Abstract
Recently, scientist study the role of surfactants for carbon steel corrosion protection. In the present study, newly tetra-cationic surfactant (CS4: 1,N1'-(ethane–1,2-diyl) bis (N1, N2—didodecyl–N2–(2- (((E)-3-hydroxy-4-methoxy-benzylidene)amino)ethyl)ethane-1,2-diaminium) chloride) based on Schiff-base compound(5,5'-((1E,17E)-2,5,8,11,14,17-hexaazaoctadeca-1,17-diene-1,18-diyl)bis(2-methoxyphenol) was synthesised, purified and characterized using FTIR and 1HNMR spectroscopy. The synthesized Tetra-cationic surfactant (CS4) was evaluated as anti-corrosion for carbon steel (CS-metal) in aggressive 1 M HCl using electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization techniques (PDP). CS4 compound had a good surface-active property by reducing the surface tension as a result to the hydrophobic chains role. The prepared CS4 behaved as hybrid inhibitor (mixed-type) by blocking the anodic and cathodic sites. CS4 exhibited good inhibition efficiency reached 95.69%. The surface morphology of CS-metal was studied using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS)confirming the anti-corrosive effect of CS4 compound returned into the adsorption process of CS4 molecules over CS-metal which obeyed Langmuir adsorption isotherm. The inhibitive effect of CS4 was supported by theoretical quantum chemical studies using the density functional theory (DFT), Monte Carlo (MC) and Molecular Dynamic (MD) simulation.
Similar content being viewed by others
Introduction
Carbon steel (CS-metal) is the most important portion of engineering alloys and steel production besides its application, it is widely used in different areas of industry such as petroleum industry1. Carbon steel corrosion process in acidic surroundings is a big issue in petrochemical, marine or chemical production because of the use of acidic solution of hydrochloric acid (HCl) for oil well and pipelines cleaning2. So, carbon steel protection is a desired process. One of the most attractive option that can be applied is using of Surfactants that are characterized by their unique chemical complex which qualifies it to have a great importance in many fields, whether industry or research3,4. Surfactants (surface active agents) are organic compounds with two various parts with different polarities attached together, one of them is high polar (hydrophilic head) while the other featured by its hydrophobicity (hydrophobic tail)5,6. Surfactants are known with their ability to reduce the effect of surface tension on surface between two phases besides their ability to either gathered in micelle forms and adsorb in the interfaces. The unique structures of surfactant with numerous properties play an important role in many of different applications such as pharmaceutical industry, detergent, petroleum oil recovery, demulsifier and corrosion inhibitors7. One of these types of surfactants is cationic surfactant where the hydrophilic part bears appositive charge. This type is characterized by its ability to use as corrosion inhibitor in acidic medium8,9.
Corrosion is as a destructive attack of metals due to interaction with environment during industrial process10,11. Metals in contact with corrosive environment suffer from short life extent because of corrosion process occurs12.
Corrosion inhibition is an important strategy for long-term protection of metals. In order to reduce the corrosion process of metals, several techniques have been applied. Corrosion inhibitors are one of the simplest and most efficient approaches to protect CS-metal during treatment process (cleaning, pickling and descaling). A large number of molecules have been tested for corrosion inhibition in past decades. According to the numerous advantages of using corrosion inhibitors for CS-metal protection from corrosive surrounding. Therefore, it has received extensive attention from corrosion protection scientists13. Corrosion inhibitors are materials added to corrosive environment in small concentrations, decrease the corrosion rate of metals by decreasing the reaction of the metal with the environment via formation of a protective adsorbed layer13,14. Scientists concern is to design an appropriate highly efficiencies, low cost and eco-friendliness corrosion inhibitor, fundamentally depends on the chemical structure of the surfactant which contain electronic rich functional groups like hetero atoms (N, O, S or P), double bond and aromatic rings as an active centres during the adsorption process1,2,7. Surfactants based on Schiff-base containing C = N15,16,17 have many of the above characteristics paired with a structure which make them promising effective corrosion inhibitors18,19.
Hegazy et al.17 studied the anti-corrosion of three new synthesized cationic surfactants (I(4 N), II(4 N) and IV(4 N)) for carbon steel using weight loss, electrochemical impedance spectroscopy (EIS) and polarization measurements in 1 M HCl. The prepared compounds exhibit high protection against corrosive HCl. EIS result show that the inhibition efficiency of IV(4 N) compound reached to about 96%. El-Dougdoug et al.20 discussed the inhibition performance of mono, di and tetra cationic surfactants for carbon steel in 1 M HCl. The inhibition efficiency reached to about 96 and 91% using mono and tetra cationic surfactants respectively. The inhibition performance of the prepared compounds was studied through harsh conditions (different temperatures and immersion time) showed high protection for carbon steel due to protective film layer of the adsorbed inhibitors. Ting Zhou et al21. discussed the inhibitory behaviour of imidazolium Gemini surfactant on carbon steel in HCl solution using electrochemical EIS, polarization curves, weight loss measurement and the quantum chemical study. Results reveal that the inhibition efficiency of the inhibitor reaches 96% and the quantum chemical calculation was employed to interpret the possible inhibition mechanism of the prepared inhibitor.
The novelty of our study is the synthesis of tetra-cationic surfactants (CS4) enriched with variable function groups N = C, NH, OH, O–CH3 and aromatic ring through two simple steps. The prepared compound was evaluated as corrosion inhibitor for CS-metal in 1 M HCl solution using EIS (electrochemical impedance spectroscopy) and PDP (potentiodynamic polarization). The surface analysis of CS-metal was studied using scanning electron microscope (SEM) and X-ray photoelectron spectroscopy (XPS) which confirmed the inhibition performance of the prepared CS4 in 1 M HCl. The computational investigation of CS4 as corrosion inhibitor was studied using the density functional theory (DFT), Monte Carlo (MC) and Molecular Dynamic (MD) Simulation in gas and liquid phases.
Materials and experimental techniques
Materials
All chemicals were used without further purification, 3-hydroxy-4-methoxybenzaldehyde, pentaethylenehexamine, and chlorododecane were purchased from Sigma Aldrich Company.
Synthesis of cationic surfactant
Tetra-Cationic surfactant (CS4) was prepared as in Scheme 1 through two steps. First step: 0.02 M 3-hydroxy-4-methoxybenzaldehyde was refluxed with 0.01 M pentaethylenehexamine in presence of ethanol as a solvent for 8 h. The solvent was evaporated then washed using petroleum ether to obtain a reddish-brown semisolid compound (Schiff-base)22.
Second step: The obtained Schiff base was refluxed with 1-chlorododecane in ratio 1:4 and ethanol as a solvent for 48 h to produce the corresponding cationic surfactant (CS4). The obtained CS4 compound was purified using diethyl ether and n-hexane17. The chemical structure was confirmed by FTIR and 1HNMR.
Surface active parameters
Surface tension (γ) for different concentrations of CS4 compound was measured in 1 M HCl solution at room temperature using Theta instrument. Surface active parameters such as πCMC (The effectiveness), Γmax (The maximum surface excess), Amin (minimum surface area), CMC (critical micelle concentration), and the change in free energy of micellization \({(\Delta G}_{\mathrm{mic}}^{^\circ })\) and adsorption \({(\Delta G}_{\mathrm{ads}}^{^\circ })\) were calculated and discussed.
Electrochemical measurements
Mild carbon steel specimens with chemical composition in wt.%; C = (0.12∼0.20), Si = (0.20∼0.55), Mn = (1.20∼1.60), P = (≤ 0.045), S = (≤ 0.045), Cr = (≤ 0.30), Ni = (≤ 0.30), Cu = (≤ 0.30) and Fe balanced with a surface pre-treatment procedure was carried out prior to each experiment, The corrosion behavior of CS-metal was studied in 1 M HCl solution with and without different concentrations (1 ppm: 50 ppm) of CS4 compound using OrigaMaster 5 potentiostat/galvanostat. Three electrode system of CS-metal as working electrode, Pt (platinum) electrode as auxiliary electrode and Ag/Agcl as a reference electrode were connected to origalys instrument. After OCP (30 min), EIS (electrochemical impedance spectroscopy) and PDP (potentiodynamic polarization) were performed. EIS was measured in frequency range (100 kHz and 0.05 Hz) and PDP was measured using potential range ± 300 mV around OCP value at 2 mV/s.
Computational studies
In the computational investigation, Accelrys, Inc.’s BIOVIA Materials Studio (7.0) program was employed. The optimization geometry of CS4 was carried out in two different phases (gas and solution) using the DMol3 module with Perdew and Wang (LDA) exchange–correlation functional and DND-3.5 basis set. The interaction between CS4 and iron surface (1 1 0) with 30 Å vacuum layer was achieved and simulated in a corrosion environment23. Some quantum chemical parameters of CS4 such as: the electron density, HOMO (the highest occupied molecular orbital), LUMO (the lowest unoccupied molecular orbital) and dipole moment which are used to predict the most reactive centres in CS4 compound were calculated.
SEM
The CS-metal surface morphology was examined for more information about the corrosion process and the inhibition performance of CS4 using SEM (Scanning Electron Microscope, ZEISS) as a powerful tool to confirm the performance of CS4 as an efficient inhibitor. The CS-metal samples were scanned after immersion in 1 M HCl solution with and without 50 ppm of CS4 at room temperature for 6 h.
XPS
XPS is a surface quantitative spectroscopic technique for interface of metal/solution analysis. CS-metal samples with exposed surface area 1 cm2 were immersed in 1 M HCl solution for 24 h in the absence and presence of CS4 inhibitor and analysed using a KRATOS XSAM-800 to determine the bonding characteristics of CS4 molecules on the metal surface.
Result and discussion
Structure characterization
FTIR
CS4 chemical structure was confirmed by Fourier transform infrared spectroscopy as in Fig. 1 showed bands at 3254.74 cm−1 (ν OH-stretching), 3058.6 cm−1 (νC-H aromatic stretching), 2925.9 cm−1 and 2855.19 cm−1 (νC–H aliphatic stretching), 1668.22 cm−1 (νC = N), 1582.59 cm−1 (ν C–C aromatic), 1514.95 cm−1 (νC = C aromatic), 1448.9 cm−1 (νCH2), 1029.12 cm−1 (νC-O), 638.34 cm−1 (νC–Cl).
1HNMR
1HNMR spectra of CS4 was characterized by Bruker High Performance Digital as in Fig. 2 (δ, ppm): 0.84 (m, 12H, (CH2)nCH3), 1.06 (m, 36H, (CH2)9CH3), 1.24 (m, 8H, CH2(CH2)9CH3), 3.4 (m, 8H, +NHCH2CH2(CH2)9CH3), 3.75 (t, 6H, OCH3), 3.86 (m, 4H, CH2N = C), 6.86–7.93 (d, 6H, Ar), 7.39 (s, 4H,+NH), 8.06 (s, 2H, N = CH), 9.78 (s, 2H, OH).
Surface active parameters
Surface tension values (γ) of the prepared CS4 compound was measured at room temperature (298 K) as in Fig. 3 showing the relationship between Surface tension (γ) and various concentrations of CS4 (-log C). The surface tension (\({\gamma }_{\mathrm{o}}\)) decreased with addition of various concentrations till CCMC as seen in Fig. 3 (γ = 31.5 at CCMC = 2.5 × 10−3 M) while no change was observed after CCMC. This decrease was due to the migration of CS4 molecules to the solution interface because of presence of four hydrophobic fatty chain in its structure24. CMC was determined by the intersection between two lines as in Fig. 3 (before and after CCMC)25. Values of πCMC, Γmax, Amin, \({\Delta G}_{mic}^{o}\) and \({\Delta G}_{\mathrm{ads}}^{^\circ }\) were calculated and listed in.
Table 1 as the following equation:
where γCMC, n, R, T and NA are surface tension of CS4 at CMC, the slope of the straight line (γ Vs –log C), number of ions dissociation (n = 5), the gas constant, the absolute temperature (K) and Avogadro’s number26. The value of \({\pi }_{\mathrm{CMC}}\) in Table 1 showed that CS4 compound decreased surface tension effectively due to presence of large hydrophobic part in its structure27. the values of \({\Gamma }_{max}\) and \({A}_{min}\) indicated that CS4 molecules occupied large surface area at interface as result in the role hydrophobic carbon chain which mean that CS4 compound has adsorption affinity at interface28. The -ve values of \({\Delta G}_{mic}^{^\circ }\) and \({\Delta G}_{\mathrm{ads}}^{^\circ }\) as in Table 1 indicated that spontaneous process of micellization and adsorption occurred. Also, \({\Delta G}_{\mathrm{ads}}^{^\circ }\) value was more than that of \({\Delta G}_{mic}^{^\circ }\) indicating that the prepared CS4 prefer adsorption than micellization confirming that CS4 can act as corrosion inhibitor16,26.
Electrochemical corrosion tests
Electrochemical impedance spectroscopy
Nyquist and bode diagrams as in Figs. 4 and 5 exhibit corrosion behaviour of CS-metal in destructive free acidic HCl solution and with CS4 inhibitor at room temperature. It can see that the diameter of semicircle increases as concentration of CS4 compound increase which can be explained as number of adsorbed molecules on CS-metal surface increase and more surface coverage of CS4 on CS-metal through adsorption process which verifies that corrosion behaviour is controlled and affected by Rct (charge transfer resistance)16,29. No change in Nyquist diagram in absence and presence of CS4 indicating that the corrosion mechanism of CS-metal not affected by the addition of CS4 compound and controlled by Rct30,31. The increase in capacitive loop diameter confirms the higher inhibition/protection of CS-metal and subsequently decrease in corrosion rate in acidic HCl solution in presence CS4 compound32. Furthermore, the compressed semicircle shape of Nyquist diagram as in Fig. 4 may be a result of surface roughness and in-homogeneities of CS-metal33.
At low frequency region, Rct value increase after the addition of CS4 concentrations compared to the blank solution. Also, the noticed change in bode-phase curve can be attributed to the relaxation effect resulting by the adsorption of CS4 molecules34. As seen in Fig. 5 the gap between bode modulus at lower frequency increase with rising of concentration compared to that in free HCl solution. This demonstrates adsorption of CS4 and protection of CS-metal by formation of protective layer against aggressive HCl33. While at higher frequency, reduction in corrosion rate of CS-metal and high protection efficiency are observed as shift of phase angle to wards -90 with rising of concentration35,36. The proposed equivalent circuit was presented as in Fig. 6 using Rs, Rct and CPE which were extracted and listed in Table 2 indicating that one constant phase element clarified by Yo and n. In general, for \(n=0 \&-1\), ZCPE represents resistance \((R={Y}_{o}^{-1})\) and inductive \((L={Y}_{o}^{-1})\) respectively. while for \(n=0.5 \& 1\), Warburg impedance (W = Yo) and capacitance with (C = Yo) for \(n=1\)17,34,35]. From Table 2, the value of n decreased by the addition of CS4 indicated that the surface heterogeneity increases due CS4 adsorption at the CS-steel/solution interfaces. Also, Yo decreased with the addition of CS4 due to increase thickness of adsorbed layer on CS-metal15,16. The following equation was used to determine CPE impedance (ZCPE):
where Q = constant phase element constant, ωmax = the angular frequency39. From Table 2, RS values increase after the addition of CS4 more than that of blank solution indicating that, the solution conductivity decreases with the addition of the studied CS4. This denotes that, shielding of CS-metal from corrosive solution and greater blocking of the active sites at CS-metal surface by CS4 compound40,41. Rct value increase and reached 138.3 Ω.cm2 and 681.4 Ω.cm2 in presence of 1 ppm and 50 ppm of CS4 compound respectively, compared to Rct of free acid solution 38.605 Ω.cm2 indicating that strongly shield of CS-metal by adsorbed CS4 molecules from destructive action of HCl species and formation of barrier layer between CS-metal and corrosive media15,29. The surface coverage (θ) and the inhibition efficiency (η) values were calculated based on Rct values according to equation:
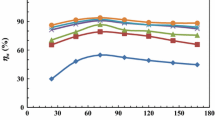
The values of η increase with concentration due to adsorption of CS4 on CS-metal till reach 72.08 and 94.33% in presence of 1 ppm and 50 ppm of CS4 compound respectively confirming that CS4 retard the corrosion process of CS-metal effectively42,43. On the other hand, Corrosion mitigation and inhibition performance increase with concentration due to surface coverage of CS-metal with CS4 molecules by blocking active centre of CS-metal44,45,46.
Replacement of water molecules by CS4 over CS-metal increase with concentration which lead to increase thickness (T) of adsorbed layer of CS4 according to Helmholtz Equation44,45,46.
where A, ε and ε◦ are electrode surface area, the permittivity of local and air dielectric constant of the electric double layer, respectively16,34. The ε value of CS4 is smaller than that of H2O, while the volume is obviously larger than that of H2O. So, the water molecules over CS-metal were replaced with the adsorbed molecules of CS4 subsequently decrease of \({C}_{\mathrm{dl}}\)34.
Potentiodynamic polarization (PDP)
I-V curves as in Fig. 7 sowed the inhibition performance of CS4 compound for CS-metal after fixed immersion time in 1 M HCl. Some electrochemical parameters were extracted from Fig. 7 such as Ecorr (corrosion potential), βc (cathodic slope), βa (anodic slope), icorr (corrosion current density), and η (inhibition efficiency) listed in Table 3 The values of η were calculated from equation:
where icorr.blank and icorr.inh are the corrosion current densities of free acid solution and CS4 respectively47. Cathodic reaction (H2 evolution) and anodic reaction were reduced after CS4 addition to acidic media confirming the inhibition effect of CS416. I-V curves were shifted to lower current values with rising concentration. Parallel line of cathodic Tafel lines confirming that corrosion mechanism not affected by addition of CS4 inhibitor while the anodic Tafel line reflected the inhibition potency of CS4 compound48,49. It was noticed that at higher anodic potential more than -0.285 V the desorption rate of CS4 molecules increased or became higher than their adsorption in a phenomenon called desorption potential. This resulted as increase the dissolution rate of CS-metal more than protection by inhibitor16.
icorr decreased with CS4 addition till reached 0.1571 mA/cm2 and 0.0299 mA/cm2 in presence of 1 ppm and 50 ppm respectively compared with uninhibited corrosion current 0.6949 mA/cm2. This indicated the adsorption process of CS4 compound over CS-metal forming barrier protective layer against corrosive HCl50. From Table 3, the change in Ecorr values were less than 85 mV implying that CS4 inhibitor acted as hybrid inhibitor (mixed-type inhibitor)51. The I-V curves shape didn’t change after the addition of CS4 concentrations, in addition to there was slightly change in βa and βc values which mean that the corrosion mechanism of CS-metal didn’t change52. η values increased with rising concentration till reached 95.69% due to the adsorption of CS4 molecules decreased the contact between CS-metal and aggressive solution by blocking the anodic and cathodic site at CS-metal during the adsorption process of CS4 molecules and decrease the corrosion rate of CS-metal25,51.
Adsorption isotherm
The adsorption of CS4 molecules at CS-metal/1 M HCl interface can be explained by the replacement of water molecules by organic molecules (CS4) as the following equation:
where CS4sol and CS4ads are organic molecules (CS4 compound) in the solution (liquid phase) and adsorbed on CS-metal (adsorption phase), respectively, and n is the number of replaced water molecules37.
The corrosion mitigation potency of organic compounds mainly depends on their adsorption capability over CS-metal surface. To clarify the adsorption properties (nature & strength) of CS4 compound, the experimental data calculated from EIS and PDP were fitted to a various adsorption isotherms. Langmuir adsorption isotherm was the best fit isotherm with linear association coefficients very close to 1 (R2 = 0.9988) which was presented in Fig. 8 as a straight line with slope was nearly 1 (slope = 1.03). This indicated that, the adsorption of CS4 compound on CS-metal surface obeys Langmuir adsorption isotherm. Also, a protective monolayer film of CS4 formed over CS-metal and no interactions between adsorbed CS4 molecules occurs37,39. The values of Kads (adsorption equilibrium constant) and \(\Delta {G}_{\mathrm{ads}}^{^\circ }\)(standard free energy) were listed in Table 4 and were calculated from the following equations:
where 55.5, C, \(\uptheta ,\) R and T are molar concentration of water, the concentration of inhibitor, the surface coverage, the gas constant, the absolute temperature (k) and the standard free energy respectively. The high value of Kads, reflects the high adsorption ability of CS4 compound and the high stability of the adsorbed layer on CS-metal53. \(\Delta {G}_{\mathrm{ads}}^{^\circ }\) values that obtained from PDP and EIS data were − 44.071 kJ mol−1 and − 43.274 kJ mol−1 respectively, indicating a strong interaction between CS4 and the surface of CS-metal. Also, the −ve sign reflected that the adsorption of CS4 was spontaneous process38. It is known that, the value around − 40 kJ mol-1 or higher related to formation of a new bonds (coordinate bond) between inhibitor and CS-metal through charge transfer or sharing (chemisorption)22. \(\Delta {G}_{\mathrm{ads}}^{^\circ }\) value indicating that, chemical adsorption of CS4 over the surface of CS-metal via hetero atoms (N & O) and aromatic ring in its structure30,33.
Computational studies
Frontier molecular orbitals
Quantum chemical calculations were used to understand how the material's structural and electrical characteristics affect the way it prevents corrosion. Additionally, the study’s objective was to understand more about the donor–acceptor interactions between inhibitor molecules and metal atoms. The frontier molecular orbitals and charge density distributions of chemical compounds can be used to help comprehend their molecular reactivity. The EHOMO orbital is most highly associated to an inhibitor’s ability to give electrons, whereas the ELUMO orbital is most typically associated to an inhibitor's capacity to receive electrons. High values of EHOMO are thought to represent a molecule’s propensity to give electrons to suitable acceptor molecules with energy level and an empty molecular orbital rather than a molecule’s propensity to strongly accept electrons from other molecules. Therefore, a molecule has a higher chance of receiving electrons the lower the ELUMO value is. The binding capacity of the inhibitor to the metal surface is increased by lowering the ELUMO energy values and raising the EHOMO energy values. As a result, the metal surface’s ability to accept charges rises, which causes a specific detail between the inhibitor’s acceptor anti-bonding orbital and the donor iron atoms.
HOMO and LUMO values explained the ability of CS4 molecules to donate and accept electrons. The value of ΔE (energy gap) of FMO (Frontier Molecular Orbital) gives information about the kinetic stability and chemical reactivity of CS4 structure. Also, FMO helps in the prediction of the most reactive sited in CS4 structure. Figure 9 show the optimized geometry of the investigated compound. Some quantum chemical parameters of the studied CS4 were calculated based on HOMO and LUMO values such as: I (ionization potential), A (electron affinity) x (electronegativity) and ηH (chemical hardness) and listed in Table 5.
The HOMO and LUMO densities were found in the Nitrogen-containing region of CS4 structure as seen in Fig. 10 are responsible for the electron sharing (donation and acceptation) between CS4 and Fe (1 1 0) surface54,55,56.
ΔE value explained the relationship of CS4 electronic properties with chemical structure such as frontier electron density and chemical stability by explanation of charge transfer57. The high value of ΔE (7.77 eV) reflects the metal ion complex’s stability over the Fe surface. The graphical representation of molecular orbitals can provide insight into the aromaticity and lone pair. The + ve and − ve phase of wave-functions are represented in red and blue colour, respectively.
A significant correlation may be shown in terms of molecular hardness; that is, when molecular hardness decreases, molecules react with surfaces more easily and have a smaller amount of corrosion-causing power. The molecule with the largest dipole moment is also more effective in inhibiting other compounds. It was discovered that the quantum indices, such as the magnitudes of A and I, play an important role in evaluating the effectiveness of the CS4. The lower the values of (I), the greater the ability of the inhibitor molecule to offer electrons to the surface of CS-metal. In a similar fashion, having high values of (A) encourages the inhibitor to host the electrons that have been accepted from the substrate surface. The fact that the N value is 0.768 (< 3.6), indicates that the inhibition efficiency related to the ability of CS4 to donate electrons22. Another index that is used to measure the likelihood of bond formation is the dipole moment, denoted by the symbol. There is a lot of controversy surrounding the application of µ values to correlate the experimental findings for the inhibition performance. The higher µ values, the lower the inhibition efficiency, which reflects that lower µ will help in accumulation of CS4 molecules over CS-metal surface. On the other hand, the other view suggests that, the higher µ values related to a higher interaction between CS4 molecules and CS-metal surface which will increase the inhibition performance. One of the contending opinions is that higher dipole moment values result in lower inhibition efficiency. The theoretical approach is consistent with the quantifiable parameters that have been discussed.
Molecular electrostatic potential (MEP)
MEP is a tool that was used for predicting the nucleophilic and the electrophilic attack sites, visualize the charge distribution and charge-related characteristics of target inhibitors A colour grading system was used to illustrate the molecular interactions and chemical composition. MEP surface analysis of CS4 was determined using DFT calculations with the optimized structure and B3LYP/6-31G + (d, p) basis set. Fig. 10 illustrates the electrostatic potential surface of the investigated CS4. The compound’s colour code is in the range of 0.284e3 to +1.618e3. The MEP structure’s red and blue colours denote more electron-rich and electron-poor regions, respectively. The polarization effect was observed, the − ve potential areas of the MEP are localized around electronegative atoms (oxygen and nitrogen), while the + ve potential regions are localized around hydrogen atoms. The + ve electrostatic potential and the negative electronegative potential sites are more favourable for the attraction of nucleophilic and electrophilic species.
Monte Carlo (MC) and molecular dynamic (MD) simulation
Using the simulated corrosion environment, the lowest energy configurations of the various protonated forms of CS4 on CS-metal surface were determined as shown in Fig. 11. Based on the adsorption geometries, heteroatoms (O and N) were involved in the CS4 adsorption process. CS4 adsorption affinity resulted in the formation of a protective anticorrosion layer over CS-meal surface. The adsorption energies were calculated using the following Equation57,58:
where \({E}_{Fe \left(110\right)inh}{, E}_{Fe \left(110\right)}\) and \({E}_{inh}\) are the total energy of the simulated corrosion system, the total energy of Fe (1 1 0) surface and that CS4 molecule, respectively. The obtained data from the simulation method reveals that:
-
\({E}_{tot}\) (total energy) is equal to the summation of the internal and the adsorption energy of the adsorbate.
-
\({E}_{ads}\) (adsorption energy) is the energy released when the adsorbate (CS4 molecule) is relaxed on the substrate (CS-metal surface) and the equal rigid adsorption energy plus the deformation energy.
-
\({E}_{rig}\) (rigid adsorption energy) is the energy released (or required) when the unrelaxed adsorbate CS4 molecules are adsorbed on the substrate.
-
\({E}_{def}\) (deformation energy) is the energy released when the adsorbed CS4 molecules are relaxed on the CS-metal surface.
The adsorption energy of CS4 was listed in Table 6, indicating a more stable and stronger interaction between the Fe surface and CS4, and therefore better inhibition efficiency.
The theoretical data obtained from Monte Carlo simulations were matched with the experimental data. The –ve value of the adsorption energies as seen in Fig. 12, indicated that CS4 molecules adsorbed over CS-metal surface spontaneously59,60.
To examine the interaction between CS4 and metal surfaces, MD simulations were used to predict the binding energy \({({\varvec{E}}}_{{\varvec{b}}{\varvec{i}}{\varvec{n}}}\)) of CS4 on the Fe surface and explains the significant association between experimental inhibition efficiency and binding energy the studied CS4. The interaction energy (\({E}_{int}\)) between the CS4 molecules and Fe (110) surface has been obtained61,62.
The calculated \({{\varvec{E}}}_{{\varvec{b}}{\varvec{i}}{\varvec{n}}}\): \({E}_{ads}={-E}_{inh}=-{E}_{ads}\) was listed in Table 7, indicated that a strong interaction between CS4 and CS-metal surface occurred and more stable inhibitor/surface interaction.
Surface analysis (SEM)
The surface morphology examination of the CS-metal surface in 1 M HCl solution with and without the optimum concentration (50 ppm) of CS4 compound was performed by SEM as in Fig. 13. The blank solution (free acid) showed the badly damaged surface due to the aggressive action of corrosive media (1 M HCl) as result in dissolution of CS-metal (corrosion process) and roughness surface layer was observed. In contrast, the appearance of CS-metal surface was different in presence of CS4 compound. It can be seen that after the addition of CS4 compound, an improvement in carbon steel surface morphology (more smooth) and less damaged and roughness surface was observed, resulting in good inhibition performance of CS4 which reduce the corrosive effect of HCl. This may be due to the adsorption process over CS-metal forming a protective barrier layer which decreases the contact between CS-metal and aggressive solution37,38,43.
XPS analysis
XPS measurements were performed on both inhibited and uninhibited CS-metal as shown in Fig. 14. The existence of Fe, O, and Cl elements in the XPS results of CS-metal samples in blank solution (without CS4) reflected the occurrence of CS-metal dissolution (corrosion process) in 1 M HCl. Figure 14 presented the spectrum of Fe 2p revealed satellite peaks at 730.56 and 719.37 eV. The peaks at 710.71, 712.83, 715.77, 724.42, and 727.73 eV corresponded to Fe2O3, FeOOH, FeCl3, FeO, and Fe3O4, respectively63,64. The XPS spectra of CS-metal treated with CS4 inhibitors showed Fe at 707.15 eV indicating that the high surface coverage provided by CS4 molecules which can effectively isolate Fe atoms from corrosive HCl solution65.
As in Figs.14, the spectrum of O 1 s revealed satellite peaks at 530.26 and 531.76 eV, which could be attributed to the corrosion products such as iron oxides (Fe2O3 and Fe3O4) and hydrous iron oxides (FeOOH) respectively. The presence of O–Fe bond indicating the occurrence of donor acceptor interactions between the vacant d orbitals of Fe and sp2 electron pairs of OH and O-CH3 group. The peak at 532.97 eV could be attributed to oxygen of adsorbed H2O and C–O bond. Also, the intensity decreased gradually, thereby indicating that addition of CS4 reduces the erosion of corrosion particles to some extent65,66.
Figure 14 represents C1s spectrum of CS-metal surface in presence of CS4 molecules at 284.92, 286.02 and 287.78 eV. The peak at 284.92 eV could be attributed to C–C, C = C and C–H bonds, the peak at 286.02 eV can be attributed to C–O bond and the peak at 287.78 eV can be partly attributed to aromatic rings (π-π * shakeup satellite) and partly associated to the coordination of oxygen with Fe of CS-metal surface. These bonds exist in CS4 compound, proving that CS4 compound adsorbed and shielded CS-metal surface from corrosive HCl solution.
Cl 2p spectrum as in Fig. 14 has included two peaks at 197.24 eV and 198.43 eV associated to Cl–Fe bond in FeCl367.
N 1 s spectrum as in Fig. 14 revealed satellite peaks at peaks at 401.02 and 399.5 eV, which could be attributed to N–Fe and N–C, respectively54,55. This result indicated that, the lone electron pairs of distributed N atoms in CS4 inhibitor formed chemical bonds with the empty Fe d-orbital which allow CS4 molecules to adsorb onto the CS-metal surface65.
The inhibition mechanism
It is known that, the organic inhibitors have suppression performance due to their adsorption at the metal/solution interface via their chemical structure, the charge distribution of the inhibitor, nature, and charged metal surface. Generally, the single adsorption mechanism between the inhibitor (CS4) and carbon steel surface is unachievable because of the complexity of the studied inhibitor’s structure. Therefore, the adsorption mechanism of CS4 can be explained in 3 modes of adsorption:
-
1.
Physical adsorption: electrostatic interaction which helps CS4 molecules to be adsorbed on metal surface between + ve quaternary N (cationic head) of CS4 and − ve sites of carbon steel surface (cathodic sites → cathodic inhibition) besides electrostatic interaction between chloride ions Cl- and + ve sites of carbon steel (anodic sites → anodic inhibition). CS4 has a high inhibition efficiency due to having many charged parts in its structure.
-
2.
Chemical adsorption: formation of co-ordination bond between hetero atoms (N and O) and π-electrons (C = N and pyridine ring) with vacant d-orbital of carbon steel through electron sharing process (donor–acceptor interactions).
-
3.
Aliphatic chain: the hydrophobic chains play an important role in the mitigation process, not only increase the thickness of the adsorption film layer but also enhances its density. CS-metal was inhibited effectively by CS4 due to the presence of multiple numbers of hydrophobic chains in its structure. The aliphatic chains attached to quaternary N+ forces molecule towards carbon steel surface by displacement of water molecules and increase the surface coverage of CS-metal away from the corrosive effect of HCl solution and forms more compacted and denser protective layer.
Conclusion
A newly tetra-cationic surfactant (CS4) was synthesized through two simple steps by condensation reaction of vanillin with pentaethylenehexamine followed by quaternization reaction of chlorododecane with the obtained Schiff-base in molar ratio 1:4. The main objective of the present work was to study the corrosion performance of the prepared CS4 for carbon steel in 1 M HCl. CS4 exhibited high inhibition efficiency was 95.69% which can be explained by the highly adsorption capability of CS4 molecules over CS-metal surface. The adsorption of CS4 followed Langmuir adsorption isotherm which can explained the chemical adsorption of CS4 over CS-metal via hetero atoms in N = C, OH, NH and O-CH3 besides presence of the aromatic rings which can be explained by the high Kads and \(\Delta {G}_{\mathrm{ads}}^{^\circ }\) values. Various electrochemical techniques such as EIS and PDP that were carried out were matched with other which suggested that, CS4 acted as mixed type inhibitor according to Tafel data. Also, the theoretical quantum studies (DFT, MC and MD) confirmed the adsorption capacity of CS4 over Fe (110) in 1 M HCl as well as SEM and XPS analysis.
Data availability
All data generated or analysed during this study are included in this manuscript.
Change history
19 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-33409-3
References
Mao, T. et al. Novel cationic Gemini ester surfactant as an efficient and eco-friendly corrosion inhibitor for carbon steel in HCl solution. J. Mol. Liq. 339, 117174 (2021).
Kaczerewska, O., Leiva-garcia, R., Akid, R. & Brycki, B. Efficiency of cationic gemini surfactants with 3-azamethylpentamethylene spacer as corrosion inhibitors for stainless steel in hydrochloric acid. J. Mol. Liq. 247, 6–13 (2017).
Lee, S., Lee, J., Yu, H. & Lim, J. Journal of industrial and engineering chemistry synthesis of environment friendly nonionic surfactants from sugar base and characterization of interfacial properties for detergent application. J. Ind. Eng. Chem. 38, 157–166 (2016).
Abd-Elaal, A. A., Elbasiony, N. M., Shaban, S. M. & Zaki, E. G. Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J. Mol. Liq. 249, 304–317 (2018).
Tizpar, A. & Ghasemi, Z. The corrosion inhibition and gas evolution studies of some surfactants and citric acid on lead alloy in 12.5 M H 2 SO 4 solution. Appl. Surf. Sci. 252, 8630–8634 (2006).
Haladu, S. A. et al. Inhibition of mild steel corrosion in 1 M H 2 SO 4 by a gemini surfactant 1, 6-hexyldiyl-bis- (dimethyldodecylammonium bromide ): ANN, RSM predictive modeling, quantum chemical and MD simulation studies. J. Mol. Liq. 350, 118533 (2022).
Pakiet, M., Kowalczyk, I., Leiva Garcia, R., Akid, R. & Brycki, B. Cationic clevelable surfactants as highly efficient corrosion inhibitors of stainless steel AISI 304: Electrochemical study. J. Mol. Liq. 315, 113675 (2020).
Pinazo, A. et al. New cationic vesicles prepared with double chain surfactants from arginine: Role of the hydrophobic group on the antimicrobial activity and cytotoxicity. Colloids Surf. B Biointerfaces 141, 19–27 (2016).
Hegazy, M. A., Badawi, A. M., El, S. S. A. & Kamel, W. M. Corrosion inhibition of carbon steel using novel N - (2- (2-mercaptoacetoxy ) ethyl ) - N, N -dimethyl dodecan-1-aminium bromide during acid pickling. Corros. Sci. 69, 110–122 (2013).
Shaban, S. M., Saied, A., Tawfik, S. M., Abd-Elaal, A. & Aiad, I. Corrosion inhibition and Biocidal effect of some cationic surfactants based on Schiff base. J. Ind. Eng. Chem. 19, 2004–2009 (2013).
Al-Sabagh, A. M., Nasser, N. M., El-Azabawy, O. E. & El-Tabey, A. E. Corrosion inhibition behavior of new synthesized nonionic surfactants based on amino acid on carbon steel in acid media. J. Mol. Liq. 219, 1078–1088 (2016).
Hejazi, S. et al. Electrochemical and quantum chemical study of Thiazolo-pyrimidine derivatives as corrosion inhibitors on mild steel in 1M H2SO4. J. Ind. Eng. Chem. 25, 112–121 (2015).
Tan, B. et al. Passiflora edulia Sims leaves extract as renewable and degradable inhibitor for copper in sulfuric acid solution. Colloids Surf. A Physicochem. Eng. Asp. 645, 128892 (2022).
Sayın, K. Experimental and theoretical investigation of inhibition behavior of 2- ((4- (dimethylamino) benzylidene) amino) benzenethiol for carbon steel in HCl solution Hülya Keles. Corres. Sci. 184, 109376 (2021).
El Basiony, N. M., Badr, E. E., Baker, S. A. & El-Tabei, A. S. Experimental and theoretical (DFT&MC) studies for the adsorption of the synthesized Gemini cationic surfactant based on hydrazide moiety as X-65 steel acid corrosion inhibitor. Appl. Surf. Sci. 539, 148246 (2021).
El-Tabei, A. S., El-Tabey, A. E. & El Basiony, N. M. Newly imine-azo dicationic amphiphilic for corrosion and sulfate-reducing bacteria inhibition in petroleum processes: Laboratory and theoretical studies. Appl. Surf. Sci. 573, 151531 (2022).
Hegazy, M. A., Abd El-Rehim, S. S., Badr, E. A., Kamel, W. M. & Youssif, A. H. Mono-, Di- and tetra-cationic surfactants as carbon steel corrosion inhibitors. J. Surfactants Deterg. 18, 1033–1042 (2015).
Hegazy, M. A. A novel Schiff base-based cationic gemini surfactants: Synthesis and effect on corrosion inhibition of carbon steel in hydrochloric acid solution. Corros. Sci. 51, 2610–2618 (2009).
Ansari, K. R., Quraishi, M. A. & Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 79, 5–15 (2014).
El-Dougdoug, W. I. et al. Synthesis and assessment of Gemini cationic surfactants as inhibitors for corrosion of carbon steel in hydrochloric acid. Green Chem. Lett. Rev. 15, 796–812 (2022).
Zhou, T., Yuan, J., Zhang, Z., Xin, X. & Xu, G. The comparison of imidazolium Gemini surfactant [C14-4-C14im]Br 2 and its corresponding monomer as corrosion inhibitors for A3 carbon steel in hydrochloric acid solutions: Experimental and quantum chemical studies. Colloids Surfaces A Physicochem. Eng. Asp. 575, 57–65 (2019).
El-Tabei, A. S., El-Azabawy, O. E., El Basiony, N. M. & Hegazy, M. A. Newly synthesized quaternary ammonium bis-cationic surfactant utilized for mitigation of carbon steel acidic corrosion; theoretical and experimental investigations. J. Mol. Struct. 1262, 133063 (2022).
Karton, A. & Spackman, P. R. Evaluation of density functional theory for a large and diverse set of organic and inorganic equilibrium structures. J. Comput. Chem. 42, 1590–1601. https://doi.org/10.1002/jcc.26698 (2021).
Mao, J. et al. Effect of spacer hydroxyl number on the performance of Gemini cationic viscoelastic surfactant for fracturing fluids. J. Mol. Liq. 346, 117889 (2022).
Eldougdoug, W. I., Ali, A. I., Elaraby, A. & Mabrouk, E. M. Corrosion inhibition of Tri-cationic surfactant on carbon steel in hydrochloric acid solution. J. Basic Environ. Sci. 5, 289–300 (2018).
Shaban, S. M., Abd, S., Taw, S. M., Abdel-rahman, A. A. & Aiad, I. Studying surface and thermodynamic behavior of a new multi-hydroxyl Gemini cationic surfactant and investigating their performance as corrosion inhibitor and biocide. J. Mol. Liq. 316, 113881 (2020).
Ahmady, A. R. et al. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 299, 102581 (2022).
Verma, C., Quraishi, M. A. & Rhee, K. Y. Hydrophilicity and hydrophobicity consideration of organic surfactant compounds: Effect of alkyl chain length on corrosion protection. Adv. Colloid Interface Sci. 306, 102723. https://doi.org/10.1016/j.cis.2022.102723 (2022).
Mobin, M., Parveen, M. & Aslam, H. R. Effect of different additives, temperature, and immersion time on the inhibition behavior of L-valine for mild steel corrosion in 5% HCl solution. J. Phys. Chem. Solids 161, 110422 (2022).
Wang, C., Zou, C. & Cao, Y. Electrochemical and isothermal adsorption studies on corrosion inhibition performance of β-cyclodextrin grafted polyacrylamide for X80 steel in oil and gas production. J. Mol. Struct. 1228, 129737 (2021).
Migahed, M. A. & Nassar, I. F. Corrosion inhibition of Tubing steel during acidization of oil and gas wells. Electrochim. Acta 53, 2877–2882 (2008).
Huang, L. et al. Corrosion resistance and antibacterial activity of procyanidin B2 as a novel environment-friendly inhibitor for Q235 steel in 1 M HCl solution. Bioelectrochemistry 143, 107969 (2022).
Tantawy, A. H., Soliman, K. A. & Abd El-Lateef, H. M. Novel synthesized cationic surfactants based on natural piper nigrum as sustainable-green inhibitors for steel pipeline corrosion in CO2–3.5%NaCl: DFT, Monte Carlo simulations and experimental approaches. J. Clean. Prod. 250, 119510 (2020).
Guo, L. et al. Multidimensional insights into the corrosion inhibition of 3,3-dithiodipropionic acid on Q235 steel in H2SO4 medium: A combined experimental and in silico investigation. J. Colloid Interface Sci. 570, 116–124 (2020).
Haddadi, S. A., Alibakhshi, E., Bahlakeh, G., Ramezanzadeh, B. & Mahdavian, M. A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution. J. Mol. Liq. 284, 682–699 (2019).
El Basiony, N. M. et al. Synthesis, characterization, experimental and theoretical calculations (DFT and MC) of ethoxylated aminothiazole as inhibitor for X65 steel corrosion in highly aggressive acidic media. J. Mol. Liq. 297, 111940 (2020).
Hegazy, M. A. Novel cationic surfactant based on triazole as a corrosion inhibitor for carbon steel in phosphoric acid produced by dihydrate wet process. J. Mol. Liq. 208, 227–236 (2015).
Yildiz, R., Döner, A., Doǧan, T. & Dehri, I. Experimental studies of 2-pyridinecarbonitrile as corrosion inhibitor for mild steel in hydrochloric acid solution. Corros. Sci. 82, 125–132 (2014).
Abdallah, M., Eltass, H. M., Hegazy, M. A. & Ahmed, H. Adsorption and inhibition effect of novel cationic surfactant for pipelines carbon steel in acidic solution. Prot. Met. Phys. Chem. Surf. 52, 721–730 (2016).
Aslam, R., Mobin, M., Aslam, J., Lgaz, H. & Chung, I. M. Inhibitory effect of sodium carboxymethylcellulose and synergistic biodegradable gemini surfactants as effective inhibitors for MS corrosion in 1 M HCl. J. Mater. Res. Technol. 8, 4521–4533 (2019).
Mobin, M. & Aslam, R. Experimental and theoretical study on corrosion inhibition performance of environmentally benign non-ionic surfactants for mild steel in 3.5% NaCl solution. Process Saf. Environ. Prot. 114, 279–295 (2018).
Elbasiony, N. M. Journal of molecular structure controlling C-steel dissolution in 1M HCl solution using newly synthesized ρ- substituted imine derivatives: Theoretical ( DFT and MCs ) and experimental investigations. J. Mol. Struct. 1274, 134357. https://doi.org/10.1016/j.molstruc.2022.134357 (2022).
Döner, A. & Kardaş, G. N-Aminorhodanine as an effective corrosion inhibitor for mild steel in 0.5M H2SO4. Corros. Sci. 53, 4223–4232 (2011).
Migahed, M. A., Nasser, A., Elfeky, H. & El-Rabiei, M. M. The synthesis and characterization of benzotriazole-based cationic surfactants and the evaluation of their corrosion inhibition efficiency on copper in seawater. RSC Adv. 9, 27069–27082 (2019).
Zhu, H. et al. Efficiency of Gemini surfactant containing semi-rigid spacer as microbial corrosion inhibitor for carbon steel in simulated seawater. Bioelectrochemistry 140, 107809 (2021).
Wang, D., Li, Y., Chen, B. & Zhang, L. Novel surfactants as green corrosion inhibitors for mild steel in 15% HCl: Experimental and theoretical studies. Chem. Eng. J. 402, 126219 (2020).
Han, P. et al. The anticorrosion of surfactants toward L245 steel in acid corrosion solution: Experimental and theoretical calculation. J. Mol. Liq. 348, 118044 (2022).
Jin, X. et al. The study of surface activity and anti-corrosion of novel surfactants for carbon steel in 1 M HCl. J. Mol. Liq. 353, 118747 (2022).
El-Tabei, A. S., Hegazy, M. A., Bedair, A. H., El Basiony, N. M. & Sadeq, M. A. Novel macrocyclic cationic surfactants: Synthesis, experimental and theoretical studies of their corrosion inhibition activity for carbon steel and their antimicrobial activities. J. Mol. Liq. 345, 116990 (2022).
Sargolzaei, B. & Arab, A. Synergism of CTAB and NLS surfactants on the corrosion inhibition of mild steel in sodium chloride solution. Mater. Today Commun. 29, 102809 (2021).
Zaki, E. G. & Zidan, T. A. Methyl Acrylate Derivatives as Corrosion Inhibitors for X-65 Type Carbon Steel in 1 M HCl. 16, (2021).
Solmaz, R., Kardaş, G., Çulha, M., Yazici, B. & Erbil, M. Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochim. Acta 53, 5941–5952 (2008).
Fouda, A. S., Megahed, H. E., Fouad, N. & Elbahrawi, N. M. Corrosion inhibition of carbon steel in 1 M hydrochloric acid solution by aqueous extract of Thevetia peruviana. J. Bio- Tribo-Corrosion 2, 1–13 (2016).
Hsissou, R. et al. Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution. J. Mol. Struct. 1182, 340–351 (2019).
Mehmeti, V. & Podvorica, F. I. Experimental and theoretical studies on corrosion inhibition of niobium and tantalum surfaces by carboxylated graphene oxide. Materials 11(6), 893 (2018).
Molhi, A. et al. Contribution to the corrosion inhibition of c38 steel in 1 m hydrochloric acid medium by a new epoxy resin pgeppp. Int. J. Corros. Scale Inhib. 10, 399–418 (2021).
Khaled, K. F. Monte Carlo simulations of corrosion inhibition of mild steel in 0.5 M sulphuric acid by some green corrosion inhibitors. J. Solid State Electrochem. 13, 1743–1756 (2009).
Yu, L. J. et al. Effect of alkyl chain length on inhibition performance of imidazoline derivatives investigated by molecular dynamics simulation. Mater. Corros. 64, 225–230 (2013).
Sun, H. et al. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 22, 1–10 (2016).
El Faydy, M. et al. An experimental-coupled empirical investigation on the corrosion inhibitory action of 7-alkyl-8-Hydroxyquinolines on C35E steel in HCl electrolyte. J. Mol. Liq. 317, 113973 (2020).
El Faydy, M. et al. Experimental and computational investigations on the anti-corrosive and adsorption behavior of 7-N, N’-dialkyaminomethyl-8-Hydroxyquinolines on C40E steel surface in acidic medium. J. Colloid Interface Sci. 576, 330–344 (2020).
Saha, S. K. & Banerjee, P. A theoretical approach to understand the inhibition mechanism of steel corrosion with two aminobenzonitrile inhibitors. RSC Adv. 5, 71120–71130 (2015).
Zarrouk, A. et al. New 1H-pyrrole-2,5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: Electrochemical, XPS and DFT studies. Corros. Sci. 90, 572–584 (2015).
Nam, N. D. et al. The behaviour of praseodymium 4-hydroxycinnamate as an inhibitor for carbon dioxide corrosion and oxygen corrosion of steel in NaCl solutions. Corros. Sci. 80, 128–138 (2014).
Zheng, T. et al. Synergistic corrosion inhibition effects of quaternary ammonium salt cationic surfactants and thiourea on Q235 steel in sulfuric acid: Experimental and theoretical research. Corros. Sci. 199, 110199 (2022).
Berrissoul, A. et al. Assessment of corrosion inhibition performance of origanum compactum extract for mild steel in 1 M HCl: Weight loss, electrochemical, SEM/EDX, XPS, DFT and molecular dynamic simulation. Ind. Crops Prod. 187, 115310 (2022).
Zhang, Y. et al. Novel Schiff base-based cationic Gemini surfactants as corrosion inhibitors for Q235 carbon steel and printed circuit boards. Colloids Surf. A Physicochem. Eng. Asp. 623, 126717 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.E.: supervision, investigation, methodology, data curation, writing—original draft, review and editing. S.A.E-S.: investigation, methodology, data curation, writing—original draft. E.A.K.: investigation, methodology, data curation. E.G.Z.: supervision, investigation, methodology, data curation, writing -original draft, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Affiliation 2, which was incorrectly given as ‘University College of Umluj-Tabuk University, Umluj, Saudi Arabia’. The correct affiliation is: University College of Umluj, University of Tabuk, Tabuk, Saudi Arabia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elaraby, A., El-samad, S.A., khamis, E.A. et al. Theoretical and electrochemical evaluation of tetra-cationic surfactant as corrosion inhibitor for carbon steel in 1 M HCl. Sci Rep 13, 942 (2023). https://doi.org/10.1038/s41598-023-27513-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27513-7
- Springer Nature Limited
This article is cited by
-
Some New Synthesized Gemini Cationic Surfactants as Corrosion Inhibitors for Carbon Steel in Hydrochloric Acid Solution
Journal of Bio- and Tribo-Corrosion (2023)