Abstract
Ultra-stable fluoropolymer glasses were created using vacuum pyrolysis deposition that show large fictive temperature Tf reductions relative to the glass transition temperature Tg of the rejuvenated material. Tf was also found to be 11.4 K below the dynamic VFT temperature TVFT. Glass films with various thickness (200–1150 nm) were deposited onto different temperature substrates. Glassy films were characterized using rapid-chip calorimetry, Fourier-transform infrared spectroscopy and intrinsic viscosity measurements. Large enthalpy overshoots were observed upon heating and a Tf reduction of 62.6 K relative to the Tg of 348 K was observed. This reduction exceeds values reported for a 20-million-year-old amber and another amorphous fluoropolymer and is below the putative Kauzmann temperature TK for the material as related to TVFT. These results challenge the importance of the Kauzmann paradox in glass-formation and illustrates a powerful method for the exploration of material dynamics deep in the glassy state (Tf < T < Tg).
Similar content being viewed by others
Introduction
Thin films have become a vital component in our daily lives, whether it be in microelectronics, auto-mobiles, household appliances or food packaging. Thin film properties can differ drastically from bulk material properties, and this has resulted in considerable interest in the science and engineering communities. Thin film applications depend on the properties of the film and these depend on the fabrication technique. The effects of an enhanced mobility surface layer on such films has an increasing effect on the film properties with decreasing film thickness. Some of these effects include Tg depression and enhanced optical properties. Not only do thin films allow for easier integration into different devices, but they also allow fabrication to be tunable to the desired application requirements as these important film properties change with decreasing thickness, generally at sizes below 100 nm1,2,3,4,5,6,7,8,9,10,11,12,13,14.
While spin coating is a common method of making ultrathin polymer films, in the present work we focus on vapor deposition of polymers. This approach differs somewhat from the physical vapor deposition of small molecule organics, but builds on those ideas as a means of creating very stable glassy states. Furthermore, the interest in thin glassy polymer films has increased due to their enhanced properties that include wear resistance, thermal stability and tunable optical properties15.
Glasses are non-equilibrium materials that have a molecular structure that is kinetically trapped and that, consequently, shows a constant evolution towards equilibrium that is determined by both absolute temperature and the history of the glass formation and use conditions16,17. The glass transition temperature (Tg) is the temperature at which the molecular mobility becomes slow relative to the cooling rate and the thermodynamic state-like variables (e.g., volume and enthalpy) begin to depart from the equilibrium state. Once below Tg, the molecules evolve towards equilibrium through a process called structural recovery, also termed as aging. The fictive temperature (Tf) is similar to Tg but is prominent for materials that have undergone structural recovery and is used as a measure of the non-equilibrium state that reflects the 'frozen-in' liquid structure of the glass. Tf is used to describe the material Tg for that structure, and the limiting Tf (Tf′) is the Tg of the material measured after cooling by a heating experiment at the same rate. The Tf evolution with cooling rate or with isothermal aging is a measure of a material’s structural recovery towards equilibrium; the greater the reduction in Tf during an aging or slow cooling treatment, the more thermally stable the material is17,18,19,20,21,22,23.
Earlier work from the McKenna laboratory on a 20-million-year-old amber has shown that aging of the amber for this time scale resulted in a Tf reduction of 43.6 K relative to the Tg = Tf′ and this accompanied a densification of the ancient amber of 2.1% relative to the density of the thermally rejuvenated material23. Experiments on the dynamics in the temperature window between the reduced fictive temperature and the Tg also showed a breakdown in the Vogel24-Fulcher25-Tammann26 (VFT) relationship which is associated with apparently diverging timescales at the notional thermodynamic temperature (Kauzmann27 temperature, TK) above 0 K, and which developed from the observation that the entropy of the supercooled liquid extrapolates to values lower than that of the crystal. This deviation of the dynamics from the VFT extrapolation is consistent with literature data obtained from long time aging experiments17,18,19,20,21,22,23,28,29,30 nearer to the glass transition temperature.
Another way to obtain low fictive temperature glasses has recently become of interest, that of physical vapor deposition. For example, Ediger and coworkers31,32,33,34,35,36,37,38,39 were able to “hike down the energy landscape” using physical vapor deposition (PVD) methods to produce stable films of small molecule glass formers grown at different deposition temperatures. Postulated to be due to an enhanced mobility of the depositing material, Ediger and coworkers’ results identified an optimum substrate temperature for deposition to be in the vicinity of 0.85Tg, Tg being in K and is the nominal glass transition temperature generally obtained at a cooling rate of 10 K/min. Although Boucher et al.40 were successful in creating low fictive temperature polystyrene films by aging, the time associated with conducting such experiments is considerably longer than VPD experiments (orders of magnitude). In addition, those results did not account for the possibility that the extremely thin films have a lower TK than the bulk material as is suggested by dynamical measurements in the thin film and nanoconfined polymer literature1,11,12,13,14.
Swallen et al.32 conducted PVD experiments with 1,3,5-(tris)napthylbenzene (TNB) and indomethacin (IMC) and reported Tf reductions of 33 K and 29 K, respectively. León-Gutierrez et al.41,42,43,44 worked on creating stable glasses of toluene and ethylbenzene and observed Tf reductions as well as a shift to the onset or devitrification temperature to higher temperatures, indicating an increase in glassy stability. Raegen et al.45 deposited poly (oligomeric) styrene and report an approximately 25 K Tf reduction as well as densification as large as 1.6%.
Bowie and Zhao46 simulated linear polymer thin film growth under vapor deposition polymerization where they varied the ratio, G, of the diffusion coefficient to the deposition rate and very high G values yielded high density polymer films. Lin et al.47 used molecular dynamics simulations to investigate properties of vapor deposited glasses created from short polymer chains and found that the deposition rate plays an important role in the stability of the deposited polymer glass and the deposited glass experienced higher density as well as higher kinetic stability. Additional work from Samanta et al.48 explored the differences in stability of vapor deposited organic glasses, 1,3-bis(1-naphthyl)-5-(2-naphthyl)benzene (TNB) and 9-(3,5-di(naphthalen-1-yl)phenyl)anthracene (α,α-A), with their liquid quenched counterparts, observing a density increase as well as improved kinetic stability for the VPD glasses. Dalal et al.49 used spectroscopic ellipsometry to better understand the properties vapor deposited ααβ-TNB glasses and found that the vapor deposited glass was denser as well as had a higher onset temperature for devitrification.
The above works have been generally interpreted to imply that the freedom allowed by VPD for the deposited molecules to explore a lower potential energy on the substrate’s surface allows packing densities to be obtained that would require orders of magnitude less time than that associated with physical aging experiments, yet leads to a glass that can be considered to have the same physical properties as a very long-time aged material. This interpretation, while acceptable, remains to be fully validated.
In recent work50 from the McKenna laboratory, vapor deposition experiments were performed using a high molecular weight (MW) amorphous fluoropolymer (Teflon AF 1600, MW = 400 kg/mol) and the results showed very large enthalpy overshoots corresponding to Tf reductions of up to 57 K, just above the reported TK (the associated TVFT) by 0.2 K. The idea behind the vapor deposition and formation of high MW stable polymer films was that the polymer chains pyrolyze and repolymerize on the substrate temperature, resulting in stable glasses that have lower MW than the virgin material, but also remain polymeric, thus improving mechanical toughness needed for viscoelastic testing. They50 referred to this as vacuum pyrolysis deposition (VPD).
In the current work, we used vacuum pyrolysis deposition (VPD) to create highly stable glasses from a different perfuorinated polymer (CYTOP, fluoropolymer). The potential advantage of this material over the AF 1600 is that the glass transition temperature of the CYTOP is over 50 K lower than that of the AF 1600, which made viscoelastic testing in the atomic force microscope potentially easier. The stable glasses were characterized using rapid-chip calorimetry, Fourier transform infrared spectroscopy (FTIR) and intrinsic viscosity measurements. These latter measurements provided the picture of the VPD methodology leading to decreased molecular weight of the polymer, but that the thin film remained polymeric and had the same chemical structure as the original Teflon AF 1600. We compare results with those for the virgin CYTOP material using the same techniques, except for the calorimetry, in which case conventional DSC was also employed for some of the calorimetric measurements. We describe how the stable glasses were created and how we characterized their stability, as well as provide evidence that the material is still polymeric in nature.
Experimental section
Sample preparation
CYTOP (Type S) was purchased directly from Japan through AGC inc. (formerly Asahi Glass Co.). The reported \({M}_{W}\) from the manufacturer is 250,000–300,000 g/mol with a reported Tg of 108 \(^\circ\)C (381.2 K). The CYTOP was received in a solution of 9% CYTOP on weight basis. The solvent used for the solution was CT-Solv-180 (perfluorotributylamine). To get CYTOP in solid form, a known quantity of the solution was placed in a Pyrex container and subjected to a series of vacuum oven drying steps at different temperatures: 80 \(^\circ\)C × 24 h + 120 \(^\circ\)C × 12 h to remove dissolved air, 180 \(^\circ\)C × 24 h to evaporate the solvent and 240\(^\circ\)C × 24 h to remove bubbles by annealing. The drying process was considered complete once there was no decrease in weight after annealing at 240\(^\circ\)C. Once the sample was dried, it was allowed time to cool and the bulk CYTOP was removed from the mold. VPD was used to create ultra-stable CYTOP films with thickness ranging from 180 to 1150 nm. The VPD process was achieved using a Varian high vacuum evaporator (Varian 3118) and the deposition rate was tracked and confirmed using a quartz crystal microbalance (QCM). A temperature-controlled surface was used upon which the substrates were attached. Silicon wafers, mica sheets and Flash-DSC (FDSC) chips were used as the substrate at which the films were grown. Due to the design of the FDSC chips, film growth occurs on the back side of the chip with a mask used to ensure that the deposition area is limited only to the chip’s sensor area. The temperature of the substrate surface was varied between 0.79 and 0.91 Tg, where Tg is in K and is the Tg measured in our labs of 104 °C (377.2 K) for the bulk material. We remark that the deposition temperatures were originally chosen based upon the manufacturer’s reported Tg = 108 °C (381.2 K), but we report our results based on our measured value. The deposition rate was maintained at approximately 0.1 nm/s by adjusting the current to the basket in the vacuum jar but basket temperature was not measured. The vacuum pressure achieved was in the range of 10–7 Torr50.
Bulk CYTOP samples (oven-dried) used in FTIR measurements were created using a mica sheet to spread a drop of CYTOP solution on the surface of a CaF2 IR window followed by oven drying. The VPD samples produced for FTIR analysis were deposited directly on NaCl and CaF2 windows. Handling of these windows with deposited samples was done with great care and after deposition the windows were placed in a desiccator to minimize exposure to humidity.
The as-received CYTOP solution was diluted to make solutions of the bulk polymer for intrinsic viscometry measurements. Solution concentrations varied between 0.01 and 0.18 mg/mL. For for the VPD samples, 1.1 µm thick films, grown on mica sheets, were placed directly in the solvent for 24 h to allow for the sample to dissolve into the solvent, yielding concentrations varying from 0.05 to 0.18 mg/mL.
Calorimetric measurements
The fictive temperatures of the ultra-stable CYTOP were measured using a Mettler Toledo Flash Differential Scanning Calorimetry (FDSC) with Freon intercooler and nitrogen purge. Relevant temperature scans were conducted at a cooling and heating rate of 600 K/s. The heating scans were run in the temperature range of − 10–210 \(^\circ\)C, where the sample was held for 5 s and cooled back down to − 10\(^\circ\)C. The approximate sample mass of 180–220 nm thick CYTOP films (\(\rho\) = 2.03 g/cm3) ranged between 91 and 112 ng. Information regarding the calibration of the Flash DSC is mentioned elsewhere51. For the ultra-stable CYTOP samples, 2 rejuvenated scans were conducted to ensure stability and reproducibility. The virgin material experiments were conducted using a Mettler Toledo DSC 822e. In this case the heating scans were conducted at 10 K/min on a 40 \(\mu\)L aluminum pan containing 15.76 mg of virgin CYTOP.
FTIR measurements
FTIR measurements were performed on the VPD CYTOP as well as bulk CYTOP, both at different thicknesses, using a Bruker Optics VERTEX 70 Fourier transform infrared (FTIR) spectroscope. Each spectrograph is an average of 64 scans with a resolution of 4 cm−1.
Intrinsic viscosity measurements
Intrinsic viscosity measurements were conducted to estimate the molecular weight of the VPD CYTOP. Bulk CYTOP solution was diluted to the desired concentration levels and used to determine the relative (\({\eta }_{r}\)) and specific (\({\eta }_{sp}\)) viscosities. A micro-Ostwald viscometer (2 mL) was used in a water bath with constant temperature of 25 °C. Each concentration viscosity was an average of three to five measurements. The average efflux time (\({t}_{0}\)) for pure solvent CT-Solv-180 was 1395 \(\pm\) 4 s.
Analysis
Calorimetry
The fictive temperature, \({T}_{f}\), is the temperature at which the extrapolated equilibrium liquid line intersects the glass line on an enthalpy-temperature or volume-temperature plot. In calorimetry studies, \({T}_{f}\) is measured from a heating heat flow scan after cooling and is a function of the cooling rate. As the cooling rate decreases, \({T}_{f}\) also decreases as more time is allowed for the molecules to maintain equilibrium. \({T}_{f}\) is also a function of aging time and at long aging times, \({T}_{f}\) approaches the aging temperature \({T}_{a}\). Determination of \({T}_{f}\) was carried out using Moynihan’s method51,52 (Eq. 1) of area matching:
here \({C}_{pl}\) and \({C}_{pg}\) are the liquid and glass heat capacities and \({C}_{p}\) is the apparent heat capacity of the material, in this case CYTOP.
Upon integration of Eq. (1) and applying it for 2 scans, the first (ultra-stable response) and second (rejuvenated response) heating scans, we reach to Eq. (2) from which the \({T}_{f}\) reduction is calculated:
here \({T}_{f, rejuv.}\) and \({T}_{f, VPD}\) are the fictive temperatures of the rejuvenated and stable materials, \({H}_{rejuv.}\) and \({H}_{VPD}\) are the enthalpies obtained from the heating scans for the rejuvenated and stable materials and \(\Delta {C}_{p}\) is the difference between heat capacities of the liquid and glass lines at \({T}_{f}\).
The cooling rate dependence of \({T}_{f}\) was calculated using a variation51,52 of the Vogel-Fulcher-Tammann (VFT) Equation24,25,26 that is expressed in terms of cooling rate, as shown in Eq. (3):
here \(q\) is the cooling rate, \(A\) and \(B\) are fitting parameters and \({T}_{0}\) is the temperature at which the dynamics diverge to infinity.
Using the VFT parameters, the fragility (\(m\)) and the activation energy (\({E}_{g}\)) were calculated using Eqs. (4), (5)51,52,53,54:
The Mauro-Yue-Ellison-Gupta-Allan (MYEGA)55 viscosity model was also used for comparison, expressed in terms of cooling rate:
here K and C are fitting parameters.
Intrinsic viscosity measurements
The relative (\({\eta }_{r}\)) and specific (\({\eta }_{sp}\)) viscosities were determined using Eqs. (6), (7) shown below:
here \({t}_{0}\) is the solvent’s measured efflux time and \(t\) is the efflux time for the dilute polymer solution.
The intrinsic viscosity (\(\left[\eta \right]\)) is obtained by extrapolating to 0 polymer concentration the data resulting from normalizing \(\mathrm{ln}{\eta }_{r}\) and \({\eta }_{sp}\) with concentration (\(C\)) as shown in Eq. (7).
The Mark-Houwink Staudinger-Sakurda56 relation allows us to estimate the viscosity average molecular weight using the intrinsic viscosity as shown Eq. (8):
here \(K\) is the Mark-Houwink parameter and \(\alpha\) is the exponent, typically ranging from 0.6 to 0.8 for most polymers.
Applying Eq. (8) to both the VPD and virgin materials and subtracting them from each other results in Eq. (9) from which the VPD material’s viscosity average molecular weight could be estimated:
here \(\overline{{M }_{v, VPD}}\) and \({M}_{v, VM}\) are the viscosity average molecular weight of the VPD and virgin materials.
Results and discussion
Fourier-transform infrared spectroscopy (FTIR)
VPD was used to create stable glasses of amorphous fluoropolymer CYTOP. Due to the pyrolysis process, it is of interest to ensure that the stable glass formed has the same chemical structure as the virgin material (Though, importantly, for the purposes of the present ultra-stable glass investigation, this is a side issue as the physics of the VPD ultra-stable glass are compared with those of the same material but after thermal rejuvenation, i.e., after heating above Tg). FTIR was utilized to explore the structure of the stable glasses formed. Figure 1 show the IR spectrograms for bulk and stable CYTOP films of various thickness. The main peaks shown in Fig. 1 are that of CF2 (symmetric at 1100 cm-1 and asymmetric at 1200 cm−1)57 and a CF stretch mode at 1340 cm-1. High thicknesses resulted in transmittance saturation in the spectral regions of interest and fabrication of a thinner sample (1.06 m\(\mu\)) was necessary to be able to compare the very thin VPD CYTOP samples to the virgin material. The spectrographs for the stable and rejuvenated CYTOP are in good agreement, while that of the bulk CYTOP agrees with peak position but differs slightly in intensity. Importantly, after rejuvenation, the VPD CYTOP film’s spectrograph agreed well with the bulk CYTOP film. The results shown in Fig. 1 show that the chemical structures of the VPD material and the as-received material are similar, concluding that the VPD of CYTOP didn’t produce a material of a different chemical structure. Similar results have also been reported for studies on stable and bulk fluoropolymers50,58.
The FTIR and intrinsic viscosity studies were carried out to provide information about the present polymer. While we could not determine the molecular weight distribution we could determine/estimate the intrinsic viscosity based molecular weight and the major result for these parts of the present investigation is that the CYTOP seems to retain its chemical structure (FTIR) and it remains polymeric, though with a lower molecular weight than the virgin material. Since perfluoropolymers are highly stable, once the vapor deposited material has formed, there is no evidence that the thermal exposures during subsequent testing would chemically change the material response. Nason et al.59 created amorphous fluoropolymer films using VPD and found little change in overall composition when compared to the virgin material. The results led to the conclusion that the volatile fragments resulting from cleavage takes place between two dioxole fragments (there are more of these junctions per chain than any other type), due to the high steric congestion at those points, and the free radical created can be stabilized by a neighboring O atom. Blanchet58 caried out laser ablation experiments on amorphous fluoropolymers in which the laser-induced pyrolytic decomposition yielded monomers that ejected at high velocities that impinge on the substrate and repolymerize to form a film. Regardless, the important aspect of the present work is that the vapor deposited, stable film is chemically the same as its rejuvenated counterpart. Thus, differences in physical response between the VPD films and their rejuvenated references are relevant to the comparisons to be made in the present work. Furthermore, fluoropolymers are quite stable in general, thus the mild heating treatments for the calorimetric measurements are not likely to lead to chemical degradation. Furthermore, the fact that the thermograms of the samples overlap in the liquid states after multiple heating cycles is consistent with there being no mass loss during the experiments.
Intrinsic viscosity measurements
Intrinsic viscosity measurements were conducted to determine the molecular weight of the stable CYTOP glasses created using VPD. Since in the prior work it was found that the chains of the AF 1600 amorphous Teflon pyrolyze and re-polymerize on the deposition substrate. Calculating the MW allows us to determine whether if the material is still polymeric in nature. Capillary viscometry measurements were made on different concentration solutions of both the untreated CYTOP and the VPD stable CYTOP materials and the relative and specific viscosities were calculated, from which the intrinsic viscosities were extracted. Intrinsic viscometry results show that \(\left[\eta \right]\) dropped by approximately 38%, from 0.52 \(\pm\) 0.01 to 0.32 \(\pm\) 0.01 dL/g. Rearrangement of the Mark-Houwink-Sakurada relation, Eq. (8), which relates the intrinsic viscosity to the viscosity average MW, \(\overline{{M }_{v}}\), results in a \(\overline{{M }_{v}}\) in the range of 100–163 kg/mol, which indicates that the material is still polymeric in nature. Figure 2 below summarizes the intrinsic viscosity measurement results.
Intrinsic viscosity measurement results for VPD and bulk CYTOP. (A) Ratio of the specific viscosity (\({\eta }_{sp}\)) to concentration plotted versus concentration, with the intercept being the intrinsic viscosity. (B) Ratio of the natural logarithm of the relative viscosity (\({\eta }_{r}\)) to concentration plotted against concentration, with the intercept being the intrinsic viscosity. Best fit lines also shown for each data set.
An analysis to determine if the cause of the molecular weight decrease was due to branching of the polymer chains during re-polymerization was carried out and is presented in Section 1 of the Supplemental Information 1. The analysis illustrates that the apparent MW decrease was not due to branching.
Calorimetry
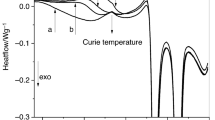
The major interest of the VPD method is the creation of glasses that are stable in the sense of having a reduced fictive temperature relative to that of the rejuvenated counterpart. It then is important to be able to characterize the stability of the glasses created and the determination of the Tf was the route chosen. Fast-scanning differential scanning calorimetry (FDSC) as well as regular differential scanning calorimetry (DSC) were used to characterize the stability of the stable CYTOP films as well as the virgin material, respectively. The stable glasses were grown by vapor deposition on substrates having different temperatures in order to explore the possibility of an optimum deposition TDep/Tg,Bulk ratio. Figure 3A shows the heat flow curves for the stable glasses deposited at different deposition temperatures along with the rejuvenated material’s heating curve. Figure 3B shows the enthalpy evolution for the respective heat flow scans, the product of integrating the heat flow scans. Figure 3C shows the reduction in Tf for the stable material when compared to the rejuvenated sample’s Tf, measured at the nominal heating rate of 10 K/min, after cooling at 10 K/min, being 75.2 \(\pm\) 1.1 °C (348.3 K).
Summary of the VPD CYTOP calorimetry results. (A) Heat flow curves for stable CYTOP films of similar thickness (185–230 nm) deposited at different substrate temperatures. (B) Enthalpy curves for the stable CYTOP films created compared to the rejuvenated material. Enthalpy curves were calculated by integrating the heat flow curves. (C) Tf reduction (delta Tf) relative to the rejuvenated CYTOP as a function of the ratio of deposition temperature and Tg, Bulk. Tdep was determined based on reported Tg not the measured Tg.
A summary of the results obtained through the calorimetry measurements is shown in Table 1. The largest Tf reduction was observed at a Tdep/Tg,Bulk ratio of 0.86, which is consistent with literature reports. Tf reductions at lower ratios were also very large as evident from their undershoots in Fig. 3A. The highest Tdep/Tg,Bulk ratio showed the smallest reduction in Tf, which is also consistent with literature reports. Of importance to mention, the rejuvenated Tf for the stable film grown at 50.8 °C has been adjusted by + 3 °C. We associate the observed difference in the rejuvenated Tf as being due to variability in the chip sensors used for the vapor deposition. For example, Koh et al.60 performed structural recovery experiments on a single polystyrene thin film using nano-calorimetry and out of the four chips used in the study, differences of up to 4.46 K were observed from the instrument reported temperature.
A cooling rate dependence (CRD) of the fictive temperature of the rejuvenated VPD material was also carried out to determine the Vogel-Fultcher-Tammann (VFT) parameters, which includes a temperature at which the viscosity or relaxation time diverges to infinity. Figure 4 shows the heating scans for the rejuvenated VPD CYTOP and the virgin CYTOP.
From the VFT parameters the fragility (m) and the apparent activation energy (Eg) were calculated. Figure 5 shows the inverse of the fictive temperatures for the VPD CYTOP as a function of cooling rate at different deposition rates along with their respective VFT fits. Figure 5 also shows the results for CRD for the virgin CYTOP. We also note that Badrinarayanan et al.61 compared Tg values measured on cooling with the limiting fictive temperature, Tf′, measured on heating and found that the Tf′ is systematically lower than Tg, citing the breadth of the relaxation on cooling presumably as the cause. The CRD Tf data for the virgin material were shifted by − 29 °C, which is the difference between the Tg,bulk and the VPD Tf at nominal cooling rates of 10 K/min. From the intrinsic viscosity measurements. The results showed us that the material is still polymeric in nature, which allows us to assume that the shape of the VFT would be the same. Also, combining the FDSC and conventional DSC data provides a larger number of decades, in terms of cooling rate, which gives a better determination of the VFT parameters and the fragility62.
Cooling rate dependence of Tf for VPD and bulk CYTOP. (A) Cooling rate dependence of rejuvenated VPD CYTOP films along with their VFT fits. (B) All CRD data for the VPD CYTOP films and the virgin material CRD data along with the VFT and MYEGA fits on the data. Here, data for the deposition temperature of 50.9 °C have been shifted by + 3 °C and the virgin (bulk) material fictive temperatures has been shifted by -29 °C. VFT parameters for individual data sets are presented in Section 2 of the Supplemental Information 1.
The CRD data set for the 50.8 °C deposition temperature was shifted by 3 °C to bring the fictive temperatures at the nominal cooling rate for samples of all deposition temperatures into alignment because of the variability in the chip response60. The VFT fit was applied to all the data, after alignment, and the parameters were calculated to be LogA = 16.6 \(\pm\) 5.1, B = 1981 \(\pm\) 1250 K, and \({T}_{\infty }\) = 297.2 \(\pm\) 16.2 K. These results produced a fragility value of m = 116 and an apparent activation energy at Tg of Eg = 773 kJ/mol, which are similar values to those of high fragility polymers63. If we attempt to quantify the thermodynamic stability of the most stable glass created using the relationship shown in Eq. (12)31, and
equating \({\mathrm{T}}_{\mathrm{K}}={\mathrm{T}}_{\infty }\), we obtain a value for \({\theta }_{K}\) of the CYTOP 1.20 \(\pm\) 0.38. A value of 1 for \({\theta }_{K}\) would nominally indicate that the molecules occupy lowest possible position in the energy landscape assuming that the \({\mathrm{T}}_{\mathrm{K}}\) or TVFT correspond to the point of a thermodynamic glass transition, thus the present work suggests the possibility of creating a glass having a fictive temperature below the Kauzmann temperature. This further suggests that the fact that glass-formation in non-crystallizable materials such as the perfluoropolymer used here and that forms such an ultra-stable glass either form a different class of glass-forming materials than do crystallizing systems or that the Kauzmann paradox/paradigm/conundrum may not be very relevant to glass-formation itself. Table 2 summarizes an analysis of literature data for the values of \({\theta }_{K}\), for glasses stabilized in different ways. The present work is the only one we found for which \({\theta }_{K}\) > unity.
The results shown in this paper indicate that the stable CYTOP glass grown at a substrate temperature of 0.86Tg has a Tf of approximately 12.6 °C, which is 11.4 °C below the VFT divergence temperature \({\mathrm{T}}_{\infty }\) = 24 °C. The result of having a Tf value that is lower than the TK questions underlying ideal glass transition theories. Is the Kauzmann paradox relevant to non-crystallizable glass formers? For example, Gibbs-DiMarzio70,71 theory hypothesizes that for non-crystallizable polymers, at a certain temperature below Tg, the stiffening (shrinking) of the polymer chains ceases in order to leave the system with at least one configuration, i.e., the configurational entropy goes to zero. This temperature, called T2 has been related to an ideal glass transition and the Adam-Gibbs72 ansatz that relates the diverging dynamics to the decreasing entropy, viz., the VFT temperature and TK (or T2) are related.
To our knowledge, this discovery is the first of its kind with regards to stable high MW polymers and questions classical theories that predict molecular behavior deep in the glassy region. Although we realize that there is uncertainty in our results, such a Tf reduction allows for a larger temperature range of experimentation to explore molecular dynamics far below Tg. In addition, the possible observation that Tf < TK suggests that the Kauzmann paradox may not be important in the understanding of glass-formation, at least in non-crystallizable materials such as that studied here. Consistent with this view are density data for vapor deposited ethylbenzene from Ishii et al.73 which McKenna74 analyzed and found that the fictive temperature is 8 K below the TK. This observation along with the present findings for the VPD CYTOP material pave an exploration avenue in the deep glassy state that is filled with challenges and interest.
Further investigations should be carried out to examine, e.g., density and dynamics of these ultra-stable glasses. AFM or ellipsometric thickness change dilatometry to explore thickness evolution of ultra-stable CYTOP films with temperature to obtain volumetric information to match with the enthalpy studies are such avenues of research. In addition, dynamic measurements such as the viscoelastic response provided from the TTU nanobubble inflation experiment75,76 are anticipated to provide the means to explore the dynamics of the ultrastable and extremely thin CYTOP films in the glassy regime, capturing relaxation behavior in the temperature range between Tf,VPD and Tg,rejuvenated. It is also suggested that other polymers that might be more dielectrically active than the fluorinated materials used here and in prior VPD work would also provide a possibility to investigate the ultra-stable glasses in the deep glassy state.
The study of ultrastable glasses remains an area of research and the present work suggests there is a regime in the case of vacuum pyrolysis deposited polymers to create systems of higher stability than heretofore reported. In this work we used calorimetry measurements to investigate the Tf reduction. The origins behind why Tf of the stable VPD glasses below Tdep are not known and clearly require further exploration. We think that this shows that the vapor deposition is a powerful method of creating ultrastable polymer glasses, as originally suggested by McKenna and co-workers50, 76 using an amorphous fluoropolymer and subsequently confirmed by Raegen et al.45 for polystyrene and poly(methyl methacrylate).
Summary and conclusions
We have created ultra-stable glassy CYTOP films using vapor pyrolysis deposition at varying substrate temperatures. Thickness of the stable films grown varied from 185 to 220 nm and the substrate temperature varied from 26 to 69.9 °C. FTIR spectroscopy indicated that both VPD films and the virgin material are of the same chemical composition and structure. Intrinsic viscosity measurements showed a MW decrease of approximately 50% but the material was still polymeric in nature. Heat flow scans of the stable VPD CYTOP films showed large enthalpy undershoots, one major signature of stable glasses. The stable CYTOP film deposited at a deposition temperature corresponding to Tdep/Tg,Bulk = 0.86 exhibited a Tf reduction of 62.6 °C relative to the glass temperature, making Tf of the stable film lower than the VFT divergence temperature by 11.4 °C. The work provides a potential challenge to ideas related to the ideal glass transition that are based on the Gibbs-DiMarzio model and on the the Kauzmann paradox and suggests a route to making deep glassy state materials that can provide important new avenues of research and understanding.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author (GBM) upon reasonable request.
References
Alcoutlabi, M. & McKenna, G. B. Effects of confinement on material behavior at the nanometer size scale. J. Phys. Condens. Matter 17, R461 (2005).
Keddie, J. L., Jones, R. A. & Cory, R. A. Size-dependent depression of the glass transition temperature in polymer films. Europhys. Lett. 27, 59 (1994).
Keddie, J. L., Jones, R. A. & Cory, R. A. Polymer dynamics in thin films. In Modern Aspects of Colloidal Dispersions (eds Ottewill, R. H. & Rennie, A. R.) (Springer, Dordrecht, 1998).
McKenna, G. B. Glass transition: Challenges of extreme timescales and other interesting problems. Rubber Chem. Technol. 93, 1 (2020).
Forrest, J. A., Dalnoki-Veress, K., Stevens, J. R. & Dutcher, J. R. Effect of free surfaces on the glass transition temperature of thin polymer films. Phys. Rev. Lett. 77, 4108 (1996).
Forrest, J. A., Dalnoki-Veress, K. & Dutcher, J. R. Interface and chain confinement effects on the glass transition temperature of thin polymer films. Phys. Rev. E 56, 5705 (1997).
Forrest, J. A., Dalnoki-Veress, K. & Dutcher, J. R. Brillouin light scattering studies of the mechanical properties of thin freely standing polystyrene films. Phys. Rev. E 58, 6109 (1998).
Roth, C. B. & Dutcher, J. R. Hole growth as a microrheological probe to measure the viscosity of polymers confined to thin films. J. Polym. Sci. B Polym. Phys. 44, 3011–3021 (2006).
Roth, C. B. & Dutcher, J. R. Glass transition and chain mobility in thin polymer films. J. Electroanal. Chem. 584, 13–22 (2005).
Rossnagel, S. M. Thin film deposition with physical vapor deposition and related technologies. J. Vac. Sci. Technol. A 2(5), S74–S87 (2003).
O’Connell, P. A., Hutcheson, S. A. & McKenna, G. B. Creep behavior of ultrathin polymer films. J. Polymer Sci. Part B Polymer Phys. Ed. 46, 1952–1965 (2008).
O’Connell, P. A., Wang, J., Ishola, T. A. & McKenna, G. B. Exceptional property changes in ultrathin films of polycarbonate: Glass temperature. Rubbery Stiffening Flow Macromol. 45, 2453–2459 (2012).
Varnik, F., Baschnagel, J. & Binder, K. Glassy dynamics in thin polymer films: Recent MD results. J. Non-Cryst. Solids 307–310, 524–531 (2002).
Schönhals, A., Goering, H., Schick, C., Frick, B. & Zorn, R. Glass transition of polymers confined to nanoporous glasses. Colloid Polym. Sci. 282, 882–891 (2004).
Sproul, W. D. Physical vapor deposition tool coatings. Surf. Coat. Technol. 81, 1–7 (1996).
Angell, C. A., Ngai, K. L. & McKenna, G. B. Relaxation in glass forming liquids and amorphous solids. J. Appl. Phys. 88, 3113–3157 (2000).
McKenna, G. B. & Zhao, J. Accumulating evidence for non-diverging time-scales in glass-forming fluids. J. Non-Cryst. Solids 407, 3–13 (2015).
McKenna, G. B. Glass dynamics: Diverging views on glass transition. Nat. Phys. 4(9), 673–674 (2008).
Zhao, J. & McKenna, G. B. Temperature divergence of the dynamics of a poly (vinyl acetate) glass: Dielectric vs mechanical behaviors. J. Chem. Phys. 136(15), 154901 (2012).
O’Connell, P. A. & McKenna, G. B. Arrhenius-type temperature dependence of the segmental relaxation below Tg. J. Chem. Phys. 110, 11054 (1999).
McKenna, G. B. Glass formation and glassy behavior. Compr. Polymer Sci. 2, 311 (1989).
Simon, S. L., Sobieski, J. W. & Plazek, D. J. Volume and enthalpy recovery of polystyrene. Polymer 42, 2555–2567 (2001).
Zhao, Z., Simon, S. L. & McKenna, G. B. Using 20-million-year-old amber to test the super-Arrhenius behaviour of glass-forming systems. Nat. Commun. 4, 1783 (2013).
Vogel, H. The law of the relation between the viscosity of liquids and the temperature. Phys. Z. 22, 645–646 (1921).
Fulcher, G. S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 8, 339–355 (1925).
Tammann, G. & Hesse, W. The dependence of viscosity upon the temperature of supercooled liquids. Z. Anorg. Allg. Chem. 156, 245 (1926).
Kauzmann, W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem. Rev. 43, 219 (1948).
Zhao, J. & McKenna, G. B. Response to “comment on ‘temperature divergence of the dynamics of a poly (vinyl acetate) glass: Dielectric vs mechanical behaviors”. J. Chem. Phys. 139, 137101 (2013).
Richert, R. Comment on “Temperature divergence of the dynamics of a poly (vinyl acetate) glass: Dielectric vs. mechanical behaviors”. J. Chem. Phys. 139(13), 137101 (2013).
Wagner, H. & Richert, R. Dielectric relaxation of the electric field in poly (vinyl acetate): A time domain study in the range 10–3−106 s. Polymer 38(13), 255–261 (1997).
Kearns, K. L. et al. Hiking down the energy landscape: Progress toward the Kauzmann temperature via vapor deposition. J. Phys. Chem. B 112, 4934–4942 (2008).
Swallen, S. F. et al. Organic glasses with exceptional thermodynamic and kinetic stability. Science 315, 353–356 (2007).
Kearns, K. L., Still, T., Fytas, G. & Ediger, M. D. High-modulus organic glasses prepared by physical vapor deposition. Adv. Mater. 22, 39–42 (2010).
Kearns, K. L., Whitaker, K. R., Ediger, M. D., Huth, H. & Schick, C. Observation of low heat capacities for vapor-deposited glasses of indomethacin as determined by AC nanocalorimetry. J. Chem. Phys. 133, 014702 (2010).
Kearns, K. L., Swallen, S. F., Ediger, M. D., Wu, T. & Yu, L. Influence of substrate temperature on the stability of glasses prepared by vapor deposition. J. Chem. Phys. 127, 154702 (2007).
Sepulveda, A., Swallen, S. F., Kopff, L. A., McMahon, R. J. & Ediger, M. D. Stable glasses of Indomethacin and α, α, β-trisnaphthylbenzene transform into ordinary supercooled liquids. J. Chem. Phys. 137, 204508 (2012).
Sepulveda, A., Swallen, S. F. & Ediger, M. D. Manipulating the properties of stable organic glasses using kinetic facilitation. J. Chem. Phys. 138, 12A517 (2013).
Yu, H. B., Tylinski, M., Guiseppi-Elie, A., Ediger, M. D. & Richert, R. Suppression of β relaxation in vapor-deposited ultrastable glasses. Phys. Rev. Lett. 115(18), 185501.15 (2015).
Beasley, M. S., Bishop, C., Kasting, B. J. & Ediger, M. D. Vapor-deposited ethylbenzene glasses approach “ideal glass” density. J. Phys. Chem. Lett. 10, 4069–4075 (2019).
Boucher, V. M., Cangialosi, D., Alegria, A. & Colmenero, J. Reaching the ideal glass transition by aging polymer films. Phys. Chem. Chem. Phys. 19, 961 (2017).
León-Gutierrez, E. et al. In situ nanocalorimetry of thin glassy organic films. J. Chem. Phys. 129, 181101 (2008).
Leon-Gutierrez, E., Sepúlveda, A., Garcia, G., Clavaguera-Mora, M. T. & Rodríguez-Viejo, J. Stability of thin film glasses of toluene and ethylbenzene formed by vapor deposition: an in situ nanocalorimetric study. Phys. Chem. Chem. Phys. 12, 14693–14698 (2010).
Leon-Gutierrez, E., Sepúlveda, A., Garcia, G., Clavaguera-Mora, M. T. & Rodríguez-Viejo, J. Correction: Stability of thin film glasses of toluene and ethylbenzene formed by vapor deposition: An in situ nanocalorimetric study. Phys. Chem. Chem. Phys. 18, 8244–8245 (2016).
Leon-Gutierrez, E., Sepúlveda, A., Garcia, G., Clavaguera-Mora, M. T. & Rodríguez-Viejo, J. Glass transition in vapor deposited thin films of toluene. Thermochim. Acta 492, 51–54 (2009).
Raegen, A. N., Yin, J., Zhou, Q. & Forrest, J. A. Ultrastable monodisperse polymer glass formed by physical vapour deposition. Nat. Mater. 19, 1110–1113 (2020).
Bowie, W. & Zhao, Y.-P. Monte Carlo simulation of vapor deposition polymerization. Surf. Sci. 563(1–3), L245–L250 (2004).
Lin, P., Lyubimov, I., Yu, L., Ediger, M. D. & de Pablo, J. J. Molecular modelling of vapor-deposited polymer glasses. J. Chem. Phys. 140, 204504 (2014).
Samanta, S. et al. Exploring the importance of surface diffusion in stability of vapor-deposited organic glasses. J. Phys. Chem. 123, 4108–4117 (2019).
Dalal, S. S., Sepulveda, A., Pribil, G. K., Fakhraai, Z. & Ediger, M. D. Density and birefringence of a highy stable α, α, β-trisnapthylbenzene glass. J. Chem. Phys. 136, 204501 (2012).
Yoon, Hd., Koh, Y. P., Simon, S. L. & McKenna, G. B. An ultrastable polymeric glass: Amorphous fluoropolymer with extreme fictive temperature reduction by vacuum pyrolysis. Acromolecules 50(11), 4562–4574 (2017).
Shamim, N., Koh, Y. P., Simon, S. L. & McKenna, G. B. Glass transition temperature of thin polycarbonate films measured by flash differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 52(22), 1462–1468 (2014).
Moynihan, C. T., De Easteal, A. J., Bolt, M. A. & Tucker, J. Dependence of the fictive temperature of glass on cooling rate. J. Am. Ceram. Soc. 59, 12–16 (1976).
Gao, Q. & Jian, Z. Fragility and vogel-fulcher-tammann parameters near glass transition temperature. Mater. Chem. Phys. 252, 123252 (2020).
Qin, Q. & McKenna, G. B. Correlation between dynamic fragility and glass transition temperature for different classes of glass forming liquids. J. Non-Cryst. Solids 352(28–29), 2977–2985 (2006).
Mauro, J. C., Yue, Y., Ellison, A. J., Gupta, K. & Allan, D. C. Viscosity of glass-forming liquids. Proc. Natl. Acad. Sci. U. S. A. 106(47), 19780–19784 (2009).
Billmeyer, F. W. Textbook of Polymer Science 79–85 (Wiley, New Jersey, 1969).
Wang, X., Harris, H. R., Bouldin, K., Temkin, H. & Gangopadhyay, S. Structural properties of fluorinated amorphous carbon films. J. Appl. Phys. 87, 621 (2000).
Blanchet, G. B. Deposition of amorphous fluoropolymers thin films by laser ablation. Appl. Phys. Lett. 62, 5 (1993).
Nason, T. C., Moore, J. A. & Lu, T. M. Deposition of amorphous fluoropolymer thin films by thermolysis of teflon amorphous fluoropolymer. Appl. Phys. Lett. 60, 1866 (1992).
Koh, Y. P., Grassia, L. & Simon, S. L. Structural recovery of a single polystyrene thin film using nanocalorimetry to extend the aging time and temperature range. Thermochim. Acta 603, 135–141 (2015).
Badrinarayanan, P., Zheng, W., Li, Q. & Simon, S. L. The glass transition temperature versus the fictive temperature. J. Non-Cryst. Solids 353, 2603–2612 (2007).
Arellano, A. K. T. & McKenna, G. B. Extremely fragile glass-formers? Calorimetric and rheological determinations. J. Polym. Sci. Part B Polym. Phys. 53, 1261–1272 (2015).
Qin, Q. & McKenna, G. B. Correlation between dynamic fragility and glass transition temperature for different classes of glass forming liquids. J. Non-Cryst. Solids 352, 2977–2985 (2006).
Kovacs, A. J. Transition vitreuse dans les polymères amorphes. Etude Phénomènologique. Fortschr. Hochpolym. Forsch. 3, 394–507 (1964).
Ramos, S. L. L. M., Oguni, M., Ishii, K. & Nakayama, H. Character of devitrification, viewed from enthalpic paths, of the vapor-deposited ethylbenzene glasses. J. Phys. Chem. B. 115, 14327–14332 (2011).
Koh, Y. P. & Simon, S. L. Enthalpy recovery of polystyrene: Does a long-term aging plateau exist?. Macromolecules 46, 5815–5821 (2013).
Haruyama, O. et al. Volume and enthalpy relaxation in Pd42.5Cu30Ni7.5P20 bulk metallic glass. Mater. Trans. 55, 466–472 (2014).
Wang, J. Q., Shen, Y., Perepezko, J. H. & Ediger, M. D. Increasing the kinetic stability of bulk metallic glasses. Acta Mater. 104, 25–32 (2016).
Zhao, Y., Tong, X., Ke, H., Bai, H. & Wang, W.H. Ultrastable metallic glass by room temperature aging. Sci. Adv. 8(33), 3623 (2022).
Gibbs, J. H. Nature of the glass transition in polymers. J. Chem. Phys. 25, 185 (1956).
Gibbs, J. H. & DiMarzio, E. A. Nature of the glass transition and the glassy state. J. Chem. Phys. 28, 373–383 (1958).
Adam, G. & Gibbs, J. H. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 43, 139–146 (1965).
Ishii, K., Nakayama, H., Hirabayashi, S. & Moriyama, R. Anomalously high-density glass of ethylbenzene prepared by vapor deposition at temperatures close to the glass-transition temperature. Chem. Phys. Lett. 459, 109 (2008).
McKenna, G. B. Looking at the glass transition: Challenges of extreme time scales and other interesting problems. Rubber Chem. Technol. 93(1), 79–120 (2020).
O’Connell, P. A. & McKenna, G. B. Novel nanobubble inflation method for determining the viscoelastic properties of ultrathin polymer films. Rev. Sci. Instrum. 78, 013901 (2007).
Yoon, H. & McKenna, G. B. Testing the paradigm of an ideal glass transition: Dynamics of an ultrastable polymeric glass. Sci. Adv. 4, eaau5423 (2018).
Acknowledgements
The authors gratefully acknowledge the National Science Foundation under Grants DMR-2022732 and DMR-1610495 and the John R. Bradford Endowment at Texas Tech, each for partial support of this work. The Office of Naval Research through the DURIP program Grant No. N00014-12-1-0876, is also thanked for instrumental support. The authors are also grateful to Clifton Dey, Dr. Jing Li and Dr. Hongxing Jiang for the thickness measurement on the bulk sample used in the FTIR.
Author information
Authors and Affiliations
Contributions
G.B.M. led the project, developed the original ideas and secured funding. A.E.B. carried out the research experiments, analyzed the data, and wrote the original drafts of the manuscript. A.E.B. and G.B.M. jointly finalized the manuscript writing and composition. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Banna, A.A., McKenna, G.B. Challenging the Kauzmann paradox using an ultra-stable perfluoropolymer glass with a fictive temperature below the dynamic VFT temperature. Sci Rep 13, 4224 (2023). https://doi.org/10.1038/s41598-023-31074-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31074-0
- Springer Nature Limited