Abstract
Farmyard manure is the most common type of organic fertilizer, and its properties depend mainly on the type of livestock, bedding material and the conditions of fermentation. Co-maturing of manure with other amendments to modify its final properties has been seen as a win–win strategy recently. This study aimed to evaluate the differences in the effect of unenriched manure and manures co-matured with biochar, elemental sulfur or both amendments on the soil physico-chemical and biological properties, and plant (barley, maize) biomass production. For this purpose a pot experiment was carried out in a time-dependent way. Samples were taken from 12 week-lasting (test crop barley) and 24 week-lasting (test crop maize) pot cultivation carried out in a growth chamber. Co-matured manure with biochar showed the highest rate of maturation expressed as humic to fulvic acid ratio, its amendment to soil significantly increased the dry aboveground biomass weight in the half-time (12 weeks) of experiment. However, the effect vanished after 24 weeks. We received for this variant highest long-term (24 weeks) contents of total carbon and nitrogen in soil. Contrarily, co-matured manure with biochar and elemental sulfur led to short-term carbon sequestration (the highest total carbon in 12 weeks) due to presumed retardation of microbial-mediated transformation of nutrients. We conclude that the prolonged pot experiment with biochar or elemental sulfur enriched manure led to the increased recalcitrancy of soil organic matter and retardation of soil nutrient transformation to the plant-available form.
Similar content being viewed by others
Introduction
One of the main negative consequences of poor soil management, unsustainable agricultural practices, pollution, wind and water erosion is the loss of soil organic matter (SOM)1,2,3. That is closely related to decrease in soil fertility and decline in biological activity and quality of soil. Application of organic fertilizers can restore and maintain a suitable SOM content in soil4. Farmyard manure is the most common type of organic fertilizer with key role in maintaining of quality and healthy arable soil in sustainable agriculture5. Manure amendment to the agricultural soil positively affects formation of SOM, improves the soil structure as well as the fertility of the soil6,7. Moreover, manure contributes significantly to increasing soil water storage, crop yield and soil microbial activity8,9,10. It represents a good source of nutrients, especially carbon, nitrogen, phosphorus, and other minerals for both, plants and soil organisms, including microbes11. The properties of manure are variable and depend mainly on the type of livestock, bedding material and the conditions of fermentation, which can be modified to achieve the intended quality of the product12.
However, co-composting is another way how to modify the properties of final manure. Co-composting is based on the affecting the processes and manure quality via amendment of another mostly organic materials13. For example, biochar has a great potential for enhancing composting of manure, it modifies the thermodynamics and heat generation in fermentation process14, improve compost mixture physicochemical properties15, enhance microbial activities16, promoted organic matter decomposition17, reduced greenhouse gas emissions18, namely ammonia19 upgrade compost quality via increased total/available nutrient content20, enhanced maturity, and decreased phytotoxicity and nutrient leaching21. The time-related effect of biochar-based organic amendments is another issue which has been in the focus of several studies22,23,24. The primary effect of biochar on the soil properties was evaluated and reported by several studies25,26,27,28 as well as long-time effect22,29,30, but the research focused on the comparison and contrasting the time-shifted impact on soil23 might be broaden.
Amendment of manure with elemental sulfur have also shown promising results in previous studies. Application of elemental sulfur incerases nutrient availability31,32, namely in calcareous soils33,34, and serves as an additive changing the physico-chemical properties of soil35,36. Moreover, it has a potential to increase crop yields37. Europe's agricultural faces recently an emerge of sulfur deficiency due to decline in SO2 emissions, which has been reduced to 20% compared to levels 30 years ago38. The general data of soil analyses carried out in the Czech Republic show that 85% of soil has low sulfur content39. Nevertheless, the effect of elemental sulfur on manure quality, soil properties and plant growth upon the combined treatments with manure or biochar have been reported in few studies40,41,42, and still raises a need for further investigation.
The objectives of this study were to evaluate: (I) the differences in the effect of unenriched manure and manures co-matured with amendments (biochar, elemental sulfur, and combination of both) on the soil physico-chemical and biological properties, and plant biomass production, (II) the time-effect of soil amendment with these various manure types on the respective properties and potential changes in the impact in a shorter time (12 weeks) and longer time (24 weeks) of pot experiment. It was hypothesized that early effects of amendments would be more significant and may become weakened and less demonstrable with longer period of interaction with soil. A presumption was adopted that sulfur and/or biochar enhanced the growth and activity of manure-derived microflora which would accelerate the nutrient transformation processes in the amended soil and expedited changes in soil chemistry would culminate in the early, not in the later phase of the whole trial.
Materials and methods
Production and analyses of co-matured manures
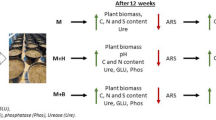
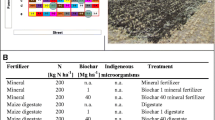
Farmyard manure was collected from cattle breeding farm without marketable milk production at Research institute for cattle breeding Ltd., located in village Rapotin, Czech Republic, Central Europe (49°58′46.4″ N, 17°0′26,6″ E). The basic physical and chemical properties of manure before activation (via co-maturation) were as follows: pH (H2O) 9.035 ± 0.298, Nkjeldahl 2.578 ± 0.085 g kg−1, Ctot 9.100 ± 0.300%, HA:FA 0.565 ± 0.019, Stot 0.786 ± 0.026 g kg−1, Ctot : Stot 11.578 ± 0.039, dsr 10⋅106 ± 0.01⋅106 copies⋅g−1. Manures were prepared in these variants: unenriched manure (M), manure mixed with elemental sulfur (M + S), manure mixed with biochar (M + B), manure mixed with biochar and elemental sulfur (M + B + S), more detailed description of the variants in (Table 1).
Biochar used in the experiment was commercially produced from agricultural waste (cereal husks, sunflower peels and fruit mud) at 600 °C (Sonnenerde GmbH, Riedlingsdorf, Austria). According to manufacturer, the properties of the biochar were as follows: elements (g·kg−1) C 866, N 3.0, O 10.0, H 14.2, P 2.5; Ash550 °C 11.7%, salts 0.42%, pH (CaCl2) 8.5, BET surface area 288.5 m2⋅g−1, bulk density 120 kg⋅m3. Elemental sulfur was produced as waste product during desulfurization of biogas in THIOPAQ scrubber (Paques, Netherlands).
Manure and amendments were dosed into the 50-L tightly closable barrels according to (Table 1), thoroughly kneaded and mixed. For a further manure application to the soil equivalent to 50 t ha−1, doses equal to 20 t ha−1 of biochar and 0.7 t ha−1 of elemental sulfur were calculated. Each variant was prepared in 3 replicates and the mixtures tightly closed to decrease desiccation and prevent air contamination from the surroundings. Activation process was similar as reported previously43, carried out for 8 weeks under room temperature (25 ± 2.5 °C) and aerobic conditions. Temperature and relative air humidity were monitored (Table 2) every 2 weeks and the content of each barrel was mixed.
Manure pH in H2O was determined by electrolytic measurement according to ISO 10,390:200544, total Kjeldahl N (NKjeldahl) was determined by Kjeldahl procedure according to ISO 11,261:199545, total carbon (Ctot) was measured by dry combustion according to ISO 10,694:199546, humic and fulvic acids were determined in the extract according to ISO 19,822:201847, Stot was determined by ICP-OES according to EN 15,74948, sulfur-reducing microorganisms (dsr) were determined by real-time qPCR detection of gene coding for sulfur reductase according to49. The determined parameters for manure amendments are listed in (Table 3).
Collection of soil, treatment plan and pot experiment
The experimental soil was collected from the rural area near the town Troubsko, Czech Republic (49°10′28"N 16°29′32"E). The soil was a silty clay loam according to USDA Textural Triangle, Haplic Luvisol according to WRB soil classification (FAO, 2014) same as in the previous study50, content of nutrients in g·kg−1: total carbon (TC) 14.00, total nitrogen (TN) 1.60, P 0.10, S 0.15, Ca 3.26, Mg 0.24, K 0.23, pH (CaCl2) 7.29.The topsoil was dug up to the depth of 15 cm, big parts (stones, lumps) were removed on a sieve (2 mm). The sieved soil was mixed with fine quartz sand (0.1–1.0 mm) in weight ratio 1:1.
The three tested variants were prepared by thorough mixing of 5 kg of experimental soil with 200 g of particular manure type per a pot, equals to 50 t⋅ha−1. Unamended control (control) contained only 5 kg of experimental soil. Each variant was prepared in four pots of volume 5 L and marked equally to the used manure type.
To investigate the effectiveness of prepared variants, barley (Hordeum vulgare L.), a crop with lower requirements of sulfur, was grown for first 12 weeks of the experiment. Each pot was sown with 16 barley seeds 2 mm under soil surface. All pots were placed randomly into growth chamber (CLF Plant Climatics GmbH, Germany) and rotated every other day to ensure homogeneity of conditions for the treatments. Controlled conditions were set as follows: 12 h long photoperiod, light intensity 370 µmol·m−2·s−1, temperature (day/ night) 20/12 °C, relative air humidity (day/ night) 45/70%. Pots were watered with distilled water. Soil moisture was determined gravimetrically and maintained at 65% of water holding capacity throughout the experiment51. The number of plants were reduced to 12 in each pot after 2 weeks. After 12 weeks, the aboveground biomass of barley plants was harvested, and 100 g of soil was taken from each pot for analyses.
Then, each pot with remaining soil and barley roots was sown with five maize (Zea mays L) seeds, for maize is a crop with higher demand to sulfur content in soil. Seedlings were reduced to 2 plants after 2 weeks. The experiment was carried out under the same growth conditions for further 12 weeks, i.e., 24 weeks in total, following same sampling protocols as described above.
Plant biomass and soil properties determination
Harvested barley and maize biomass were dried at 60 °C to constant weight. The dry above ground biomass (AGB) was determined gravimetrically using the analytical scales.
The soil samples were homogenized by sieving through a 2 mm mesh. Air-dried samples were used for determination of soil pH in CaCl243, total soil carbon (TC) and nitrogen (TN) content45,52. The samples stored at 4 °C were used for determination of soil basal (BR) and substrate induced respirations: D-glucose (Glc-SIR), D-trehalose (Tre-SIR), N-acetyl-β-D-glucosamine (NAG-SIR), L-alanine (Ala-SIR), L-lysine (Lys-SIR), L-arginine (Arg-SIR), according to53. The freeze-dried samples were prepared for the enzymes, arylsulfatase (ARS), N-acetyl-β-D-glucosaminidase (NAG), and urease (Ure), activity assays:54.
Statistical analyses
Data obtained from the performed measurements were statistically analyzed using the principal component analysis (PCA), one-way analysis of variance (ANOVA), Tukey HSD post-hoc test (at significance level p = 0.05), and Pearson correlation analysis (Program R, version 3.6.1)55.
The Rohlf’s PCA Analysis was used to evaluate the mutual dependence among the properties and their values in individual compared variants of amended soil. The results of Pearson’s correlation analysis were mentioned when value of the correlation coefficient r was: 0.5 < r < 0.7 (moderate correlation) and 0.7 < r < 0.9 (high correlation)56.
Results
Plant traits under applied amendments
After 12 weeks of cultivation, the significantly highest weight of dry aboveground biomass (AGB dry) of barley was found in the variant M + B, but M and M + S variants also exerted AGB dry significantly increased as compared to the unamended control, (Fig. 1). Significant (p ≤ 0.001; p ≤ 0.01) moderate positive correlation (Fig. S2a) was observed between AGB dry and N-acetyl-b-D-glucosamine-induced, D-glucose-induced, and L-lysine-induced respiration (NAG-SIR, Glc-SIR, Lys-SIR; r was 0.77, 0.69, 0.67, respectively). At the end of experiment (24 weeks), there was no statistical difference among all amended soil variants, which exerted significantly increased AGB dry of maize in comparison to the control, (Fig. 1). The final values of AGB dry significantly (p ≤ 0.001) and moderately positively correlated with soil total carbon (TC, r = 0.64) and highly negatively correlated with pH (r =− 0.72), (Fig. S2b).
Soil physico-chemical properties
After 12 weeks of cultivation, soil pH of was significantly decreased (as compared to the control and variants M and M + B) in both variants amended with elemental sulfur, M + S and M + B + S. Moreover, the M + B + S variant showed significantly lowest pH, (Fig. 2). The pH after 12 weeks correlated (Fig. S2a) significantly (p ≤ 0.001) and moderately negatively with TC (r = − 0.67) content. However, this assumed sulfur-derived effect seemed short-timed again, thus pH after 24 weeks of cultivation exerted no significant difference among all amended variants, which all were significantly decreased compared to the control, (Fig. 2). The pH after 24 weeks negatively correlated (Fig. S2b) with TC less strongly albeit still apparently (r = − 0.53, p ≤ 0.001). The overall effect of manure on pH was more significant than the impact of any amendment in the end of experiment.
After 12 weeks of cultivation, the total soil carbon (TC) was significantly the highest in the M + B + S and variant M + B contained significantly more TC compared to the variant M + S, M and control, Fig. 2a. These results correspond to the total carbon content in the applied manure variants, which were (in average): 24.1% (M + B + S), 21.0% (M + B), 10.0% (M + S), 9.0% (M). A putative contribution of sulfur addition to the carbon sequestration was assumed, however, only increase in biochar-derived carbon was significantly detectable. The higher TC availability likely anticipated higher carbon utilization, as far as we can assume from the moderate positive correlation of TC and respirations (e.g., BR, NAG-SIR, Arg-SIR; r was 0.61, 0.62, 0.64, respectively; p ≤ 0.001); furthermore, TC correlated with TN (r = 0.55; p ≤ 0.001). Again, vastly different was the determination of TC at the end of experiment (24 weeks), when the highest value was detected in M + B variant. TC values of M + B and M + B + S variant were significantly higher compared to the variants M + S and control, Fig. 3a. At the end of experiment, TC correlated (Fig. S2b) most with total soil nitrogen (TN, r = 0.65) and with AGB dry (r = 0.64), which corroborated the presumed mutual relation of nutrient content (or sequestration) and high plant (maize) biomass yield. Higher TC content was again a putative prerequisite for higher transformation activity, as indicated by TC and respiration correlation (i.a. Arg-SIR, NAG-SIR, Ala-SIR; r was 0.65, 0.58, 0.53, respectively; p ≤ 0.001).
Soil basal (a) and D-glucose-induced (b) D-trehalose (c) and N-acetyl-b-D-glucosamine (d) induced respiration, L-alanine (e), L-lysine (f) and L-arginine (g) induced respiration after 12 and 24 weeks of cultivation. Average values (n = 4) are displayed, error bars are standard error of mean. The different letters of variant values indicate a statistical difference among them at the level p ≤ 0.05.
In the early phase of experiment, the soil TN was significantly increased in variants M, M + S, and M + B + S as compared to the control, Fig. 2b. These results correspond (with exception of co-matured manure M + B) to the values of TN content in the respective manure variants: M (2.48%), M + S (2.39%), M + B + S (1.88%), M + B (1.99%). The presumed role of enhanced nutrient oxidation in mitigation of nitrogen volatilization was ascribed from the significant positive moderate correlation between TN and N-acetyl-b-D-glucosamine-induced respiration (NAG-SIR, r = 0.61), Fig. S2a. The final TN values of amended variants after 24 weeks exerted no significant increase compared to the control, except of the statistically highest TN in the M + B variant, Fig. 2b. The only detected markable positive correlation was between TN and TC (r = 0.65), (Fig. S2b).
Soil microbial activity
Microbial activity in soil was determined as soil respiration (basal and induced by various substrates) and activity of soil enzymes. In the first half of the experiment (12 weeks) the significantly increased basal respiration (BR), compared to the control, was received in all manure-amended variants. However, the BR values of variants M + B and M + B + S were significantly higher in comparison to the M and M + S variants, Fig. 3a. The BR correlated (Fig. S2a) significantly (p ≤ 0.001), positively and moderately with TC (r = 0.61), and with all respiration types, the most with NAG-SIR (r = 0.65), Lys-SIR and Arg-SIR (r was 0.68 both). At the end of experiment (24 weeks), the previously enhanced BR in the variant M + B + S was mitigated to values comparable to the control, the significantly increased BR was observed in the M and M + B soil, (Fig. 3a). The data from the 24th week of cultivation showed also a significant positive moderate (to high) correlation between BR and NAG-SIR, Lys-SIR, Arg-SIR (r was 0.67, 0.65, 0.78, respectively), (Fig. S2b). Very similar results as the basal respiration showed almost all types of substrate-induced respiration.
The M + B variant showed the significantly highest Glc-SIR, Tre-SIR, NAG-SIR and Lys-SIR compared to all other variants after 12 weeks of barley cultivation. The control exerted significantly decreased Glc-SIR, Tre-SIR, NAG-SIR, Ala-SIR and Lys-SIR compared to the variants amended with any manure type, (Fig. 3a-g),. After 24 weeks, the significantly highest values of all substrate-induced respirations were found in the M + B. Nevertheless, the control variant exerted no significant difference to the M, M + S, and M + B + S variants in parameters Glc-SIR, and Tre-SIR, (Fig. 3b). After 12 weeks, Glc-SIR significantly (p ≤ 0.001) positively correlated with all respiration types—the most with Tre-SIR, NAG-SIR (r were 0.72 and 0.73, respectively)—and after 24 weeks with NAG-SIR, Ala-SIR, Lys-SIR (r were 0.51, 0.65, 0.72, respectively), (Fig. S2a, b).
After 12 weeks, significant positive correlation was found between Lys-SIR (Arg-SIR) and AGB dry (r was 0.65 and 0.67, respectively), Ala-SIR correlated (p ≤ 0.001) positively with all respiration types (e.g., Lys-SIR and Arg-SIR; r was 0.59 and 0.60, respectively) and with Ure (r = 0.46), whereas negatively with arylsulfatase (ARS, r = 0.50) (Fig. S2a). At the end of experiment, the mutual correlations of Ala-SIR with both other respiration types (e.g., Lys-SIR and Arg-SIR, r was 0.88 and 0.72, respectively) and Ure (r = 0.46) were again revealed, even higher than in the early cultivation. Whereas the relation to TC in soil was more apparent: TC and Ala-SIR, Arg-SIR showed r value 0.53 and 0.65, respectively, (Fig. S2b).
After 12 weeks of experiment, the highest average soil ARS activity was received in the control variant, which showed comparable value to M + S variant and significantly increased ARS compared to the variants M, M + B, M + B + S, Fig. 4a. The PCA biplot showed that ARS was antagonistic to the BR in soil, (Fig. S1a). The end-values (24th week) of ARS were comparable among the control and all other variants, from which M + S had a significantly increased ARS in comparison to the variants M and M + B + S (Fig. 4a).
Soil arylsulfatase (a), N-acetyl-b-D-glucosaminidase (b), and urease (c) activity after 12 and 24 weeks of cultivation. Average values (n = 4) are displayed, error bars are standard error of mean. The different letters of variant values indicate a statistical difference among them at the level p ≤ 0.05.
In the early phase of the experiment (12 weeks), NAG activity did not differ significantly among all variants except of M + B + S, which was significantly decreased compared to the others (Fig. 4b). The long-termed cultivation (24 weeks) preserved the low NAG activity in M + B + S, which only was comparable to the control. The other variants (M, M + B, M + S) exerted significantly increased NAG compared to the control (Fig. 4b). The PCA biplot revealed synergism between NAG and Lys-SIR, Ala-SIR, (Fig. S1b).
Urease (Ure) activity was already presented as the significantly highest in the M and M + S variants, but also M + B variant was significantly increased compared to the control after 12 weeks, (Fig. 4c). A significant positive correlation (Fig. S2a) was received between Ure and respirations (Tre-SIR, NAG-SIR, Ala-SIR; r were 0.5, 0.39, 0.46; p ≤ 0.001), enzymes (Phos, GLU; r were 0.57, 0.33; p ≤ 0.001) as well as AGB dry (r = 0.52). The end-values (after 24 weeks of the experiment) of Ure in control and variants with enriched manure (M + B, M + S, M + B + S) were significantly increased as compared to control variant, the highest average value of Ure activity showed M + B variant (Fig. 4c).
Discussion
Plant traits under applied amendments
In the early phase of experiment, only the co-matured manure with biochar (M + B) had a significantly higher beneficial effect on plant (barley) biomass yield in comparison to the application of unenriched manure (M), thus it increased the barley AGB dry. It was in the line with the reported results55. Nevertheless, the observed biochar-derived benefit cannot be coupled with the increased soil water holding capacity. The other types of enriched co-matured manures (M + B + S, M + S) increased AGB dry compared to the control soil variant, but not compared to the unenriched manure (M). We presumed the beneficial effect of elemental sulfur supplement to the soil used in this experiment, because the chosen soil type had relatively low total S content, compared to other arable soils in the Czech Republic56. The M + B manure showed the highest rate of maturation determined as humic:fulvic acid ratio—HA:FA = 2.04 (Table 3). These results agreed with the referred enrichment of manure humic acids in relation to the fulvic acid fraction in manure–biochar mixture57. Putatively, these properties, together with high amount of pyrolyzed carbon matter, anticipated the most demonstrable contribution to soil nutrient availability and plant biomass yield. Nevertheless, it was assumed (similarly as in57) that manure–biochar mixture featured high polymerization degree of humic-like substances at the end of the maturation process, therefore amendment of soil with this respective manure (+ biochar) reduced in the later phase of the experiment (12th to 24th week) the degradability of total soil (mainly organic) carbon. It presumably resulted from the relative enrichment of recalcitrant organic matter due to the preferential degradation of labile, non-aromatic organic matter, similarly as referred by58. Contrarily, the co-matured manure with combined biochar and elemental sulfur was found the least beneficial to the soil fertility and barley biomass, putatively due to the adverse co-effect of the lowest pH, nutrient transformation rate indicated by the lowest values of Ure, Phos, NAG, and the highest C:N ratio. These features documented assumed retarded mineralization and mediation of nutrients acquisition by plants.
Nevertheless, the differences derived by application of variable manures were mitigated in the second half of the experiment, leading to the comparable maize yield in all manure-amended soils. Although, the M + B variant revealed the highest average TC, TN, Ure activity indicating increased nitrification rate, the M variant exerted the highest average AGB dry (Fig. 1). We assume that the prolonged incubation of biochar under experimental conditions led to the increased recalcitrancy of soil organic matter (SOM) and retardation of nutrient transformation to the plant-available form. However, the results could also be affected by the sensitivity of barley to soil pH and the plasticity of maize to the experimental conditions.
Soil physico-chemical properties
The significant decrease of pH value in the M + S variant was assumed and explainable as there are studies which referred to the acidifying effect of elemental sulfur on the pH of organic fertilizers42,59 or soil60. The novel finding is that combination of biochar and elemental sulfur in co-matured manure even more decreased soil pH after short period (12 weeks). That presume an expected specific impact of elemental sulfur amendment and its transformation on the biochar interaction and decomposition in soil, which differed from sole biochar interaction with manure and its impact on soil pH value. We explained this observation by the putatively increased oxidation of biochar in the M + B + S manure and soil due to the parallel oxidation of elemental sulfur. It was in the line with study61 which expected microbial induced "in situ digestion" of biochar by oxidation of elemental sulfur. This biochar digestion could subsequently reduce its pH-buffering capacity and lead to more significant pH decrease. After 24 weeks of experiment, this significant effect faded out due to the strong negative impact of all manure-based amendments (M, M + B, M + B + S, M + S) on the soil pH. Such observation is rare, thus most authors referred to pH-increasing effect of either manure62 of biochar63 soil amendment. The study64 reported of the pH-decreasing effect of biochar due to the adsorption of ammonium nitrogen on the biochar surface. We assume that its stabilization via biochar in M + B, M + B + S variants or decrease in ammonium concentrations via its consumption/conversion in M, M + S variants caused the observed change in pH.
The highest contribution of M + B + S manure amendment to soil TC was related to the highest carbon content in the end of manure maturation, (Table 3). It was in the agreement with the reported sulfur-enriched biochar positive effect on carbon sequestration65. The contribution of sole biochar in co-matured manure to the total soil carbon is well known66, less documented is the positive effect of co-matured manure with sulfur on soil organic matter, fertility, and crop growth67. However, the strong positive effect of elemental sulfur to TC sequestration was only short-termed, while the stabilizing effect on TC of the co-matured manure with sole biochar was more significantly lasting. These finding are related to references of short-termed beneficial effect of sulfur-enhanced biochar68, whereas storing carbon in soil due to the amendment of biochar was multiply evaluated as long-termed69.
Based on the previous reports42, we assumed mitigation of nitrogen (N) loss via volatilization due to higher oxidation of reduced N forms in the early phase of the experiment (12 weeks). The variants with two highest values of both, total soil and total manure nitrogen, showed the highest manure nitrate content: M + S (31.21 mg⋅kg−1) and M (14.64 mg⋅kg−1) too (Table 3). We claim that the reason was the enhanced nitrogen conversion rate compared to the putative biochar-mediated retardation, documented i.e., by positive relation between TN and NAG-SIR. The mitigation of this early-phase induced-effect of manure amendment on soil TN was observed again at the end of experiment. The only significant increase of TN content in the M + B variant repeatedly corroborated assumption that the general enhancement of nutrient sequestration by the manure + biochar amendment has the long-termed consequence.
Soil microbial activity
Soil respiration (basal and substrate-induced) is an indicator of catabolic activity of aerobic microbiota in soil70, it monitors the carbon mineralization and functional diversity in soil microbial community. In the first (12 weeks) and second (24 weeks) half of the experiment, the highest basal and induced by 3 types of carbohydrates respiration was found in M + B variant. Basal respiration was significantly increased in M + B + S variant (compared to the control) too, however induction with D-glucose, D-trehalose, and N-acetyl-β-D-glucosamine showed that the respiration potential of M + B + S soil is lower than of M + B. Elemental sulfur significantly weakened enhancing effect of biochar in manure on soil respiration. Thus, the strongest respiration-stimulatory effect of co-matured manure + biochar amendment on the soil was detectable during the whole experiment. We explained this observation by very high content of carbonaceous organic matter in the respective manure type M + B, TC (21.01%) and concurrently its highest maturity indicated by HA:FA (2.04%). It was reported that the level of manure maturity is crucial for the improvement of soil fertility71 and the significant positive effect of manure72 and biochar73 on the soil respiration was reported too. The other manure types (M, M + S) with TC (9.10%, 9.99%) content lower than M + B manure (TC 21.01%) either did not contribute so significantly to the soil oxidizable carbon pool or could stimulate also anaerobic respiration which decreased the rate of CO2 emission. These general features of soil respiration in soil variants affected putatively also particular types of SIR (NAG-SIR, Lys-SIR, Arg-SIR), which correlated (Fig. S2a, b) significantly positively with TC, the relevant marker to the available catabolizable carbon compounds in soil.
The results from determination of amino acid (AA: Ala, Lys, Arg)-induced respiration in the M + B soil shortly affected (12 weeks) by co-matured manure and biochar variant showed the increased (highest) values compared to the control. Farmyard manure was referred to increase total soil amino acids as well as soil oligopeptide immobilization74. Biochar induced elevated lysine content in rhizosphere too75. Stimulation of catabolism and carbon mineralization was putatively joined with enhanced nutrient transformation, leading to higher availability and acquisition by plants, subsequently indicated by correlating increased AGB dry (Fig. S2a). Amendments of other types of manure also stimulated soil microbiome for enhanced Ala- and Lys-SIR after 12 weeks of experiment, but the positive effect of these variants vanished at the end of experiment. Except of M + B, at the end of experiment only variant M + B + S putatively sequestered sufficient amount of organic (nitrous) matter for AA-induced respiration significantly increased in comparison to the control. Similar observation was previously reported to crop yield correlated (Fig. S2b) positively with the ratio of soil respiration to SOM76. The AAs-SIR showed higher correlation with TC in the samples taken at the end of the experiment than after 12 weeks. Moreover, the highest values of respiration in M + B variant were related to the highest soil TC value, which was likely biochar-mediated. On the contrary, a negative correlation between Ala-SIR and ARS after 12 weeks of experiment may explain the presumed negative effect of sulfur mineralization (in the M + S soil) on the soil respiration. This novel assumption was in contrast with up-to-now reported joint increase in both, soil respiration and arylsulfatase77.
The results of ARS determination in the 12 week-incubated soil samples revealed the highest activity in the control variant. We explained this finding by the lowest content of plant available sulfur in the control soil, because this variant was not supplied with any source of sulphates. Therefore, the plant and microbial demand for sulfur was coupled with its higher mineralization rate, leading to the ARS induction. The positive relation between microbial biomass sulfur and ARS was reported78. The study by79 referred to decreased sulfur mineralization upon additions of carbon or sulfur in the organic amendment. Further, ARS was enhanced in the M + S variant, assuming that the higher access of elemental S in the respective manure without any limitation due to the biochar stabilization led to the significantly increased sulfur turnover and subsequently to induction in ARS. The presumption that ARS activity is tightly negatively related to the mineralization rate in soil, is corroborated by the antagonism with BR in soil. The later phase of the experiment was characterized by increased carbon mineralization rate, BR values and plant sulfur uptake, which led to ARS decrease in the most of soil variants.
N-acetyl-β-D-glucosaminidase (NAG) is an indicator of nitrogen mineralization and fungal biomass content and its turnover in soil80. We considered adverse effects of elemental sulfur on the fungal growth and soil biomass (and concurrently also NAG activity) in this experiment, in the line with reports by81. However, subsequently we presumed a proceed in the sulfur transformation and a mitigation of the putative fungistatic effect of elemental S, and we indeed evidenced the highest increase in NAG activity in the variants M + B and M + S, which exerted high TN content and high respiration potential as well (determined as Glc-SIR).
Urease (Ure) is a key and ubiquitous enzyme in the soil nitrogen mineralization82. Thus, the early experimental phase results revealed a significant increase in Ure in soil variants M and M + S, which were amended with the manure types most abundant on the total nitrogen, 24.79 g·kg−1 (M) and 23.93 g·kg−1 (M + S), (Table 3). The positive correlation between Ure and AGB dry in the early phase of experiment was in the line with reported correlation of nitrogen mineralization rate with urease activity83. The final (24 weeks) values of Ure were comparable in variants amended with enriched manure, M + B, M + S, M + B + S and the control, and Ure and AGB dry correlated negatively but insignificantly at the end of experiment which corresponds with the generally reported contribution of urease to inhibition of crop yield84.
Conclusions
Significant diverse impact of manures co-matured with biochar, elemental sulfur or combination of both on the properties of treated soil was evaluated. This variable effect on dry plant biomass, physico-chemical and biological traits of soil variants was time-dependent, as it was evident from the differences between samples from 12 week-lasting (with barley) and 24 week-lasting (with maize) pot experiment carried in a growth chamber. Co-matured manure with biochar (M + B) showed the highest rate of maturation, its amendment to soil lead to significantly highest soil fertility and barley dry aboveground biomass in the half-time (12 weeks) of experiment, however, the effect vanished after 24 weeks. The highest long-term (24 weeks) contents of total carbon and nitrogen was received for this variant. M + B showed most significant longer-term effect of co-matured manure with biochar on nutrient sequestration and SOM stabilization in soil. Contrarily, after 12 weeks, co-matured manure with biochar and elemental sulfur (M + B + S) impaired some properties to the treated soil (e.g., the lowest pH) which led to short-term carbon sequestration (the highest TC) due to presumed retardation of microbial-mediated transformation of nutrients. This effect of M + B + S manure faded out after 24 weeks as well. Further, there was observed also a specific effect of co-matured manure with only elemental sulfur (M + S). After 12 weeks, its application led to the significantly decreased respiration induced by amino acids (L-alanine, L-arginine). Longer (24 weeks) interaction of manure M + S resulted in basal and all induced respiration values comparable to the control, the lowest basal respiration.
Therefore, we conclude that enriched co-matured manure variants exerted significantly changed properties in comparison to the unenriched manure. However, their application to soil brought only short-term (12 week) beneficial effect on soil fertility and barley plant biomass, compared to single-composed manure. The longer-term (24 weeks) benefit was not significant. We assumed that the prolonged pot experiment with biochar or elemental sulfur enriched manure led to the increased recalcitrancy of soil organic matter (SOM) and retardation of soil nutrient transformation to the plant-available form. We probably should verify our findings in further research, preferably upscaled to the small-scale-plot format.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Montanarella, L. The Importance of land restoration for achieving a land degradation-neutral world 249–258 (Elsevier, 2016).
Richmond, N. T. Chemical soil degradation as a result of contamination: A review. J. Soil Sci. Environ. Manag. 6(11), 301–308. https://doi.org/10.5897/jssem15.0499 (2015).
Virto, I. et al. Soil degradation and soil quality in western Europe: Current situation and future perspectives. Sustainability. 7(1), 313–365. https://doi.org/10.3390/su7010313 (2014).
Liu, X., Herbert, S. J., Hashemi, A. M., Zhang, X. & Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—a review. Plant Soil Environ. 52, 531–543 (2006).
Kirchmann, H. & Thorvaldsson, G. Challenging targets for future agriculture. Eur. J. Agron. 12(3–4), 145–161. https://doi.org/10.1016/s1161-0301(99)00053-2 (2000).
Mustafa, A. et al. Long-term fertilization enhanced carbon mineralization and maize biomass through physical protection of organic carbon in fractions under continuous maize cropping. Appl. Soil Ecol. 165, 103971. https://doi.org/10.1016/j.apsoil.2021.103971 (2021).
Mustafa, A. et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manage. 270, 110894. https://doi.org/10.1016/j.jenvman.2020.110894 (2020).
Ashraf, M. N. et al. Soil microbial biomass and extracellular enzyme–mediated mineralization potentials of carbon and nitrogen under long-term fertilization (> 30 years) in a rice–rice cropping system. J. Soils Sediments https://doi.org/10.1007/s11368-021-03048-0 (2021).
Hoover, N. L., Law, J. Y., Long, L. A. M., Kanwar, R. S. & Soupir, M. L. Long-term impact of poultry manure on crop yield, soil and water quality, and crop revenue. J. Environ. Manage. 252, 109582. https://doi.org/10.1016/j.jenvman.2019.109582 (2019).
Wang, X. et al. Impacts of manure application on soil environment, rainfall use efficiency and crop biomass under dryland farming. Sci. Rep. 6, 20994. https://doi.org/10.1038/srep20994 (2016).
Qaswar, M. et al. Soil carbon (C), nitrogen (N) and phosphorus (P) stoichiometry drives phosphorus lability in paddy soil under long-term fertilization: A fractionation and path analysis study. PLoS ONE 14(6), e0218195. https://doi.org/10.1371/journal.pone.0218195 (2019).
Naveed, M. et al. Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environ. Sci. Pollut. Res. Int. 28(33), 45629–45645. https://doi.org/10.1007/s11356-021-14004-3 (2021).
Zhang, L. & Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 171, 274–284. https://doi.org/10.1016/j.biortech.2014.08.079 (2014).
Czekala, W. et al. Co-composting of poultry manure mixtures amended with biochar - the effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 200, 921–927. https://doi.org/10.1016/j.biortech.2015.11.019 (2016).
Xiao, R. et al. Recent developments in biochar utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 246, 203–213. https://doi.org/10.1016/j.biortech.2017.07.090 (2017).
He, X., Yin, H., Sun, X., Han, L. & Huang, G. Effect of different particle-size biochar on methane emissions during pig manure/wheat straw aerobic composting: Insights into pore characterization and microbial mechanisms. Bioresour. Technol. 268, 633–637. https://doi.org/10.1016/j.biortech.2018.08.047 (2018).
Khan, N. et al. Maturity indices in co-composting of chicken manure and sawdust with biochar. Bioresour. Technol. 168, 245–251. https://doi.org/10.1016/j.biortech.2014.02.123 (2014).
Maurer, D., Koziel, J., Kalus, K., Andersen, D. & Opalinski, S. Pilot-scale testing of non-activated biochar for swine manure treatment and mitigation of ammonia, hydrogen sulfide, odorous volatile organic compounds (VOCs), and greenhouse gas emissions. Sustainability. 9(6), 929. https://doi.org/10.3390/su9060929 (2017).
Agyarko-Mintah, E. et al. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 61, 129–137. https://doi.org/10.1016/j.wasman.2016.12.009 (2017).
Jindo, K. et al. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci. Total Environ. 416, 476–481. https://doi.org/10.1016/j.scitotenv.2011.12.009 (2012).
Hagemann, N. et al. Effect of biochar amendment on compost organic matter composition following aerobic composting of manure. Sci. Total Environ. 613–614, 20–29. https://doi.org/10.1016/j.scitotenv.2017.08.161 (2018).
Hardy, B., Sleutel, S., Dufey, J. E. & Cornelis, J.-T. The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2019.00110 (2019).
He, L., Shan, J., Zhao, X., Wang, S. & Yan, X. Variable responses of nitrification and denitrification in a paddy soil to long-term biochar amendment and short-term biochar addition. Chemosphere 234, 558–567. https://doi.org/10.1016/j.chemosphere.2019.06.038 (2019).
Sun, D.Q., Jun, M., Zhang, W.M., Guan, X.C., Huang, Y.W., Lan, Y., et al. Implication of temporal dynamics of microbial abundance and nutrients to soil fertility under biochar application - Field experiments conducted in a brown soil cultivated with soybean, north China. In 1st International conference on energy and environmental protection (ICEEP 2012). Hohhot, PEOPLES R CHINA 2012. pp. 384–94.
Alizadeh, S., Prasher, S. O., ElSayed, E., Qi, Z. & Patel, R. M. Effect of biochar on fate and transport of manure-borne estrogens in sandy soil. J. Environ. Sci. 73, 162–176. https://doi.org/10.1016/j.jes.2018.01.025 (2018).
Jia, X. et al. N2O emission and nitrogen transformation in chicken manure and biochar Co-Composting. Trans. ASABE 59(5), 1277–1283. https://doi.org/10.13031/trans.59.11685 (2016).
Llovet, A. et al. Fresh biochar application provokes a reduction of nitrate which is unexplained by conventional mechanisms. Sci. Total Environ. 755, 142430. https://doi.org/10.1016/j.scitotenv.2020.142430 (2021).
Mikajlo, I., Antosovsky, J., Dvorackova, H., Svoboda, Z., Zahora, J. The effect of inoculated and mitigated by plants biochar on soil microbiota. In 23rd international PhD students conference (MendelNet). Mendel Univ Brno, Fac AgriSciences, Brno, CZECH REPUBLIC: Mendel Univ Brno, Fac Agronomy; 2016. p. 117–22.
Elshony, M., Farid, I., Alkamar, F., Abbas, M. & Abbas, H. Ameliorating a sandy soil using biochar and compost amendments and their implications as slow release fertilizers on plant growth. Egypt. J. Soil Sci. https://doi.org/10.21608/ejss.2019.12914.1276 (2019).
Hernandez-Soriano, M. C. et al. Long-term effect of biochar on the stabilization of recent carbon: Soils with historical inputs of charcoal. GCB Bioenergy 8(2), 371–381. https://doi.org/10.1111/gcbb.12250 (2016).
Bouranis, D. L., Venieraki, A., Chorianopoulou, S. N. & Katinakis, P. Impact of elemental sulfur on the rhizospheric bacteria of durum wheat crop cultivated on a calcareous soil. Plants 8(10), 379. https://doi.org/10.3390/plants8100379 (2019).
Skwierawska, M., Skwierawska, M., Skwierawski, A., Benedycka, Z. & Jankowski, K. Sulphur as a fertiliser component determining crop yield and quality. J. Elementol. https://doi.org/10.5601/jelem.2015.20.3.992 (2016).
Besharati, H. Effects of sulfur application and Thiobacillus inoculation on soil nutrient availability, wheat yield and plant nutrient concentration in calcareous soils with different calcium carbonate content. J. Plant Nutr. 40(3), 447–456. https://doi.org/10.1080/01904167.2016.1245326 (2017).
Soaud, A. A. et al. Effects of elemental sulfur, phosphorus, micronutrients and Paracoccus versutus on nutrient availability of calcareous soils. Aust. J. Crop Sci. 5(5), 554–561 (2011).
Abou Hussien, E., Nada, W. & Elgezery, M. Influence of sulphur compost application on some chemical properties of calcareous soil and consequent responses of Hordeum vulgare L. Plants. Egypt. J. Soil Sci. 60(1), 67–82. https://doi.org/10.21608/ejss.2019.18503.1318 (2020).
Skwierawska, M., Zawartka, L. & Zawadzki, B. The effect of different rates and forms of sulphur applied on changes of soil agrochemical properties. Plant Soil Environ. 54(4), 171–177. https://doi.org/10.17221/391-pse (2008).
Soltanaeva, A., Suleimenov, B., Saparov, G. & Vassilina, T. Effect of sulfur-containing fertilizers on the chemical properties of soil and winter wheat yield. Bulg. J. Agr. Sci. 24, 586–591 (2018).
Hoesly, R. M. et al. Historical (1750–2014) anthropogenic emissions of reactive gases and aerosols from the community emissions data system (CEDS). Geosci. Model Dev. 11(1), 369–408. https://doi.org/10.5194/gmd-11-369-2018 (2018).
Kulhanek, M., Balik, J., Sedlar, O., Zbiral, J., Smatanova, M., Suran, P. Determination of available sulphur in soil by the method Mehlich 3. Certified methodolgy. . Brno, Prague; Czech Republic: Central Institute for Supervising and Testing in Agriculture & Department of Agro-Environmental Chemistry and Plant Nutrition, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences; 2018.
Garcia de la Fuente, R. et al. Biological oxidation of elemental sulphur added to three composts from different feedstocks to reduce their pH for horticultural purposes. Bioresour. Technol. 98, 3561–3569. https://doi.org/10.1016/j.biortech.2006.11.008 (2007).
Godlewska, A. Sulphur content in test plants and arylsulfatase activity in soil after application of waste materials. Appl. Ecol. Environ. Res. 16(1), 145–152. https://doi.org/10.15666/aeer/1601_145152 (2018).
Mahimairaja, S., Bolan, N. S., Hedley, M. J. & Macgregor, A. N. Losses and transformation of nitrogen during composting of poultry manure with different amendments: An incubation experiment. Biores. Technol. 47(3), 265–273. https://doi.org/10.1016/0960-8524(94)90190-2 (1994).
Hammerschmiedt, T. et al. Manure maturation with biochar: Effects on plant biomass, manure quality and soil microbiological characteristics. Agriculture-Basel 12(3), 17. https://doi.org/10.3390/agriculture12030314 (2022).
ISO 10390:2005. Soil quality - Determination of pH. Geneva, Switzerland: International Organization for Standardization; 2005.
ISO 11261:1995. Soil quality—Determination of total nitrogen — Modified Kjeldahl method. Geneva, Switzerland: International Organization for Standardization; 1995.
ISO 10694:1995. Soil quality - determination of organic and total carbon after dry combustion (elementary analysis). Geneva, Switzerland: International Organization for Standardization; 1995.
ISO 19822:2018. Fertilizers and soil conditioners - determination of humic and hydrophobic fulvic acids concentrations in fertilizer materials. Geneva, Switzerland: International Organization for Standardization; 2018.
EN 15749. Fertilizers - Determination of sulfates content using three different methods. Brussels, Belgium: European Standardization Organization CEN-CENELEC; 2009.
Ben-Dov, E., Brenner, A. & Kushmaro, A. Quantification of sulfate-reducing bacteria in industrial wastewater, by real-time polymerase chain reaction (PCR) Using dsrA and apsA genes. Microb. Ecol. 54(3), 439–451 (2007).
Hammerschmiedt, T. et al. Biochar and sulphur enriched digestate: Utilization of agriculture associated waste products for improved soil carbon and nitrogen content, microbial activity, and plant growth. Agronomy 11(10), 2041. https://doi.org/10.3390/agronomy11102041 (2021).
Nachabe, M. H. Refining the definition of field capacity in the literature. J. Irrig. Drain. Eng. 124(4), 230–232. https://doi.org/10.1061/(ASCE)0733-9437(1998)124:4(230) (1998).
ISO 13878:1998. Soil quality - determination of total nitrogen content by dry combustion (Elemental analysis): International Organization for Standardization; Switzerland.
Campbell, C. D., Chapman, S. J., Cameron, C. M., Davidson, M. S. & Potts, J. M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol. 69(6), 3593–3599. https://doi.org/10.1128/AEM.69.6.3593-3599.2003 (2003).
ISO 20130:2018. Soil quality — Measurement of enzyme activity patterns in soil samples using colorimetric substrates in micro-well plates. Geneva, Switzerland: International Organization for Standardization; 2018.
R_CORE_TEAM. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
Hinkle, D.E., Wiersma, W., Jurs, S.G. Applied statistics for the behavioral sciences. 5th edn. Boston, Mass.: Houghton Mifflin; 2003.
Agegnehu, G., Srivastava, A. K. & Bird, M. I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil. Ecol. 119, 156–170. https://doi.org/10.1016/j.apsoil.2017.06.008 (2017).
Balík, J. et al. Differences in soil sulfur fractions due to limitation of atmospheric deposition. Plant Soil Environ. 55(8), 344–352. https://doi.org/10.17221/101/2009-PSE (2009).
Dias, B. O., Silva, C. A., Higashikawa, F. S., Roig, A. & Sanchez-Monedero, M. A. Use of biochar as bulking agent for the composting of poultry manure: effect on organic matter degradation and humification. Bioresour Technol. 101(4), 1239–1246. https://doi.org/10.1016/j.biortech.2009.09.024 (2010).
Hagemann, N. et al. Effect of biochar amendment on compost organic matter composition following aerobic composting of manure. Sci. Total Environ. 613–614, 20–29. https://doi.org/10.1016/j.scitotenv.2017.08.161 (2018).
Roig, A., Cayuela, M. L. & Sanchez-Monedero, M. A. The use of elemental sulphur as organic alternative to control pH during composting of olive mill wastes. Chemosphere 57(9), 1099–1105. https://doi.org/10.1016/j.chemosphere.2004.08.024 (2004).
Lindemann, W. C., Aburto, J. J., Haffner, W. M. & Bono, A. A. Effect of sulfur source on sulfur oxidation. Soil Sci. Soc. Am. J. 55(1), 85–90. https://doi.org/10.2136/sssaj1991.03615995005500010015x (1991).
Zimmer, D. et al. Bone char versus S-enriched bone char: Multi-method characterization of bone chars and their transformation in soil. Sci. Total Environ. 643, 145–156. https://doi.org/10.1016/j.scitotenv.2018.06.076 (2018).
Fließbach, A., Oberholzer, H.-R., Gunst, L. & Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agr. Ecosyst. Environ. 118(1–4), 273–284. https://doi.org/10.1016/j.agee.2006.05.022 (2007).
Atkinson, C. J., Fitzgerald, J. D. & Hipps, N. A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil. 337(1–2), 1–18. https://doi.org/10.1007/s11104-010-0464-5 (2010).
Sarfraz, R. et al. Impact of integrated application of biochar and nitrogen fertilizers on maize growth and nitrogen recovery in alkaline calcareous soil. Soil Sci. Plant Nutri. 63(5), 488–498. https://doi.org/10.1080/00380768.2017.1376225 (2017).
O’Connor, D. et al. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 621, 819–826. https://doi.org/10.1016/j.scitotenv.2017.11.213 (2018).
Wu, Q. et al. Biochar co-application mitigated the stimulation of organic amendments on soil respiration by decreasing microbial activities in an infertile soil. Biol. Fertil. Soils 57(6), 793–807. https://doi.org/10.1007/s00374-021-01574-0 (2021).
Orman, Ş. Agronomic biofortification of green bean (Phaselous Vulgaris L.) with elemental sulphur and farmyard manure. Appl. Ecol. Environ. Res. 15(4), 2061–2669 (2017).
Abd El-Mageed, T. A. et al. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of capsicum annuum under salt stress. Sci. Hortic. https://doi.org/10.1016/j.scienta.2019.108930 (2020).
Liu, X. et al. Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil. 373(1–2), 583–594. https://doi.org/10.1007/s11104-013-1806-x (2013).
Anderson, J. P. E., Page, A. L., Miller, R. H. & Keeney, D. R. Soil Respiration. In Methods of soil analysis, part 2 2nd edn (ed. Page, A. L.) 831–871 (ASA and SSSA, 1982).
NRCS. Composting manure – What’s going on in the dark? Manure management information sheet. Washington, USA: Manure Management Technology Development Team, East National Technology Support Center; 2007.
Zhen, Z. et al. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE 9(10), e108555. https://doi.org/10.1371/journal.pone.0108555 (2014).
Jones, D. L., Rousk, J., Edwards-Jones, G., DeLuca, T. H. & Murphy, D. V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 45, 113–124. https://doi.org/10.1016/j.soilbio.2011.10.012 (2012).
Iqbal, S. et al. Suppression of amino acid and oligopeptide mineralization by organic manure addition in a semiarid environment. Land Degrad Dev. 31(15), 1915–1925. https://doi.org/10.1002/ldr.3546 (2020).
Tian, J.-h, Rao, S., Gao, Y., Lu, Y. & Cai, K.-z. Wheat straw biochar amendment suppresses tomato bacterial wilt caused by Ralstonia solanacearum: Potential effects of rhizosphere organic acids and amino acids. J. Integr. Agric. 20(9), 2450–2462. https://doi.org/10.1016/s2095-3119(20)63455-4 (2021).
Faé, G. S., Kemanian, A. R., Roth, G. W., White, C. & Watson, J. E. Soybean yield in relation to environmental and soil properties. Eur. J. Agron. 118, 12. https://doi.org/10.1016/j.eja.2020.126070 (2020).
Silva Aragão, OOd. et al. Microbiological indicators of soil quality are related to greater coffee yield in the Brazilian Cerrado region. Ecol. Indic. 113, 106205. https://doi.org/10.1016/j.ecolind.2020.106205 (2020).
Malik, K. M., Khan, K. S., Akhtar, M. S. & Ahmed, Z. I. Sulfur distribution and availability in alkaline subtropical soils affected by organic amendments. J. Soil Sci. Plant Nutr. 20(4), 2253–2266. https://doi.org/10.1007/s42729-020-00292-0 (2020).
Ghani, A., McLaren, R. G. & Swift, R. S. Sulphur mineralisation and transformations in soils as influenced by additions of carbon, nitrogen and sulphur. Soil Biol. Biochem. 24(4), 331–341. https://doi.org/10.1016/0038-0717(92)90193-2 (1992).
Ekenler, M. & Tabatabai, M. β Glucosaminidase activity of soils: Effect of cropping systems and its relationship to nitrogen mineralization. Biol. Fertil. Soils 36(5), 367–7666 (2002).
Williams, J. S. & Cooper, R. M. The oldest fungicide and newest phytoalexin - a reappraisal of the fungitoxicity of elemental sulphur. Plant. Pathol. 53(3), 263–279. https://doi.org/10.1111/j.0032-0862.2004.01010.x (2004).
Watson CJ. Urease activity and inhibition - principles and practice. In: Watson CJ, edn. International Fertiliser Society 2000. London. UK: International Fertiliser Society; 2000. p. 1–40.
Zaman, M., Di, H. J. & Cameron, K. C. A field study of gross rates of N mineralization and nitrification and their relationships to microbial biomass and enzyme activities in soils treated with dairy effluent and ammonium fertilizer. Soil Use Manage. 15(3), 188–194 (1999).
Abalos, D., Jeffery, S., Sanz-Cobena, A., Guardia, G. & Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agr. Ecosyst. Environ. 189, 136–144. https://doi.org/10.1016/j.agee.2014.03.036 (2014).
Funding
The work was supported by the projects of Technology Agency of the Czech Republic TH04030142 and TH03030319, by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO1223, MZE-RO1722 and by Ministry of Education, Youth and Sports of the Czech Republic, grant number FCH-S-23-8297.
Author information
Authors and Affiliations
Contributions
M.B. and T.H.: conceptualization. T.H., A.K., and O.L.: methodology. T.H., T.B. and O.M.: software. T.B., P.S., P.R., and J.H.: validation. M.B., O.M. and T.H.: formal analysis. J.H., O.M. and O.L.: resources. T.H., T.B., O.L., P.R. and A.K.: data curation. J.H., T.H., A.M. and P.S.: writing—original draft preparation. T.H., A.K., A.M., M.N., P.S., P.R. and M.B.: writing—review and editing. M.B., T.H.: supervision. J.H., A.K., and M.B.: project administration. J.H., A.K., and M.B.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holatko, J., Hammerschmiedt, T., Mustafa, A. et al. Time-dependent impact of co-matured manure with elemental sulfur and biochar on the soil agro-ecological properties and plant biomass. Sci Rep 13, 4327 (2023). https://doi.org/10.1038/s41598-023-31348-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31348-7
- Springer Nature Limited
This article is cited by
-
Effects of Different Forms of Sulfur on Plant Growth and Soil Properties in Cadmium-Contaminated Soils
Journal of Soil Science and Plant Nutrition (2024)