Abstract
Polymeric hydrogel with the incorporation of nano to submicro-meter sized materials forms an exhilarating new generation of composite hydrogels. Most of the applications of hydrogels are in aqueous environments in which they swell to a very high degree. This emanates from low density of the polymer chains, making them highly inferior in terms of physical strength and their prospective applications. In order to address the weak mechanical properties, hydrogels have successfully prepared with high tensile strength and toughness by reinforcing the acrylamide (AAm) network with 3-methacryloxypropyltrimethoxysilane (MPTS) modified silica particles (MSiO2) as chemical cross-linker. The MSiO2 cross-linkers are prepared from narrow-dispersed silica particles (SiO2) of 100 nm, 200 nm, and 300 nm diameters to investigate the effect of cross-linker sizes on the mechanical strengths of hydrogels. The presence of MSiO2 remarkably increases the stretching ability and toughness of hydrogels compared to conventional hydrogels. The tensile strength, toughness, and Young’s modulus of the hydrogel decrease from 30 to 11 kPa, 409 to 231 kJ/m3, and 0.16 to 0.11 kPa, respectively, while the SiO2 particle size increase from 100 to 300 nm and the concentration of AAm and MSiO2 (%) are kept constant. The compressive strength and toughness of the hydrogel decrease from 34 to 18 kPa and 6 to 4 kJ/m3, respectively, but the Young’s modulus increases from 0.11 to 0.19 kPa. This work is excellent proof of regulating mechanical strength of hydrogel by adjusting the particle size of MSiO2 cross-linkers.
Similar content being viewed by others
Introduction
Hydrogels are three-dimensional cross-linked polymeric networks containing water or biological fluids in large amounts in their network, thus becoming swollen1,2,3,4,5,6. Generally, such extremely hydrated polymer structures exhibit both elastic and viscous behavior when deformed and resemble the structure of biological tissue. They have been drawing considerable attention from scientists and technologists because of their versatile and unique properties for multifaceted applications. For pragmatic applications, appropriate mechanical strengths are required for hydrogels. Unfortunately, in most cases, conventional hydrogels possess inferior poor mechanical strength due to several reasons, such as mesh size and inhomogeneous distribution of cross-linking throughout the gel network7,8,9. Because of this, scientists are constantly expanding their expertise, investing more time, and developing new techniques to create hydrogels that are both stretchable and mechanically robust for use in a variety of multidimensional applications.
Chemical cross-linking, adopted to fabricate first-generation conventional hydrogels, was unduly weak and brittle. The most effective design principle was based on constructing a potential energy dissipation model in the gel matrix by maneuvering sacrificial or reversible bonds that avert crack extension and damage under strain. Second-generation hydrogels with high Young’s modulus and tensile strengths have been developed by modifying the gel network structure to induce energy dissipative mechanisms at the molecular level10. Several smart and effective techniques have already been employed to increase the mechanical strength of hydrogels keeping all other desirable properties unchanged. It is worth mentioning that topological hydrogels11, slide-ring hydrogels12, nanocomposite (NC) hydrogels13, double-network hydrogels14, and macromolecular microsphere hydrogels15 are the most appropriate examples of such mechanically strong hydrogels. Among these, the fabrication of NC hydrogels has fascinated multifarious research interests paid particular attention to improving the mechanical properties of hydrogels and amplifying the scope of their applications.
Nanoparticles such as nanospheres, nanosheets, and nanotubes have been successfully incorporated into hydrogels where the polymer and nanofillers interaction is usually established through several mechanisms such as hydrogen bonding, van der Waals forces, π–π stacking, electrostatic interactions, and covalent bonding. The NC hydrogels with improved toughness and mechanical strength are formulated by either previously mentioned forces or by their combinations. The distinctive polymeric chain length and high molecular weight of the polymer network make the NC hydrogels unique. Each of the nanoparticles is associated with several polymeric chains. As a result, if any chain gets detached from the filler nanoparticle, there would be a rest of the adjacent chains to hold up the increased load without cracking and provide the gels with high stretchability. Well-dispersed, rigid, and surface-functionalized inorganic nanoparticles have been widely applied to significantly improve the strength or toughness of polymeric structure16. Most of the reported NC hydrogels have been formulated through the grafting of polymers onto the surface of inorganic components utilizing weak ionic forces, which may easily disrupt at harsh conditions or even in a suitable solvent. Some of the published reports17,18,19,20,21 prepared hydrogels by utilizing SiO2 nanoparticles as cross-linker, but none of the groups focused on the influence of the size of SiO2 nanoparticles on the mechanical properties of hydrogels.
Jamali et al. formulated pH-sensitive hydrogel microspheres of AAm and Acrylic Acid (AAc) monomers by introducing SiO2 nanoparticles via inverse suspension polymerization22. Linn et al. used SiO2 nanoparticles to improve weak mechanical properties of poly(N,N-dimethylacrylamide) (PDMA) hydrogels through the self-cross-linking method but they did not mention the features of SiO2 nanoparticles23. Genovese et al. reported the effect of particle size (small: < 125 µm, and large: 125–850 µm) on mechanical properties and structure of methoxyl pectin/apple particles composite gels24. Rheological data of this report showed that increasing the concentration of small particles enhanced the elastic modulus of the composite gels. Yang and his group reported about physical hydrogels prepared by SiO2 nanoparticle surface in situ polymerization25. They prepared a series of poly acrylic acid (PAAc) hydrogels where covalently grafted chains constructed the hydrogel networks, and SiO2 nanoparticles acted as analogous cross-linking centers. The content and diameter of SiO2 nanoparticles significantly altered the physical properties of hydrogels. They applied SiO2 nanoparticles of wide diameter variations, such as 74 nm to 772 nm, and showed the relationship between the swelling ratio of hydrogels and the diameter of SiO2 nanoparticles. Chang et al. investigated the impact of the addition of nanoparticles on the mechanical properties of hydrogels26. The incorporation of nanoparticles made double-network hydrogel stronger with higher elastic moduli but failed to clearly demonstrate particle size’s influence. Levin et al. compared the improvement of the rigidity of PAAm and PDMA hydrogels by adding a tiny amount of SiO2 nanoparticles and established that high concentrations of SiO2 nanoparticles increased the toughness of both hydrogels but did not show any data related to the influence of particle size27. Azimi et al. prepared sulfonated polyacrylamide (SPAM)/chromium(III) acetate-based NC hydrogels and improved the mechanical properties by incorporating the various concentrations and sizes of SiO2 nanoparticles28. We recently reported a facile strategy for designing SiO2 based single polymer network hydrogel via strong covalent interactions between SiO2 and polymer in which the cross-linking density and inter-crosslinking distance can be regulated exclusively to enhance their mechanical properties29. In the previously published literature studies, it has been realized to establish the correlation between the mechanical strength of AAm based hydrogels and the diameters of inorganic cross-linkers.
With the aim of achieving the above-mentioned goal, a straightforward approach have been presented here for preparing SiO2-based hydrogels through the implementation of strong covalent interactions between organic polymer and inorganic particles in which the degree and length of cross-linking can be synchronized exclusively to improve the mechanical properties of gel. A nano to sub micrometer sized SiO2 as inorganic particles has been chosen because of its biocompatibility, strong surface binding energy, superior chemical reactivity and stability, nontoxicity, large surface area, good absorbency, and versatile functionalization. SiO2 particles with diameters of 100 nm, 200 nm, and 300 nm will be modified by MPTS so that they could be used as chemical cross-linkers during free radical polymerization (Fig. 1). The effect of the size of the SiO2 particles on the mechanical properties of polyacrylamide-MSiO2 (PAAm-MSiO2) hydrogels will be studied.
Experimental section
Materials
AAm, N,N′-methylenebisacrylamide (BIS), potassium persulfate (KPS), and tetramethylethylenediamine (TEMED) were used as principal monomer, conventional cross-linker, initiator, and accelerator, respectively, for free radical polymerization. Additionally, MPTS as SiO2 particle modifier, acetic acid as pH adjuster, ethanol, and deionized water as solvents were used in this work. All the analytical grade chemicals were purchased from Sigma-Aldrich and used without further purification. The narrow-dispersed SiO2 particles having different diameters (100 nm, 200 nm, and 300 nm) were purchased from Nippon Shokubai, Japan, and used as received.
Modification of SiO2 particles
At first, 10 (wt%) MPTS was hydrolyzed in ethanol–water (9:1) solution, and the pH was maintained at 3–4 by using an acetic acid solution (0.1 mol L−1). The mixture of hydrophilic SiO2 was then added to the solution and heated at 60–70 °C in an oil bath under magnetic stirring at 500 rpm for half an hour. Subsequently, the silanized SiO2 were separated by centrifugation (Centrifuge machine, Hettich, Universal 16A) and submersed in deionized water for 24 h. To eliminate the influence of physical adsorption, the total product was washed with deionized water several times and dried at 60 °C for 24 h until reaching a constant weight to obtain MSiO2.
Synthesis of PAAm-BIS and PAAm-MSiO2 hydrogels
PAAm-MSiO2 hydrogels were synthesized by free radical polymerization at room temperature using KPS, TEMED, and MSiO2 particles as initiator, accelerator, and cross-linker, respectively. Initially, variable weight percentages of MSiO2 with respect to AAm were dispersed properly in deionized water. Then a predetermined molar concentration of AAm was added in the dispersion under an inert nitrogen atmosphere. The mixture was poured into two different types of mold, made up of two glass slides kept apart by 1–4 mm Teflon spacers and test tubes with varying diameters. The flat glass mold and test tube were kept in an ice bath while mixing the precursors. The polymerization was done at room temperature for 24 h. The PAAm-MSiO2 hydrogels were prepared by changing the concentration of AAm (3 M, 4 M, and 5 M) and keeping fixed the amount of MSiO2 with different particle diameters (100 nm, 200 nm, and 300 nm). The conventional PAAm-BIS hydrogels were fabricated using different concentrations of traditional cross-linker BIS. The formulations to fabricate various types of PAAm-MSiO2, PAAm-BIS, and PAAm-BIS-MSiO2 hydrogels by varying the concentration of monomers and other gel precursors such as cross-linker, initiator, and solvent have been listed in Table 1.
Fourier transform infrared (FT-IR) spectroscopic analysis
The functional groups analysis on the surface of SiO2 particles was investigated by FT-IR spectrophotometer (FT-IR-8400, Shimadzu, Japan). The SiO2 and MSiO2 were analyzed by FT-IR spectrophotometer in the region of 4000 cm–1 to 470 cm–1. SiO2 and MSiO2 were dried in an oven at 60 °C, and a small portion of the samples was taken into vials. The solid samples were ground in mortar by pestle and mixed with moisture-free pure KBr (Sigma-Aldrich, Germany) crystals, and the powder mixture was converted into pellets by pressing manually under a pressure of 8–10 tons. Finally, the pellet was positioned inside the sample chamber for FT-IR spectra measurements.
Nuclear magnetic resonance (NMR) spectroscopic analysis
The modification of SiO2 was verified by observing the 1H-NMR spectra recorded by Bruker BPX-400 spectrometer (400 MHz). Deuterated chloroform (CDCl3) was used as a solvent, and tetramethylsilane (TMS) was used as an internal standard. The coupling constant (j) was in Hz, and all the chemical shifts (δ) relative to TMS peak and solvent peaks (7.28 ppm) were recorded in ppm. The abbreviations for singlet, doublet, triplet, and quartet are used as s, d, t, and q, respectively.
Field emission scanning electron microscopy (FE-SEM)
The surface morphology of SiO2 and MSiO2 of different diameters was explored using FE-SEM (JSM-7600F, Tokyo, Japan). The selected samples of different characteristics were dried and coated with platinum by sputtering to ensure the conductivity of samples’ surfaces. The observation of sample surface by FE-SEM was carried out at an accelerating voltage of 5.0 kV.
Energy dispersive X-ray spectroscopy (EDXS)
Elementary composition investigation of SiO2 and MSiO2 of different diameters was performed by EDXS connected to a microscope (FE-SEM; JSM-7600F, Tokyo, Japan). The sample preparation for EDXS analysis is similar to that of FE-SEM analysis procedure.
Fiber optic ultraviolet–visible (UV–Vis) spectrophotometry
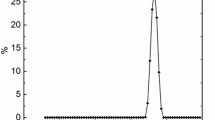
The transparency of prepared hydrogels was examined by measuring the transmittance spectra using fiber optic UV–Vis spectrometer (FLAME-T-XR1, Ocean Optics, Germany). The DH-2000-BAL was used as a balanced deuterium halogen light source. The transmittance spectra were measured in the 200–800 nm at 25 °C. The 2.0 mm thick hydrogel samples were positioned on the glass surface between the UV–Vis light source and detector perpendicular to each other.
Mechanical properties for elongation and compression
The mechanical properties of hydrogels were assessed by a universal testing machine (UTM, TestResources, Model 100-P-250-12). Flat hydrogel samples with 2.0 mm in width and 10.0 mm in length were used for uniaxial tensile measurement.
The Young’s modulus was calculated from the slope of 10% strain in the stress–strain curve. The fracture toughness was calculated by integrating the area of the stress–strain curve of each sample. Cylindrical shaped hydrogels samples with 10–15 mm diameter and 4–8 mm thickness was selected for compressive stress–strain measurements. All the mechanical properties were measured at ambient temperature. The crosshead speed was fixed at 50 mm/min. Each sample was tested at least three times to check the reproducibility of the results.
Results and discussion
Confirmation of SiO2 particle modification
The FTIR spectra confirmed that MPTS successfully modified with the bare SiO2. The peak at 1100 cm−1 for the stretching vibration of Si–O–Si, the peak at 950 cm−1 for Si–OH, the peak at 802 cm−1 for the stretching vibration of Si–O, and the peak at 470 cm−1 belonging to the vibration of Si–O groups were observed in the spectrum (Fig. 2). In MSiO2, a carboxyl group (C=O) peak at 1740 cm−1, and also peaks at 2957, 2882, and 1645 cm−1 which were because of C–H(–CH3), C–H(–CH2), and C=C stretching vibration, respectively, were observed. The appearance of all these peaks and bands indicated that the MPTS had been successfully grafted onto the surface of SiO2 particles. The decrease in peak intensity of the –OH band found in the spectrum of MSiO2 also indicates the successful incorporation of MPTS to –OH groups of SiO2. The presence of relevant characteristic signals of MPTS at δ = 5.28 ppm, 5.32 ppm (a, a′), 1.27 ppm (b), 2.35 ppm (c), 1.06 ppm (d) 0.9 ppm (e) in 1H-NMR spectrum (Fig. 2) produced by MSiO2 whereas all peaks were absent in bare SiO2. The above observations prove the successful modification of the SiO2 particles by MPTS and could be used as cross-linkers for gel preparation.
Morphology investigation of SiO2 and MSiO2 particles
The surface morphologies of all bare SiO2 and MSiO2 (100 nm, 200 nm, and 300 nm) were analyzed by FE-SEM images. The spherical shape and narrow dispersity of all SiO2 and MSiO2 were confirmed from FE-SEM micrographs (Fig. 3). Finally, the EDXS spectra of MSiO2 particles validated the successful modification of bare SiO2 particles (Fig. 3). The size of SiO2 (100 nm) ranged from 100 to 115 nm and the average particle size was 110.5 ± 4.5 nm. The size of MSiO2 (100 nm) was ranging from 100 to 120 nm and the average particle size was 117.5 ± 5.0 nm. The particle sizes were slightly increased from bare SiO2 to MSiO2 after a successful vinyl groups incorporation, but they did not induce any significant changes in the particle size distribution. Similar surface morphologies and average particle size variations were observed in cases of SiO2 (200 nm/300 nm) and MSiO2 (200 nm/300 nm). The bare SiO2 (200 nm) sizes ranged from 204 to 213 nm, and average particle size was 208.5 ± 3.0 nm. The size of MSiO2 (200 nm) ranged from 205 to 230 nm, and average particle size was 216.5 ± 7.2 nm. The bare SiO2 (300 nm) ranged from 295 to 315 nm, and the average particle size was 302.5 ± 5.3 nm. The size of MSiO2 (300 nm) ranged from 300 to 318 nm, and the average particle size was 311.5 ± 5.5 nm. The EDXS data provided information about the specific chemical elements present in bare SiO2 to MSiO2 particles. The existence of carbon, silicon, and oxygen in MSiO2 was confirmed by EDXS spectra generated by MPTS modifier.
The average zeta potential value for bare SiO2 was in the range of − 47.9 to − 53.1 mV and that of MSiO2 was − 47.1 to − 57.1 mV, which suggested that those particles were highly stable in water. Generally, colloids with a zeta potential value of more than ± 30 mV are considered stable.
Transparency of PAAm-MSiO2 hydrogels
The PAAm-MSiO2 hydrogels were highly transparent throughout the range of visible light (400 nm to 800 nm), and the transparency of the hydrogels was inversely proportional to the amount of MSiO2 cross-linker (Fig. 4). The PAAm-MSiO2 hydrogels made of 0.50% cross-linker showed more than 90% transmittance, while the transparency of PAAm-MSiO2 made of 1.25% cross-linker was around 75%. The high transparency of PAAm-MSiO2 hydrogel confirmed the homogenous dispersion of cross-linkers. The homogeneous size, shape, and stable dispersion in water of MSiO2 cross-linkers increased the possibilities of uniform polymer networks formation. The Rayleigh scattering occurs when light passes through a medium where the size of the agglomerations is smaller than the wavelength of visible light. The slopes for PAAm (4 M)-MSiO2 0.50%, PAAm (4 M)-MSiO2 0.75%, PAAm (4 M)-MSiO2 1.00%, and PAAm (4 M)-MSiO2 1.25% hydrogels (inset of Fig. 4 bottom-right) from ln(− lnT) versus ln λ plots are − 0.070, − 0.064, − 0.053, and − 0.044, respectively. The slopes of relevant trend lines imply that the hydrogels were slightly deviating from the Rayleigh scattering of light, which is inversely proportional to the fourth power of the wavelength30. Mie scattering of light was expected in the case of our hydrogels as the sizes of the aggregated clusters were similar to or slightly bigger than the wavelengths of visible light. Though all the PAAm-MSiO2 hydrogels produced Mie scattering of light, the size of clusters present in gels composed of a lower amount MSiO2 cross-linker was larger than that of gels composed of a higher amount of MSiO2 cross-linker.
Proposed structure of PAAm-MSiO2 hydrogel (top), the transmittance spectra of highly transparent hydrogel (bottom), transparent gel sample (inset-left), and the plots of ln(-lnT) versus lnλ (inset-right). In the graph, red line, green line, blue line, and purple line represent PAAm (4 M)-MSiO2 0.50%, PAAm (4 M)-MSiO2 0.75%, PAAm (4 M)-MSiO2 1.00%, and PAAm (4 M)-MSiO2 1.25% hydrogels, respectively.
The reinforcing effect of SiO2 particles can be adjusted finely and precisely by several other parameters, such as the nature of the particles, matrix properties, aspect ratio of particles, particle volume fraction, particle size, particle orientation and distribution, particle structural variations, and strength of interactions across the particles and polymer chains31. The reinforcement mechanism in polymer micro composites is similar to that present in nanocomposites except for the effect of particle size and structural parameters, which are expected to play a vital role in the mechanical behavior of nanocomposites. Micromechanical propositions such as the Halpin–Tsai32 and Mori–Tanaka33 approaches, extensively applied for micro composites, have been proposed by many researchers to evaluate the overall elastic stiffness of nanocomposite polymeric materials34. Researchers conjectured that the size of filler particles had an effect on the properties of composite materials, but reliable experimental data are still not available to support the concluding remark. Few research groups examined numerically nanocomposite properties applying spherical fillers (SiO2 particles) and observed that the composites properties could be intensified by decreasing the particle size35,36. Keeping in mind the scarcity of research data that reflects particle size’s influence on the reinforcement of PAAm-MSiO2 hydrogels, PAAm-MSiO2 hydrogels have been formulated by varying the diameters of MSiO2 cross-linker and investigated the particle size effect on mechanical properties of gels. The effect of concentration of both monomer and MSiO2 cross-linker on their mechanical strengths is also examined in detail.
Superior mechanical properties of PAAm-MSiO2 hydrogels than PAAm-BIS hydrogels
PAAm-MSiO2 and PAAm-BIS hydrogels were notably different with respect to their mechanical properties during elongation (Fig. 5). The tensile strength and Young’s modulus of PAAm-MSiO2 were low compared with the mechanically toughened PAAm-BIS hydrogels. But PAAm-BIS hydrogels readily broke at lower applied stress during elongation, and its elongation at break was found in the 135% to 160% range. Due to the inhomogeneous cross-linking density in PAAm-BIS hydrogel, the short polymer chain length failed to dissipate energy. On the contrary, PAAm-MSiO2 hydrogel demonstrated an extended elongation range reaching up to 2400% of strain (maximum instrumental limit) without breaking (Table 2). The chemical bonding, physical interactions, and the rigorous dispersion of MSiO2 cross-linker across the polymer chains of hydrogel network provided much emancipation to dissipate energy under applied stress. The tensile strength, Young’s modulus, and toughness of PAAm-MSiO2 hydrogels changed with increasing MSiO2 content. At constant MSiO2 content, the tensile strength, Young’s modulus, and toughness increased with the AAm concentration (Fig. 5a). The tensile properties of PAAm-BIS hydrogels were also dependent on the AAm concentration. PAAm-MSiO2 hydrogels and PAAm-BIS hydrogels were remarkably different with regard to their compressive strength. Typical strain–stress curves for different weight percentages of cross-linker based hydrogels have been shown in Fig. 5b. It was observed that PAAm-BIS hydrogels readily broke at lower deformation by applying force and its break was in the range of 38–45%. Contrariwise, PAAm-MSiO2 hydrogel did not get broken under 70 percent deformation. The brittleness of typical PAAm-BIS hydrogels could be trounced by employing MSiO2 as cross-linker for fabricating PAAm-MSiO2 hydrogel with soft rubber-like functions. The higher concentration of MSiO2 cross-linker increased the strength of PAAm-MSiO2 hydrogel by creating well-built and strong interactions across the polymer chains and MSiO2 particles, resulting in an increase in Young’s modulus, toughness, and compressive strength.
(a) Stress–strain curves of PAAm-MSiO2 and PAAm-BIS hydrogels prepared by varying the BIS and MSiO2 under uniaxial tension and (b) stress–strain curves of PAAm-MSiO2 and PAAm-BIS hydrogels prepared by varying the concentration of BIS and MSiO2 under uniaxial compression. In the graph, red line, green line, blue line, and purple line represent PAAm (4 M)-BIS 1.00%, PAAm (4 M)-MSiO2 1.00%, PAAm (2 M)-BIS 1.00%, and PAAm (3 M)-MSiO2 0.75% hydrogels, respectively.
Tensile and compressive strength of different PAAm-MSiO2 (100 nm, 200nnm, and 300 nm) hydrogels prepared by varying concentrations of AAm
Figure 6 shows the comparison of tensile and compressive strength of PAAm-MSiO2 NC hydrogels prepared by changing the concentration of AAm (3 M, 4 M, and 5 M) and the diameter of MSiO2 cross-linkers (117.5 nm, 216.5 nm, and 311.5 nm). The mechanical properties (toughness, tensile strength, and Young’s modulus) of PAAm-MSiO2 hydrogel significantly increased with the increase of AAm concentration under the constant weight percentage of MSiO2 cross-linker (Table 3). When the concentration of AAm increased, the entanglement of the polymer chains and the interaction between the AAm chain and MSiO2 cross-linker were also increased. The PAAm-MSiO2 hydrogel for the 3 M AAm concentration, the interaction between MSiO2 cross-linker and AAm chain was weaker than that of 4 M AAm hydrogel. As a result, it exhibited lower mechanical properties than that of 4 M hydrogels. But for the 5 M AAm concentration, it started to decrease the stress after reaching a certain point of stress. But for the other two hydrogels, the stress gradually increased after the yield point in the plastic region. Regardless of the direction in which the hydrogel was elongated, the hydrogel showed extraordinary extensibility that could reach 24 times the original length without a break. The stress–strain measurements could not be continued further due to the technical limitation of the instrument (Fig. 6a–c). The AAm concentration also significantly affected the compressive strength of PAAm-MSiO2 hydrogel. The toughness, compressive strength, and Young’s modulus of hydrogel significantly increased with increasing AAm concentration under the constant weight percentage of MSiO2 cross-linker. PAAm-MSiO2 hydrogel with high AAm concentration possessed higher compressive strength due to strong bonding between the polymer chain and MSiO2 particles and high entanglement of AAm polymer chains (Fig. 6d–f). Hence, when the AAm concentration was increased, the compressive strength, toughness, and Young’s modulus of hydrogel were increased (Table 4).
Tensile strength test results of PAAm-MSiO2 hydrogels prepared by 117.5 nm (a), 216.5 nm (b), and 311.5 nm (c) MSiO2 cross-linker and compressive strength test results of PAAm-MSiO2 hydrogels prepared by 117.5 nm (d), 216.5 nm (e), and 311.5 nm (f) MSiO2 cross-linker. In the graph, red line, green line, and blue line represent PAAm (3 M)-MSiO2 0.75%, PAAm (4 M)-MSiO2 0.75%, and PAAm (5 M)-MSiO2 0.75% hydrogels, respectively.
Tensile strength of PAAm-MSiO2 hydrogels prepared by varying particle sizes of MSiO2 (117.5 nm, 216.5 nm, 311.5 nm)
The tensile properties of the hydrogel were increased with decreasing particle size of MSiO2 cross-linkers. The smaller SiO2 particles with a large surface area require more MPTS than the larger SiO2 particle during the modification process. As a result, MSiO2 cross-linkers with shorter diameters could form more cross-linking points with the polymer chains to form high cross-linking density compared to MSiO2 cross-linkers with longer diameters. Furthermore, the interaction between polymer chains and cross-linkers increased because of a high degree of cross-linking. Consequently, the strong interactions restricted the movement of the polymer chain during elongation and demonstrated higher tensile strength, Young’s modulus, and toughness. After maintaining a constant concentration of AAm and MSiO2 but by changing the size of SiO2 particle from 117.5 to 311.5 nm; the tensile strength, toughness, and elastic modulus of the hydrogel decreased from 30 to 11 kPa, 409 to 231 kJ/m3 and 0.16 to 0.11 kPa, respectively. This trend continued for different concentrations of AAm (Fig. 7, Table 5). When the concentration of AAm was increased from 3 to 4 M, the tensile strength, toughness, and Young’s modulus increased from 11.2 to 20 kPa, 231 to 403 kJ/m3, and 0.11 to 0.17 kPa, respectively. A similar tendency was found for the other diameters of MSiO2 cross-linker (Table 5). The mechanical properties for PAAm-MSiO2 hydrogel containing 5 M AAm follow slightly different trend.
Stress–strain curves produced by tensile strength test of PAAm (3 M)-MSiO2 0.75% (a), PAAm (4 M)-MSiO2 0.75% (b), and PAAm (5 M)-MSiO2 0.75% (c), hydrogels and comparison of tensile strengths (d), toughness (e), and Young’s modulus (f) of PAAm (3 M)-MSiO2 0.75%, PAAm (4 M)-MSiO2 0.75%, and PAAm (5 M)-MSiO2 0.75 hydrogels. Red, green, and blue colors represent MSiO2 cross-linker having a diameter of 117.5 nm, 216.5 nm, and 311.5 nm, respectively.
Compressive strength of PAAm-MSiO2 hydrogels prepared by varying particle sizes of MSiO2 (117.5 nm, 216.5 nm, and 311.5 nm)
The stress–strain curves for compressive strength of PAAm-MSiO2 hydrogels prepared by varying particle sizes of MSiO2 (117.5 nm, 216.5 nm, and 311.5 nm) and different concentrations of AAm have been shown in Fig. 8a–c. When stress was applied to the hydrogel, the total energy could be dissipated throughout the homogeneously distributed hydrogel network. The compressive strength, toughness, and Young’s modulus of PAAm-MSiO2 hydrogels composed of 0.75% of MSiO2 cross-linker were increased when the diameter of MSiO2 increased (Fig. 8d–f, Table 6). We have proposed two hypotheses to address the phenomenon. Firstly, the bigger cross-linkers occupy more volume in space resulting increment of the ordered orientation of the polymer networks due to less entanglement probability and thereby provided enhanced mechanical properties. Secondly, the bigger particles are harder and more rigid than the small particles resulting improved compressive strength. When a force was applied, the hydrogel having a larger MSiO2 particle size resists more applied force than the gel cross-linked by small particles. As a result, the hydrogels were capable of resisting a tremendous compressive force and showed higher compressive strength, toughness, and Young’s modulus. The interlinkage of the SiO2 surface and polymer chain becomes stronger when the particle diameter decreases. Then the strong interaction obliges the polymer chains to enfold tightly to the SiO2 particles and makes hydrogel networks tough37,38,39. The high interfacial area between MSiO2 particles and AAm polymer matrix guides to a superior bonding between the two phases and provides excellent mechanical strength to the PAAm-MSiO2 hydrogels. Experimental investigations have confirmed that the dispersion of particles in the polymer matrix leads to an unprecedented increase in elastic stiffness, starting at a meager volume fraction of particle content16.
Stress–strain curves produced by compressive strength test of PAAm (3 M)-MSiO2 0.75% (a), PAAm (4 M)-MSiO2 0.75% (b), and PAAm (5 M)-MSiO2 0.75% (c), hydrogels and comparison of compressive strengths (d), toughness (e), and Young’s modulus (f) of PAAm (3 M)-MSiO2 0.75%, PAAm (4 M)-MSiO2 0.75%, and PAAm (5 M)-MSiO2 0.75% hydrogels. Red, green, and blue colors represent MSiO2 cross-linker having a diameter of 117.5 nm, 216.5 nm, and 311.5 nm, respectively.
Conclusion
PAAm-MSiO2 hydrogel with better mechanical properties than its conventional counterpart PAAm-BIS hydrogels has been reported. The poor mechanical strength of conventional hydrogel is improved by incorporating vinyl-modified SiO2 particles as cross-linkers, which get dispersed homogeneously throughout the internal spaces of gel matrix and create strong bonds with AAm polymer network capable of transforming from an intertwined globular shape to coiled shape having the ability to withstand against high compression and stretch. The PAAm-MSiO2 hydrogel has very high stretchability even under high applied forces. The mechanical properties of PAAm-MSiO2 hydrogel might be regulated accurately by changing the concentration of both monomer and MSiO2 cross-linker. The experimental data of our project correlates between the various mechanical parameters of PAAm-MSiO2 hydrogels and the particle size of MSiO2 cross-linker. The tensile strength, toughness, and Young’s modulus decreased with the increase of MSiO2 cross-linker size from 117.5 to 311.5 nm under uniaxial tensile deformation. The PAAm-MSiO2 hydrogel prepared by MSiO2 having diameter of 117.5 nm exhibited highest tensile strength, toughness, and Young’s modulus during elongation compared with other hydrogels. In contrast, PAAm-MSiO2 hydrogel with a large MSiO2 particle size demonstrated higher compressive strength, toughness, and Young’s modulus compared with other hydrogels under uniaxial compressive deformation. The report will be a good source for researchers exploring and searching for effective and reliable techniques to improve the mechanical properties of organic and inorganic composite hydrogels and other smart materials.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Imran, A. B. Design, development, characterization and application of smart polymeric hydrogel. In Manufacturing Systems: Recent Progress and Future Directions (ed. Mellal, M. A.) (Nova Science Publishers, Inc., 2020).
Imran, A. B., Seki, T. & Takeoka, Y. Recent advances in hydrogels in terms of fast stimuli sensitivity and mechanical strength. Polym. J. 42, 839–851. https://doi.org/10.1038/pj.2010.87 (2010).
Wichterle, O. & Lím, D. Hydrophilic gels for biological use. Nature 185(4706), 117–118. https://doi.org/10.1038/185117a0 (1960).
Osada, Y., Kawamura, R. & Sano, K. Hydrogels of Cytoskeletal Proteins 1–2 (Springer, 2016).
Li, H. Smart Hydrogel Modelling 1–2 (Springer, 2009).
Zhang, X., Lin, G., Kumar, S. R. & Mark, J. E. Hydrogels prepared from polysiloxane chains by end linking them with trifunctional silanes containing hydrophilic groups. Polymer (Guildf.) 50, 5414–5421 (2009).
Liu, C. et al. Optically transparent, high toughness elastomer using a polyrotaxane cross-linker as a molecular pulley. Sci. Adv. 4(10), 1–9. https://doi.org/10.1126/sciadv.aat7629 (2018).
Imran, A. B., Seki, T., Ito, K. & Takeoka, Y. Poly(N-isopropylacrylamide) gel prepared using a hydrophilic polyrotaxane-based movable cross-linker. Macromolecules 43(4), 1975–1980. https://doi.org/10.1021/ma902349j (2010).
Asai, M., Katashima, T., Chung, U.-I., Sakai, T. & Shibayama, M. Correlation between local and global inhomogeneities of chemical gels. Macromolecules 46(24), 9772–9781 (2013).
Okay, O. How to design both mechanically strong and self-healable hydrogels? Adv. Polym. Sci. https://doi.org/10.1007/12_2019_53 (2020).
Ito, K. Novel cross-linking concept of polymer network: Synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym. J. 39(6), 489–499 (2007).
Imran, A. B. et al. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 5, 5124 (2014).
Haraguchi, K. & Takehisa, T. Nanocomposite hydrogels: A unique organic–inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 14(16), 1120–1124 (2002).
Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 15(14), 1155–1158. https://doi.org/10.1002/adma.200304907 (2003).
Huang, T. et al. A novel hydrogel with high mechanical strength: A macromolecular microsphere composite hydrogel. Adv. Mater. 19(12), 1622–1626. https://doi.org/10.1002/adma.200602533 (2007).
Sinha Ray, S. & Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 28(11), 1539–1641. https://doi.org/10.1016/j.progpolymsci.2003.08.002 (2003).
Simão, A. R. et al. Drug polarity effect over the controlled release in casein and chondroitin sulfate-based hydrogels. Int. J. Biol. Macromol. 158, 116–126. https://doi.org/10.1016/j.ijbiomac.2020.04.179 (2020).
Zhang, Z., Wang, S., Waterhouse, G. I. N., Zhang, Q. & Li, L. Poly(N-isopropylacrylamide)/mesoporous silica thermosensitive composite hydrogels for drug loading and release. J. Appl. Polym. Sci. 137(8), 48391. https://doi.org/10.1002/app.48391 (2019).
Zhong, M. et al. Self-healable, tough and highly stretchable ionic nanocomposite physical hydrogels. Soft Matter 11(21), 4235–4241. https://doi.org/10.1039/c5sm00493d (2015).
Shi, F.-K., Wang, X.-P., Guo, R.-H., Zhong, M. & Xie, X.-M. Highly stretchable and super tough nanocomposite physical hydrogels facilitated by the coupling of intermolecular hydrogen bonds and analogous chemical cross-linking of nanoparticles. J. Mater. Chem. B 3(7), 1187–1192. https://doi.org/10.1039/C4TB01654H (2015).
Takafuji, M., Yamada, S. & Ihara, H. Strategy for preparation of hybrid polymer hydrogels using silicananoparticles as multifunctional cross-linking points. Chem. Commun. 47(3), 1024–1026. https://doi.org/10.1039/c0cc02891f (2011).
Jamali, A., Moghbeli, M. R., Ameli, F., Roayaie, E. & Karambeigi, M. S. Synthesis and characterization of pH-sensitive poly(acrylamide-co-methylenebisacrylamide-co-acrylic acid) hydrogel microspheres containing silica nanoparticles: Application in enhanced oil recovery processes. J. Appl. Polym. Sci. 137(12), 48491. https://doi.org/10.1002/app.48491 (2019).
Carlsson, L., Rose, S., Hourdet, D. & Marcellan, A. Nano-hybrid self-crosslinked PDMA/silica hydrogels. Soft Matter 6(15), 3619–3631. https://doi.org/10.1039/c0sm00009d (2010).
Genovese, D. B., Ye, A. & Singh, H. High methoxyl pectin/apple particles composite gels: Effect of particle size and particle concentration on mechanical properties and gel structure. J. Texture Stud. 41(2), 171–189. https://doi.org/10.1111/j.1745-4603.2010.00220.x (2010).
Yang, J., Wang, X.-P. & Xie, X.-M. In situ synthesis of poly(acrylic acid) physical hydrogels from silicananoparticles. Soft Matter 8(4), 1058–1063. https://doi.org/10.1039/c1sm06647a (2012).
Chang, A., Babhadiashar, N., Barrett-Catton, E. & Asuri, P. Role of nanoparticle–polymer interactions on the development of double-network hydrogel nanocomposites with high mechanical strength. Polymers 12(2), 470. https://doi.org/10.3390/polym12020470 (2020).
Levin, M., Sonn-Segev, A. & Roichman, Y. Structural changes in nanoparticle-hydrogel composites at very low filler concentrations. J. Chem. Phys. 150(6), 064908. https://doi.org/10.1063/1.5053171 (2019).
Azimi Dijvejin, Z., Ghaffarkhah, A., Sadeghnejad, S. & Vafaie Sefti, M. Effect of silica nanoparticle size on the mechanical strength and wellbore plugging performance of SPAM/chromium (III) acetate nanocomposite gels. Polym. J. 51, 693–707. https://doi.org/10.1038/s41428-019-0178-3 (2019).
Rezaul Karim, M., Harun-Ur-Rashid, M. & Imran, A. B. Highly stretchable hydrogel using vinyl modified narrow dispersed silica particles as cross-linker. ChemistrySelect 5(34), 10556–10561. https://doi.org/10.1002/slct.202003044 (2020).
Van Tent, A. & te Nijenhuis, K. Turbidity study of the process of film formation of polymer particles in drying thin films of acrylic latices: I. Intrastructure of acrylic latices studied with transmission spectrophotometry. J. Colloid Interface Sci. 150, 97–114 (1992).
Zaïri, F., Gloaguen, J. M., Naït-Abdelaziz, M., Mesbah, A. & Lefebvre, J. M. Study of the effect of size and clay structural parameters on the yield and post-yield response of polymer/clay nanocomposites via a multiscale micromechanical modelling. Acta Mater. 59(10), 3851–3863. https://doi.org/10.1016/j.actamat.2011.03.009 (2011).
Affdl, J. C. H. & Kardos, J. L. The Halpin-Tsai equations: A review. Polym. Eng. Sci. 16(5), 344–352. https://doi.org/10.1002/pen.760160512 (1976).
Mori, T. & Tanaka, K. Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Metall. 21(5), 571–574. https://doi.org/10.1016/0001-6160(73)90064-3 (1973).
Brune, D. A. & Bicerano, J. Micromechanics of nanocomposites: Comparison of tensile and compressive elastic moduli, and prediction of effects of incomplete exfoliation and imperfect alignment on modulus. Polymer 43(2), 369–387. https://doi.org/10.1016/s0032-3861(01)00543-2 (2002).
Zamani Zakaria, A. & Shelesh-Nezhad, K. Quantifying the particle size and interphase percolation effects on the elastic performance of semi-crystalline nanocomposites. Comput. Mater. Sci. 117, 502–510. https://doi.org/10.1016/j.commatsci.2016.02.026 (2016).
Blivi, A. S., Benhui, F., Bai, J., Kondo, D. & Bédoui, F. Experimental evidence of size effect in nano-reinforced polymers: Case of silica reinforced PMMA. Polym. Test. 56, 337–343. https://doi.org/10.1016/j.polymertesting.2016.10.025 (2016).
Choi, J., Shin, H., Yang, S. & Cho, M. The influence of nanoparticle size on the mechanical properties of polymer nanocomposites and the associated interphase region: A multiscale approach. Compos. Struct. 119, 365–376. https://doi.org/10.1016/j.compstruct.2014.09.014 (2015).
Jang, J.-S., Bouveret, B., Suhr, J. & Gibson, R. F. Combined numerical/experimental investigation of particle diameter and interphase effects on coefficient of thermal expansion and young’s modulus of SiO2/epoxy nanocomposites. Polym. Compos. 33(8), 1415–1423. https://doi.org/10.1002/pc.22268 (2012).
Liu, H. & Brinson, L. C. Reinforcing efficiency of nanoparticles: A simple comparison for polymer nanocomposites. Compos. Sci. Technol. 68(6), 1502–1512. https://doi.org/10.1016/j.compscitech.2007.10.033 (2008).
Acknowledgements
A.B. Imran gratefully acknowledges the support of Capacity Utilization Programme under Special Allocation for Science and Technology from the Ministry of Science and Technology, Peoples Republic of Bangladesh. The author is also thankful to the Committee for Advanced Studies and Research (CASR) in BUET and The World Academy of Sciences (TWAS), Italy, a program of UNESCO for funding.
Author information
Authors and Affiliations
Contributions
A.B.I. designed, conceptualized, supervised and finalized the project. M.R.K. wrote the initial draft and carried out the experimental part. M.H.R. help to analyze data and finalize the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karim, M.R., Harun-Ur-Rashid, M. & Imran, A.B. Effect of sizes of vinyl modified narrow-dispersed silica cross-linker on the mechanical properties of acrylamide based hydrogel. Sci Rep 13, 5089 (2023). https://doi.org/10.1038/s41598-023-32185-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32185-4
- Springer Nature Limited