practice Guideline, P., Chair, representative, E. G. B. & Panel, m. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 75, 659–689. https://doi.org/10.1016/j.jhep.2021.05.025 (2021).
Kaya, E. et al. Simple noninvasive scores are clinically useful to exclude, not predict, advanced fibrosis: A study in Turkish patients with biopsy-proven nonalcoholic fatty liver disease. Gut Liver 14, 486–491. https://doi.org/10.5009/gnl19173 (2020).
Noureddin, M. et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J. Hepatol. 76, 781–787. https://doi.org/10.1016/j.jhep.2021.11.012 (2022).
Author information
Authors and Affiliations
Contributions
I.H.J. and D.S.S. analyzed the data and wrote the first draft. D.S.S., U.I.C., and J.M.Y. contributed to the design of the study. I.H.J. and D.S.S. interpreted the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jo, I.H., Song, D.S., Chang, U.I. et al. Change in skeletal muscle mass is associated with hepatic steatosis in nonalcoholic fatty liver disease. Sci Rep 13, 6920 (2023). https://doi.org/10.1038/s41598-023-34263-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34263-z
- Springer Nature Limited