Abstract
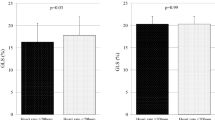
The potential associations between disease duration, glycemic control, and the echocardiographic markers of the myocardial mechanics were investigated in asymptomatic T1DM patients. Seventy T1DM patients (38.2 ± 11.7 years, 46 female) and 30 healthy volunteers were investigated. Besides the conventional and tissue Doppler measurements, left ventricular global longitudinal (GLS) and circumferential (GCS) strain as well as left and right atrial strain parameters were measured with 2D speckle tracking technique. Median HbA1c level was 7.4 (1.8)%. Even when added age and hypertension to the model, current HbA1c level remained independent predictor of left ventricular GLS (p = 0.002), GCS (p < 0.001), mitral e’ (p = 0.018), tricuspid e’ (p = 0.018) and left (p = 0.039) and right atrial conduit strain (p = 0.047) in multiple linear regression models. Correlations between disease duration and the echocardiographic variables lost their significance in multiple models. In patients with a combination of HbA1c ≤ 7.4% and no hypertension, echocardiographic findings did not differ from those in healthy volunteers. Patients with HbA1c > 7.4% and no hypertension and especially patients with coexisting hypertension and HbA1c > 7.4%, exhibited significantly impaired myocardial mechanics. Quality of glycemic control has a significant impact on myocardial mechanics in T1DM patients. Regarding disease duration this relationship was not proved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Type 1 diabetes mellitus (T1DM) is one of the most common chronic disorders affecting young adults. It is associated with serious microvascular and macrovascular complications resulting in at least tenfold increase in cardiovascular diseases as compared with the age matched healthy population1,2. Besides, heart failure develops 10–15 years earlier in T1DM patients than in the general population1,3. Although in young asymptomatic T1DM patients the standard echocardiographic parameters reflect normal cardiac size and function4, tissue Doppler imaging (TDI) and speckle tracking echocardiography seem to be useful techniques for assessing subclinical myocardial involvement in this population. Left ventricular (LV) systolic dysfunction was proved by speckle tracking-derived global longitudinal strain (GLS) data, both in T1DM children and adults5,6,7,8,9,10,11,12, whereas TDI measurements suggested impaired LV diastolic function13,14,15. Nevertheless, conflicting results were also reported5,16,17,18. Less is known about right ventricular (RV) function7,15,19, or atrial performance14,20 in this disease.
Duration of diabetes and quality of the glycemic control have been reported as critical factors contributing toward development of cardiovascular complications1,2,3. Data about their effect on myocardial mechanics, however, are scarce and controversial.
Thus, our work aimed to provide a comprehensive analysis of the myocardial size and function using standard and novel echocardiographic techniques and to investigate the potential associations between disease duration, glycemic control, and the echocardiographic markers of the myocardial mechanics in asymptomatic T1DM patients.
Methods
Study population
Seventy-five asymptomatic patients without known cardiovascular disease, diagnosed with T1DM in the tertiary center of 2nd Department of Internal Medicine, University of Pécs were recruited for our prospective study. Detailed medical history was obtained. Patients with impaired LV systolic function (LV ejection fraction (EF) < 55%), significant left-sided valvular disease, atrial fibrillation, known coronary artery disease (CAD), substantial peripheral artery disease or with diabetic nephropathy or retinopathy were excluded from the study. Treadmill exercise test was performed to exclude the patients suspicious for CAD. Blood samples for serum glycated hemoglobin (HbA1c%) and other laboratory markers were collected within a 30-day period before inclusion. An age- and gender-matched group of 30 healthy volunteers without any signs or symptoms of cardiac disease was used as control. The study complied with the Declaration of Helsinki. The Regional Research Ethics Committee, Clinical Centre, University of Pécs approved the study. All subjects had given written informed consent before inclusion in the study.
Echocardiography
Echocardiography was performed using Philips Epiq 7 ultrasound system (Philips Healthcare, Best, The Netherlands) by a single investigator. LV EF was measured by Simpson's method. End-diastolic thickness of the septum and the posterior wall and the end-diastolic diameter of the LV were measured from parasternal long-axis view by M-mode. LV mass was calculated according to the Devereux formula and corrected for body surface area (LVM index)21. Relative wall thickness was calculated as 2xposterior wall end-diastolic thickness/LV end-diastolic diameter. LV hypertrophy (LVM index > 115 g in males and > 95 g in females), elevated (≥ 0.43) relative wall thickness and enlarged LV chamber size (LV end-diastolic diameter/height [cm/m] > 3.4 in males and > 3.3 in females) were considered as signs of the hypertensive heart disease22.
In addition to the spectral Doppler parameters of the transmitral and transtricuspid flow (E, A) Fig. 1F), myocardial systolic (S), early- (e′) and late- (a′) diastolic velocities were measured from apical four-chamber view at the lateral and septal border of the mitral annulus (Fig. 1G), as well as on the lateral border of the tricuspid annulus using pulsed TDI. Lateral and septal mitral annular velocities were averaged. Mitral and tricuspid E/A and E/e′ ratios were calculated23. Basal dimension of the RV was obtained at end-diastole in RV-focused apical four-chamber view and corrected for body surface area. As parameters of the RV systolic function, tricuspid annular plane systolic excursion (TAPSE) and RV fractional area change (RVFAC) were measured. Maximal and minimal diameters of the inferior vena cava (IVC) were measured in the subxiphoid view, and collapsibility index (the percent decrease in the diameter of IVC with inspiration) was calculated. RV wall thickness was measured at end-diastole in a zoomed subxiphoid view. Systolic pulmonary artery pressure was estimated as a sum of the pressure difference across the tricuspid valve (calculated using the modified Bernoulli equation) and an estimate of mean right atrial (RA) pressure (5 to 15 mmHg) using the diameter and collapsibility index of the IVC21,24. Doppler measurements were obtained from 3 consecutive beats during end-expiratory apnea.
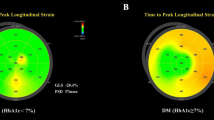
28-year-old male patient, with a 15-year history of T1DM. His HbA1c was 6.5% at inclusion. No signs of hypertensive heart disease. (A) Apical four-chamber view depicting the region of interest and the segmental longitudinal strain curves created by the speckle-tracking software. (B) Bull’s eye demonstrating the segmental peak systolic longitudinal strain values in the entire LV. Value of GLS is − 20.4%. (C) LV parasternal short-axis view at mid-papillary level depicting the region of interest and the segmental circumferential strain curves created by the software. Value of GCS is − 28.5%. (D) LA-focused apical four-chamber view depicting the region of interest and the LA strain curve created by the speckle-tracking software. LA reservoir strain: 38.9% (E) RA-focused apical four-chamber view depicting the region of interest and the RA strain curve created by the speckle-tracking software. RA reservoir strain: 50.7%. (F) Spectral Doppler curve of the mitral inflow. Mitral E: 81 cm/s, Mitral A: 62 cm/s. (G) Pulsed tissue Doppler curve measured on the septal border of the mitral annulus. Mitral e′: 11.3 cm/s.
LV diastolic function and filling pressure were classified according to the current recommendation23.
Speckle tracking echocardiography
For strain analysis, three consecutive heart cycles were recorded digitally, and processed off-line, using a dedicated software (QLab, Philips Healthcare, Andover, MA, USA). The analysis was performed by a single investigator, blinded to the clinical and conventional echocardiographic data. In segments with poor tracking, endocardial borders were manually readjusted until better tracking was achieved. To estimate LV GLS, apical four-, three- and two-chamber movies were obtained using 2D echocardiography. Care was taken to obtain true apical images using standard anatomic landmarks in each view. Foreshortening was avoided. The frame rate was set between 50 and 55 frames per second. Regional peak systolic longitudinal strain was determined in all 17 segments from the three apical views (Fig. 1A). The software automatically provided LV GLS as the average of the regional peak systolic longitudinal strain values (Fig. 1B).
Peak systolic circumferential strain was measured from the LV parasternal short-axis view at the mid-papillary level (Fig. 1C). The frame rate was set between 60 and 70 frames per second. Global circumferential strain (GCS) values were calculated as the average of the regional values in the six segments.
For atrial speckle tracking analysis, atrium-focused apical four- and two-chamber view movies (only four-chamber view for RA analysis) were obtained using 2D echocardiography (Fig. 1D,E). The frame rate was set between 80 and 90 frames per second. The onset of the R-wave was set as the reference (zero) point of the strain analysis. The first positive peak of the curve was measured at the end of the reservoir phase, just before the mitral valve opening (reservoir strain). This was followed by a plateau and a second late peak at the onset of the P wave on the electrocardiogram (contractile strain). The conduit strain was defined as the difference between the reservoir and the contractile strain. Results obtained in apical four- and two-chamber views were averaged for left atrial (LA) strain analysis25.
Using the atrial borders created for speckle tracking analysis, the same software generated left and right atrial volume curves. Atrial volumes were measured at different time points of the cardiac cycle: maximal volume (Vmax) at the end of T wave on electrocardiogram, just before the opening of the mitral or tricuspid valve; minimal volume (Vmin) at QRS complex, at the closure of the mitral or tricuspid valve; and preceding atrial contraction, at the beginning of P wave (Vp). LA volumes obtained in apical four- and two-chamber views were averaged. All volume values were corrected for body surface area (Vmax-, Vmin-, and Vp index)26.
Statistical analysis
Categorical data were expressed as n (%). The frequencies of categorical variables were compared using chi-square test or Fisher's exact test. The normality of distribution of continuous variables was tested by Shapiro–Wilk test. Continuous variables with normal distribution were presented as mean ± standard deviation; non-normal variables were reported as median (interquartile range). Means of two continuous normally distributed variables were compared by independent samples Student’s t test. Mann–Whitney U test was used to compare means of two variables not normally distributed.
Echocardiographic variables that correlate with current HbA1c or disease duration were determined using bivariate Pearson correlation. In a second step, multiple linear regression analysis (enter method) was used. Age-related changes in the echocardiographic parameters are well known, thus age was added to the multiple models. ACE inhibitors/ARBs are often used as treatment or prevention of diabetic nephropathy, regardless of the presence of systemic hypertension. Thus, medical therapy was not useful in identifying patients with hypertension. To eliminate the influence of the hypertension on the echocardiographic variables, signs of hypertensive heart disease were recoded (1 = sign(s) are present; 0 = sign(s) are not present) and used as additional variable in the multiple models. Partial regression plots were used to visualize the correlations between HbA1c and echocardiographic variables (which displays the residuals of each variable after adjusting for age and hypertension). Partial correlation coefficients were reported on the plots.
Patients were subgrouped according to the median HbA1c level and the presence/absence of the hypertensive heart disease. Four groups were created according to the number of cardiovascular risk factors: normal volunteers (NORM); patients below the median HbA1c and without hypertensive heart disease (T1DM-LOW); patients above the median HbA1c but without hypertensive heart disease (T1DM-MED); patients above the median HbA1c and with hypertensive heart disease (T1DM-HIGH). Patients with hypertensive heart disease but below the median HbA1c were excluded from sub-grouping. Comparisons of normally distributed data among multiple groups were performed using one-way ANOVA with LSD post hoc test. Kruskal–Wallis test with Dunn's multiple comparison test was used to compare means of multiple groups of variables not normally distributed.
A p-value of < 0.05 was considered significant. Data were analyzed using IBM SPSS 27 statistical software.
Results
Of a total of 75 patients, 70 (38.2 ± 11.7 years, 46 female) were eligible for the study. Four subjects were excluded due to inadequate acoustic windows. In one patient, ST changes observed during the exercise test suggested significant CAD, and this diagnosis was later confirmed by coronary angiography. Detailed clinical data of the T1DM patients are reported in Table 1.
Comparison with healthy subjects
Patients and controls were matched for age, gender, BSA and BMI. LV EF was significantly higher in T1DM patients compared with healthy controls. This difference, however, was clinically not remarkable. More negative GCS values suggested enhanced LV circumferential function in T1DM population. On the contrary, LV GLS values were similar in both groups. Averaged mitral annular S and e′ values were significantly lower, whereas LV E/e′ ratio was significantly higher in T1DM patients, but typically within the normal range. Mitral e′ values suggested impaired relaxation in 11 patients (15.7%). Six (8.6%) of these exhibited normal LV filling pressure, whereas in 5 (7.1%) patients indeterminate LV filling pressure was found.
RWT was significantly higher in T1DM patients. LV hypertrophy, abnormal RWT and elevated EDD/height were found in 6, 23 and 1 cases, respectively (altogether 27 patients).
In the right heart, tricuspid S was significantly reduced in the T1DM population. Real RV systolic dysfunction, however, was rare: RVFAC < 35%, TAPSE < 16 mm and tricuspid S < 10 cm/s were found in 1 (1.4%), 0 (0%) and 3 (4.3%) patients, respectively.
Regarding atrial size or function, no differences were found between the groups. Detailed echocardiographic data of the 70 patients compared to healthy controls are reported in Table 1.
Correlations between HbA1c or disease duration and the echocardiographic variables
Both HbA1c and disease duration showed significant correlations with various echocardiographic parameters (Table 2). Even when added age and hypertension to the model, current HbA1c level remained independent predictor of LV GLS, LV GCS, mitral and tricuspid e′ and LA and RA conduit strain in multiple linear regression models (Table 3) whereas disease duration lost its significance in similar multiple regression analyses. Partial regression plots demonstrate that HbA1c level correlates significantly with various echocardiographic variables even in age and hypertension adjusted analyses (Fig. 2).
Comparisons between subgroups
Median HbA1c level was 7.4 (1.8)% in our T1DM population. Twenty-four patients had HbA1c ≤ 7.4% and no signs of hypertensive heart disease (T1DM-LOW group). Nineteen and sixteen patients exhibited HbA1c > 7.4%, without (T1DM-MED group) or with (T1DM-HIGH group) signs of hypertensive heart disease, respectively. T1DM-HIGH-patients were significantly older than NORM and T1DM-LOW-patients and had significantly higher BMI compared with T1DM-MED and NORM groups. In T1DM-LOW-patients significantly lower LVM index was found compared with NORM. Otherwise, echocardiographic findings in T1DM-LOW-patients did not differ significantly from those in NORM. In T1DM-HIGH-patients LV GLS, mitral S, mitral E/A, mitral e′ and LA conduit strain were significantly reduced, whereas E/e′ values were significantly elevated compared with NORM, reflecting the impairment of LV longitudinal systolic and LV diastolic function. In contrast, LV EF, mitral A and (the absolute value of) GCS were significantly higher in T1DM-HIGH group compared with NORM, suggesting the compensatory behavior of these parameters. Regarding the right heart, tricuspid e′ and RA conduit strain values were significantly reduced, whereas tricuspid E/e′ was significantly elevated in the T1DM-HIGH subgroup. In general, signs of the myocardial involvement were pronounced in T1DM-MED and T1DM-HIGH subgroups compared with T1DM-LOW-patients. The differences between T1DM subgroups, however, were not always significant, especially not between T1DM-MED and T1DM-HIGH subgroups (Fig. 3). Comparison of echocardiographic variables among the study subgroups are reported in Table 4.
Discussion
Severe micro-, and macrovascular complications are common in patients with advanced T1DM. Subclinical myocardial involvement, however, may be present even in T1DM patients without manifest heart disease. Conventional echocardiographic techniques may fail, but TDI and speckle tracking echocardiography seem to be useful for recognizing subclinical myocardial involvement in this population. Disease duration and quality of the glycemic control are known as the most important factors responsible for the development of cardiovascular complications in T1DM1,2,3. Significant correlations have already been reported between disease duration, glycemic control, and the novel parameters of the myocardial mechanics5,8,15,18,20. Literature data, however, are contradictory regarding these questions. Therefore, we aimed to explore the size and mechanics of all cardiac chambers in asymptomatic T1DM patients. Potential associations between disease duration, glycemic control, and the echocardiographic parameters were also investigated. We tried to avoid the confounding effect of the age and hypertensive heart disease.
Similarly to the previous findings4, LV EF was preserved, LV size was normal in T1DM patients compared to the normal population. LVM index was enlarged only in patients with hypertensive heart disease. On the other hand, in T1DM patients with well controlled diabetes and without hypertension, LVM index was significantly lower than in healthy persons. This result is in line with the previous cardiac CT findings obtained in normoalbuminuric T1DM patients27. Similarly, Aepfelbacher et al. reported that improved glycemic control induces regression of LVM in patients with T1DM28. Better glycemic control may reduce LVM by improving intracellular calcium handling and reducing myocyte calcium overload in this population29. Another potential mechanism is the insulin-mediated reduction in elevated growth hormone levels parallel with the improved glycemic control, as elevated growth hormone levels are associated with LV hypertrophy in diabetes mellitus30.
Although Karamitsos et al. reported preserved LV longitudinal systolic function in T1DM patients15, average mitral annular S value was significantly reduced in in our patients. Similarly to their results, mitral annular S showed significant correlation with disease duration. After correcting with confounding factors, however, this significance was lost in our study.
LV GLS demonstrated subclinically impaired LV systolic function in multiple studies, not only in T1DM patients with known CAD31, but even in T1DM children and adults without the evidence of cardiovascular disease5,7,8,9,10,11,12. On the other hand, Jansen et al. reported preserved LV GLS values in T1DM patients without albuminuria (representing a relatively low cardiovascular risk profile/good glycemic control)16. Besides, in the studies of Hensel et al.17 and Weber et al.18, relatively good glycemic control was associated with preserved LV GLS. Similarly to the latter findings, mean LV GLS value was preserved in our T1DM population, compared with healthy controls. In patients with HbA1c above the median, however, significantly impaired LV GLS values were found. Bakhoum et al. found that HbA1c, but not the disease duration, was independent predictor of GLS5. Similar findings were reported by Labombarda et al.8. In our study, HbA1c levels showed significant relation with LV GLS values, even in age and hypertension adjusted multiple regression model. By reviewing the results of the previous studies this tendency is confirmed even at global level: in T1DM populations with relatively good glycemic control the mean LV GLS is preserved16,17,18, whereas worse glycemic control is associated with impaired GLS5,7,8,9,10,11,12 (Fig. 4).
Results of the previous studies suggest a linear relationship between HbA1c (%) and LV GLS (%) at global level. Good glycemic control is associated with preserved, whereas worse glycemic control is associated with impaired LV GLS. Confounding factors (e.g. age) and inter-vendor variability of the GLS were neglected. [Studies are referred by the name of the first author and by the reference number. Jensen-norm: patients without albuminuria (16), Jensen-micro: patients with microalbuminuria (16), Jensen-macro: patients with macroalbuminuria (16)].
Data regarding LV GCS are contradictory in the literature. In a pediatric T1DM population Rakha et al. reported reduced GCS values compared with controls12. In the study of Jędrzejewska et al., GCS was reduced in the basal segments, but preserved at mid-papillary and apical levels7. Preserved GCS values were reported in many T1DM populations, compared with healthy subjects5,8,17. In contrast to the previous results, elevated GCS values were revealed in our study, even at population level, but especially in patients with HbA1c above the mean. Our finding is not unique in the literature: According to the current theory, impaired LV longitudinal function is compensated by enhanced circumferential function, thus LV EF remains preserved. The shift from longitudinal to circumferential shortening was first recognized in hypertensive patients32, but it has also been reported in T1DM patients33. Significant associations between LV GCS and HbA1c or disease duration have never been reported before. In our study, however, HbA1c remained significant independent predictor of this parameter in multiple regression model.
Although both reduced mitral annular S and elevated LV GCS values reflect impaired LV longitudinal systolic function, LV GLS remained preserved in our T1DM population (with a borderline p value). These data may suggest that LV GLS is less sensitive to the mild changes in LV longitudinal systolic function than the other two parameters.
Literature data about LV diastolic function are inconsistent in T1DM patients. Di Cori et al. did not detect any diastolic functional abnormalities in young asymptomatic patients with TDM134. Prevalence of diastolic dysfunction was reported as 8% by Palmieri et al.4, whereas 27.4% of the patients had diastolic damage in the work of Raev et al.35. Discrepancies between these results may be explained not only by the variability of the populations but by the various echocardiographic methods applied in these studies. Regarding the modern, tissue Doppler-derived parameters, the result are similarly contradictory. Bakhum et al. reported, that mitral annular e′ values were preserved, but E/e′ values were higher in T1DM patients than those in healthy subjects5. In contrast, Weber et al. detected mildly reduced mitral e′ values. Mitral E/e′, however, was not elevated in their study18. Finally, in the studies of Jędrzejewska et al. and Gul et al., mitral annular e′ values were significantly lower, whereas LV E/e′ ratio was significantly higher in T1DM patients compared with healthy subjects, but typically within the normal range7,13. Our findings are in line with the latter studies: in our study-population, mitral annular e′ values were reduced, whereas mitral E/e′ values were elevated, but mainly within the normal range. LV diastolic dysfunction was found in 15.7% of our patients.
Neither HbA1c nor disease duration showed significant correlation with the parameters of LV diastolic function in the study of Bakhoum et al.5. In contrast, mitral annular e′ correlated significantly both with HbA1c values and disease duration, as reported by Weber et al.18. Although tissue Doppler parameters were not used in their study, Grandi et al. reported that diastolic dysfunction could be prevented and reversed by tight glycemic control in T1DM patients36. Our results are partially in agreement with the latter studies: both mitral annular e′ and E/e′ showed significant correlation both with HbA1c and disease duration in our population. In multiple regression model, however, only the association between HbA1c and mitral e′ remained significant.
Similarly to the left heart, Karamitsos et al. revealed preserved RV longitudinal systolic function in T1DM, whereas tricuspid e′ was reduced in their patients, suggesting impaired RV relaxation15. In contrast, in the study of Ahmed et al., a young T1DM group showed statistically significant decrease in tricuspid S and in RV longitudinal strain. Tricuspid E/e’ was significantly elevated, but tricuspid e′ was preserved in their study19. Although TDI-derived parameters of their RV function were preserved, Jȩdrzejewska et al. also reported significantly impaired RV longitudinal strain in T1DM patients7. At population level, tricuspid S was significantly reduced in our study, but no significant reduction of tricuspid e′ was proved. On the other hand, in the subgroup of T1DM patients with less well controlled diabetes and hypertensive heart disease, tricuspid e′ was significantly reduced, whereas tricuspid E/e′ was significantly elevated, suggesting RV diastolic dysfunction. Correlations between RV function and HbA1c or disease duration have not been reported in the literature. In our population tricuspid e′ showed significant association with HbA1c level, even in multiple regression model.
Nemes et al. reported enlarged LA volumes in T1DM patients37. This finding, however, was not confirmed in our study. Acar et al. investigated LA function based on volumetric measurements, in T1DM children and adolescents. LA passive emptying fraction, reflecting LA conduit function, was decreased, whereas LA active emptying fraction, reflecting contractile function, was increased in their patients compared with healthy controls14. Similarly, Ifuku et al. reported reduced LA reservoir and conduit strain in adolescents and young adults with T1DM20. Regarding RA strain, data are available only in T2DM patients: Tadic et al. reported reduced reservoir and conduit but enhanced contractile strain both in LA and RA38. In contrast, at population level, both LA and RA phasic functions were found to be preserved in our T1DM patients compared with healthy controls. Nevertheless, in the subgroup of patients with less well controlled diabetes and hypertensive heart disease, both LA and RA conduit strain were significantly reduced compared with healthy subjects, whereas compensatory increase of LA and RA contractile strain were found when compared with those having well controlled diabetes. This compensatory behavior of LA contractile function—parallel with the decline of LV diastolic function—has already been reported in other conditions39,40. LA conduit strain showed significant correlation with disease duration, as reported by Ifuku et al.20. Besides, in T2DM, LA conduit and reservoir strain, as well as RA conduit and contractile strain were independently associated with HbA1c levels38. In our T1DM population, even in multiple regression model, HbA1c levels showed significant associations with LA and RA conduit strain. Significant correlations were also proved between LA reservoir and conduit strain and disease duration. In multiple regression models, however, the latter associations were lost.
Regarding myocardial mechanics, our results are only partially in agreement with the previous findings. Nevertheless, due to the lack of uniformity in patient-selection (regarding age, disease duration, glycemic control, comorbidities) and in the use of the echocardiographic techniques, the results of the studies are not easy to compare. Based on their mean HbA1c level, our asymptomatic T1DM population exhibited a relatively well controlled diabetes compared with the populations reported in the previous studies. This may, at least partially, explain the differences.
As new finding, our comprehensive analysis suggests that in asymptomatic T1DM patients, glycemic control has significant impact on the mechanics of all cardiac chambers, even after careful correction for the main confounding factors. This early myocardial damage forms the basis of the future heart failure in patients with poor glycemic control. Our results are in line with the large observational study performed in a cohort of 20,985 T1DM patients by Lind et al. A strong positive association was found between HbA1c and the risk of heart failure in their study3.
Numerous limitations of our study need to be acknowledged. First, our study population was limited in size due to the challenging selection of patients without cardiovascular comorbidities. Neither daily physical activity, nor statin or fibrate use of the patients are reported. Treadmill exercise test was performed to exclude patients suspicious for CAD. Due to ethical reasons, use of coronary angiography was avoided in asymptomatic subjects.
Patients with well-controlled hypertension were not excluded from the study, but we intended to eliminate the confounding effect of hypertension in our analysis.
For obtaining atrial strain values, we used a software that was developed for LV strain analysis because dedicated software was not available. RV strain may better reflect the subclinical impairment of the RV systolic function than our traditional and tissue Doppler parameters. Nevertheless, in the lack of appropriate analytical software, RV strain analysis was not performed in our study.
Alpha level was not adjusted when multiple comparisons were made. This may increase the chance of type 1 error (false positives).
Conclusion
Our data suggest that quality of the glycemic control has a significant impact on the subclinical myocardial involvement in T1DM patients. Regarding disease duration, we could not prove this relationship. Thus, tight glycemic control must be a high-priority therapeutic aim for diabetic patients to minimize the risk of myocardial damage and consequential heart failure.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Laing, S. P. et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46, 760–765. https://doi.org/10.1007/s00125-003-1116-6 (2003).
Dorman, J. S. et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes 33, 271–276. https://doi.org/10.2337/diab.33.3.271 (1984).
Lind, M. et al. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: An observational study. Lancet 378, 140–146. https://doi.org/10.1016/s0140-6736(11)60471-6 (2011).
Palmieri, V. et al. Uncomplicated type 1 diabetes and preclinical left ventricular myocardial dysfunction: Insights from echocardiography and exercise cardiac performance evaluation. Diabetes Res. Clin. Pract. 79, 262–268. https://doi.org/10.1016/j.diabres.2007.09.014 (2008).
Bakhoum, S. W. G., Habeeb, H. A., Elebrashy, I. N. & Rizk, M. N. Assessment of left ventricular function in young type 1 diabetes mellitus patients by two-dimensional speckle tracking echocardiography: Relation to duration and control of diabetes. Egypt. Heart J. 68, 217–225. https://doi.org/10.1016/j.ehj.2016.02.006 (2016).
Bogdanović, J. et al. Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: An analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc. Diabetol. 18, 68. https://doi.org/10.1186/s12933-019-0876-3 (2019).
Jędrzejewska, I. et al. Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 17, 438–446. https://doi.org/10.1093/ehjci/jev164 (2016).
Labombarda, F. et al. Longitudinal left ventricular strain impairment in type 1 diabetes children and adolescents: A 2D speckle strain imaging study. Diabetes Metab. 40, 292–298. https://doi.org/10.1016/j.diabet.2014.03.007 (2014).
Hodzic, A. et al. Decreased regional left ventricular myocardial strain in type 1 diabetic children: A first sign of diabetic cardiomyopathy?. J. Transl. Int. Med. 4, 81–87. https://doi.org/10.1515/jtim-2016-0025 (2016).
Sveen, K. A. et al. Impaired left ventricular function and myocardial blood flow reserve in patients with long-term type 1 diabetes and no significant coronary artery disease: Associations with protein glycation. Diabetes Vasc. Dis. Res. 11, 84–91. https://doi.org/10.1177/1479164113518805 (2014).
Nakai, H., Takeuchi, M., Nishikage, T., Lang, R. M. & Otsuji, Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: Correlation with diabetic duration. Eur. J. Echocardiogr. 10, 926–932. https://doi.org/10.1093/ejechocard/jep097 (2009).
Rakha, S. & Aboelenin, H. M. Left ventricular functions in pediatric patients with ten years or more type 1 diabetes mellitus: Conventional echocardiography, tissue Doppler, and two-dimensional speckle tracking study. Pediatr. Diabetes 20, 946–954. https://doi.org/10.1111/pedi.12900 (2019).
Gul, K. et al. Tissue Doppler imaging must be performed to detect early left ventricular dysfunction in patients with type 1 diabetes mellitus. Eur. J. Echocardiogr. 10, 841–846. https://doi.org/10.1093/ejechocard/jep086 (2009).
Acar, G. et al. Assessment of atrial electromechanical delay, diastolic functions, and left atrial mechanical functions in patients with type 1 diabetes mellitus. J. Am. Soc. Echocardiogr. 22, 732–738. https://doi.org/10.1016/j.echo.2009.03.028 (2009).
Karamitsos, T. D. et al. Early diastolic impairment of diabetic heart: The significance of right ventricle. Int. J. Cardiol. 114, 218–223. https://doi.org/10.1016/j.ijcard.2006.02.003 (2007).
Jensen, M. T. et al. Global longitudinal strain is not impaired in type 1 diabetes patients without albuminuria: The thousand & 1 study. JACC Cardiovasc. Imaging 8, 400–410. https://doi.org/10.1016/j.jcmg.2014.12.020 (2015).
Hensel, K. O. et al. Subclinical alterations of cardiac mechanics present early in the course of pediatric type 1 diabetes mellitus: A prospective blinded speckle tracking stress echocardiography study. J. Diabetes Res. 2016, 2583747. https://doi.org/10.1155/2016/2583747 (2016).
Weber, T. R. et al. Echocardiographic evaluation in type 1 diabetes mellitus. Rev. Port. Cardiol. 40, 757–765. https://doi.org/10.1016/j.repce.2021.08.003 (2021).
Ahmed, T. A., Ahmed, Y. A., Arafa, A. I. & Salah, R. A. Detection of occult right ventricular dysfunction in young Egyptians with type 1 diabetes mellitus by two-dimensional speckle tracking echocardiography. Indian Heart J. 70, 665–671. https://doi.org/10.1016/j.ihj.2018.06.019 (2018).
Ifuku, M. et al. Left atrial dysfunction and stiffness in pediatric and adult patients with Type 1 diabetes mellitus assessed with speckle tracking echocardiography. Pediatr. Diabetes 22, 303–319. https://doi.org/10.1111/pedi.13141 (2021).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16, 233–270. https://doi.org/10.1093/ehjci/jev014 (2015).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 29, 277–314. https://doi.org/10.1016/j.echo.2016.01.011 (2016).
Rudski, L. G. et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 23, 685–713. https://doi.org/10.1016/j.echo.2010.05.010 (2010).
Badano, L. P. et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 19, 591–600. https://doi.org/10.1093/ehjci/jey042 (2018).
To, A. C., Flamm, S. D., Marwick, T. H. & Klein, A. L. Clinical utility of multimodality LA imaging: Assessment of size, function, and structure. JACC Cardiovasc. Imaging 4, 788–798. https://doi.org/10.1016/j.jcmg.2011.02.018 (2011).
Hjortkjær, H. et al. Cardiac ventricular sizes are reduced in patients with long-term, normoalbuminuric type 1 diabetes compared to the non-diabetic background population. Diabetes Vasc. Dis. Res. 16, 289–296. https://doi.org/10.1177/1479164118819961 (2019).
Aepfelbacher, F. C., Yeon, S. B., Weinrauch, L. A., D’Elia, J. & Burger, A. J. Improved glycemic control induces regression of left ventricular mass in patients with type 1 diabetes mellitus. Int. J. Cardiol. 94, 47–51. https://doi.org/10.1016/j.ijcard.2003.04.012 (2004).
Barbagallo, M., Gupta, R. K. & Resnick, L. M. Cellular ions in NIDDM: Relation of calcium to hyperglycemia and cardiac mass. Diabetes Care 19, 1393–1398. https://doi.org/10.2337/diacare.19.12.1393 (1996).
Bereket, A., Lang, C. H. & Wilson, T. A. Alterations in the growth hormone-insulin-like growth factor axis in insulin dependent diabetes mellitus. Horm. Metab. Res. 31, 172–181. https://doi.org/10.1055/s-2007-978716 (1999).
Van Berendoncks, A. M. et al. Abnormal longitudinal peak systolic strain in asymptomatic patients with type I diabetes mellitus. Echocardiography 36, 478–485. https://doi.org/10.1111/echo.14257 (2019).
Kraigher-Krainer, E. et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 63, 447–456. https://doi.org/10.1016/j.jacc.2013.09.052 (2014).
Bradley, T. J. et al. Early changes in cardiovascular structure and function in adolescents with type 1 diabetes. Cardiovasc. Diabetol. 15, 31. https://doi.org/10.1186/s12933-016-0351-3 (2016).
Di Cori, A. et al. Left ventricular function in normotensive young adults with well-controlled type 1 diabetes mellitus. Am. J. Cardiol. 99, 84–90. https://doi.org/10.1016/j.amjcard.2006.07.063 (2007).
Raev, D. C. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care 17, 633–639. https://doi.org/10.2337/diacare.17.7.633 (1994).
Grandi, A. M. et al. Effect of glycemic control on left ventricular diastolic function in type 1 diabetes mellitus. Am. J. Cardiol. 97, 71–76. https://doi.org/10.1016/j.amjcard.2005.07.110 (2006).
Nemes, A. et al. Complex evaluation of left atrial dysfunction in patients with type 1 diabetes mellitus by three-dimensional speckle tracking echocardiography: Results from the MAGYAR-Path Study. Anatol. J. Cardiol. 16, 587–593. https://doi.org/10.5152/AnatolJCardiol.2015.6225 (2016).
Tadic, M. et al. Left and right atrial phasic function and deformation in untreated patients with prediabetes and type 2 diabetes mellitus. Int. J. Cardiovasc. Imaging 31, 65–76. https://doi.org/10.1007/s10554-014-0536-3 (2015).
Porpáczy, A. et al. Impairment of left atrial mechanics is an early sign of myocardial involvement in systemic sclerosis. J. Card. Fail. 24, 234–242. https://doi.org/10.1016/j.cardfail.2018.02.012 (2018).
Brecht, A. et al. Left atrial function in preclinical diastolic dysfunction: Two-dimensional speckle-tracking echocardiography-derived results from the BEFRI trial. J. Am. Soc. Echocardiogr. 29, 750–758. https://doi.org/10.1016/j.echo.2016.03.013 (2016).
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
R.F. and N.V.V. designed the study. M.H., V.V. and G.M. carried out subject recruitment. R.F. performed echocardiography. M.H. performed the offline analysis of the echocardiographic images. M.H. and R.F. analyzed and interpreted the data and drafted the manuscript. M.O.K. helped in literature analysis, contributed to discussion of the results. G.M. and I.W. reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajdu, M., Knutsen, M.O., Vértes, V. et al. Quality of glycemic control has significant impact on myocardial mechanics in type 1 diabetes mellitus. Sci Rep 12, 20180 (2022). https://doi.org/10.1038/s41598-022-24619-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24619-2
- Springer Nature Limited