Abstract
Many studies on ionizing radiation (IR) exposure during childhood have shown deleterious effects on the central nervous system (CNS), however results regarding adult exposure are inconsistent, and no systematic reviews have been performed. The objectives are to synthesize the findings and draw evidence-based conclusions from epidemiological studies on the risk of benign and malignant brain and CNS tumors in humans exposed to low-to-moderate doses (< 0.5 Gy) of IR during adulthood/young adulthood. A systematic literature search of four electronic databases, supplemented by a hand search, was performed to retrieve relevant epidemiological studies published from 2000 to 2022. Pooled excess relative risk (ERRpooled) was estimated using a random effect model. Eighteen publications were included in the systematic review and twelve out of them were included in a meta-analysis. The following IR sources were considered: atomic bombs, occupational, and environmental exposures. No significant dose-risk association was found for brain/CNS tumors (ERRpooled at 100 mGy = − 0.01; 95% CI: − 0.05, 0.04). Our systematic review and meta-analysis did not show any association between exposure to low-to-moderate doses of IR and risk of CNS tumors. Further studies with histological information and precise dose assessment are needed.
Similar content being viewed by others
Introduction
Exposure to ionizing radiation (IR) is ubiquitous and can be anthropogenic (medical procedures, releases from nuclear facilities, nuclear accidents, or nuclear weapons tests) or natural (radon, telluric and cosmic rays, radionuclides in soils). The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) has estimated the global average annual effective dose of about 3.0 millisievert (mSv) per person, including 2.4 mSv from natural sources and 0.6 mSv from artificial sources1. While most of the population is exposed to relatively low levels of background IR, some people may be additionally exposed to IR due to their lifestyle (e.g., frequent flyers), occupation, health condition (diagnostic or therapeutic medical exposure), or environment (e.g., high background radiation areas). Many epidemiological studies have demonstrated that exposure to IR can increase the risk of cancer for some specific sites2, including central nervous system (CNS)1.
Exposure to moderate-to-high-dose of IR (> 0.5 Gy) during childhood is an established risk factor for CNS cancers (all brain/CNS tumors, gliomas and meningiomas)2,3, as reported in studies of survivors of pediatric primary tumors4 and in children irradiated for benign medical conditions5,6. At lower doses (< 0.1 Gy), such as those received during computerized tomography (CT) scans, some studies reported a dose–response relationship between exposure to IR and CNS tumors7,8,9. A summary of these studies completed by a meta-analysis concluded to a positive dose–response relationship10. Regarding exposure to medical diagnostic X-ray in utero, the relationship with brain cancer incidence was significant in the Oxford Survey, and only marginally non-significant for the combined other studies11. However, caution should be exercised when evaluating diagnostic medical exposures as study results have been shown to be subject to significant uncertainties and biases (e.g., poorly documented historical exposure data, limited non-target organ dosimetry, bias by indication, etc.)12.
The extrapolation of results from pediatric patients to adults should be considered with caution since for a given radiation dose, children are generally at greater risk of tumor than adults, because of their higher radiosensitivity13. Moreover, the consequences of IR low-dose exposures regarding adulthood on CNS tumors risk are less clearly demonstrated than in children. The Life Span Study of atomic bomb survivors exposed to moderate radiation during adulthood found no significant association with brain cancer occurrence14, whereas a significant dose-dependent excess of schwannoma for those exposed from age 40 onwards had been previously observed15. Because of the increase of the use of IR in the workplace since the 1950s, workers (e.g., nuclear and medical workers, aircrew) are prone to be exposed to low-to-moderate dose range (< 0.5 Gy) of IR but received at low-dose rate. Accordingly, the effect of adult exposure to IR on the risk of CNS tumors have been studied in several epidemiological studies in the recent years. However, results of those studies are inconsistent, with some reporting no association between IR exposure and malignant or benign CNS tumors risk16,17,18. However, some cases reports raised concerns about potential higher incidences of brain tumor in healthcare professionals19,20.
Thus, the objectives of the present systematic review are to (1) identify pertinent studies, synthesize their results, and draw evidence-based conclusions from epidemiological studies carried out on the risk of malignant and benign brain and CNS tumors (including malignant neoplasm of spinal cord, cranial nerves and other parts of the nervous system) in people exposed to low-to-moderate doses of IR (< 0.5 Gy) during adolescents above 16 years old and during adulthood, and (2) to provide a quantitative summary of the overall risk estimate.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to guide our literature review and synthesis (supplementary material, Table S1)21. The protocol was recorded in the PROSPERO database (Registration Number: CR42021215479).
Data source and search
This work is part of a larger research on tumoral and non-tumoral22 effects in the brain/CNS system following exposure to IR in young adulthood and adulthood. This study considers only the effects relevant to tumor development.
An online-based literature search was conducted in May 2022 in PubMed, Scopus, Web of Science, and Google Scholar databases. The first query included a combination of outcome, exposure, and population keywords: (neuro* OR nervous OR brain OR brain cancer OR cerebro*) AND (ionizing radiation OR medical radiation OR cosmic radiation OR nuclear OR radon OR background radiation) AND (patient* OR human OR worker OR cohort OR epidemiolog*). To complete this query, additional queries were performed to catch studies with no keywords in the title nor in the abstract: cosmic radiation AND mortality OR incidence; (nuclear worker OR nuclear facility OR nuclear industry) AND mortality OR incidence; ionizing radiation AND mortality OR incidence. Also, additional articles were searched from the references cited by relevant publications and international reports1,2. Duplicates from the different databases were removed.
For the selection process, we proceeded as follows: (1) the articles obtained through the queries were selected on the title; (2) the abstracts of the selected articles were read, and a further selection was performed; (3) the articles were selected on the full-text screening. The selection was carried out by two independent investigators (J.L. and C.B.), whereas a third investigator (M.-O.B.) made a decision in case of disagreement.
Inclusion and exclusion criteria
Eligible studies were cohort, case–control, and cross-sectional studies, published in English between January 2000 and May 2022. The publication period criterion allows for the inclusion of studies whose radiation exposure is more similar to the current IR exposures, especially in medical professionals, given improved radiation protection regulations and decreasing doses23. Furthermore, older good quality studies are regularly updated and would be found as their last updated publication. Last, it allows the exclusion of some ancient studies with poor quality design and ensure uniformity of studies in their structure (introduction, methods, results, discussion, conclusions) and the way they were reviewed (homogeneous judgment criteria), thus facilitating the comparison of studies between them. All the studies concerned exposures to low-to-moderate doses of IR (< 0.5 Gy), during adulthood or adolescence (at least 16 years old) as companies involved in some of the studies included in this work allowed for work at age 16 or older. To be eligible, a study had to report on incidence or on mortality from CNS tumors coded according to the International Classification of Diseases (ICD), either its revision 9 (ICD-9: 191-192)24 or 10 (ICD-10: C70-C72, D32-D33, D42-D43)25. Because most studies of ICD-9 codes 191-192 have reported results for both codes together, non-cranial tumors (i.e., code 192) were also included in the present work. The definitions of the ICD codes are provided in supplementary data (Table S2).
Exclusion criteria were the followings: studies with less than 10 cases; conference abstracts, reports, meta-analyses, letters, and ecological studies; all the studies with no dose assessment, or in which exposure assessment was only based on self-reports or questions about IR exposure (e.g., “How many dental X-rays have you been exposed to in your lifetime?”). However, the references of these excluded studies were checked to retrieve potential studies that met the inclusion criteria of the present review. In case of publications on overlapping populations or study updates, only data from the most complete study were considered. Studies with quantitative estimates were included in the meta-analysis section. Meta-analysis was performed when at least three studies were eligible.
Quality assessment of individual studies
The quality of the included studies was assessed using the Newcastle Ottawa Scale (NOS) for quality assessment of epidemiological studies26, which is usually used in systematic review process. This evaluation is based on eight items, which are categorized into three groups: selection of study groups, comparability of groups, and outcome of interest, for cohort studies. Stars are attributed for each item depending on the quality and a score (0 to 9) is obtained by adding the stars of each item. A study with an average NOS score of at least 6 stars out of 9 is considered as good quality.
Statistical analysis
Details on sample size, number of exposed/unexposed people, total cases or deaths, information about gender, age at exposure, and estimates of measures of risk such as excess relative risk (ERR), relative risk (RR), hazard ratio (HR), standardized rate ratio (SRR), proportional incidence ratio (PIR), or incidence rate ratio (IRR) were collected for each study when available. A pooled ERR was estimated to assess the strength of the association when available from the individual studies, using an alternative of the DerSimonian and Lair-based model proposed by Richardson et al.27. This method uses a parametric transformation of published results to improve the normal approximation used to estimate confidence intervals. This approach provides less biased summary estimates than the traditional meta-analysis approach, with more precise confidence interval coverage.
Heterogeneity across studies was tested using Cochran’s Q test at p < 0.1 and quantified using the I2 statistics. The latter reflects the proportion of total variance estimated to be attributable to between-study heterogeneity. Heterogeneity was considered as null, low, moderate, and high for I2 values < 25, 25–50, 50–75, and > 75%, respectively. Publication and selection biases were assessed and tested using the Egger test. Statistical significance was defined by p < 0.05.
Meta-analyses were based on the original values reported by the studies. Whereas a study reported only a 90% (as opposed to 95%) confidence interval18, this was introduced in the main meta-analyses, and a sensitivity analysis was performed with a 95% confidence interval calculated by ourselves.
Statistical analyses were conducted with the R 3.6.3 software (R Foundation for Statistical Computing, Vienna, Austria) using the metafor and Metaan packages.
Results
From 11,485 articles retrieved, 2,063 were excluded as being duplicates and 9,422 articles were screened on title; next, the abstract of 528 of them were reviewed. Finally, 201 articles were full read, of which 17 were selected. Briefly, full texts were excluded because of overlaps (62 studies), outcome not in the scope of the review (48 studies), unsuitable exposure (46 studies), study design not meeting inclusion criteria (20 studies), or for other reasons (8 studies, for combination of several exclusion criteria).
One more article was obtained through search in bibliographic references as described above, for a total of 18 articles included in this systematic review. Of those, 12 presented quantitative results that were included in the meta-analyses (Fig. 1). The characteristics, key findings, and NOS score assessments of the 18 articles included in the present review are detailed in Table 1. Each of them was based on cohorts of IR exposed people, and the majority was published after the year 2010. Most studies investigated CNS tumors risk in relation to occupational exposures (16 studies), while others addressed environmental background exposure or atomic bomb exposure (2 studies).
Occupational worker studies
Nuclear workers and uranium miners
Five articles were focused on nuclear workers18,28,29,30,31. In a cohort of 26,328 Los Alamos National Laboratory workers exposed to a combination of photons, neutrons, tritium and plutonium (among which 17,053 workers were monitored for a combination of external and internal source for plutonium and had dose information available; cumulative brain radiation absorbed dose: mean: 11.6 mGy; max: 760 mGy), Boice et al. (2021) reported a non-significant positive dose–response relationship among the whole cohort (ERR at 100 mGy: 0.20; 95% CI: − 0.27, 0.67; ndeaths = 94)28. Similarly, Richardson et al. 2018 reported a non-significant negative dose–response relationship between IR (median cumulative brain dose of 20.2 mSv) and brain/CNS cancers in the international pooled study of radiation workers from the UK, the USA and France including 308,297 workers (ERR = − 0.92; 90% CI: < − 0.92, 1.14; ndeaths = 594) (INWORKS cohort)18. Likewise, Sokolnikov et al. observed a non-significant negative dose–response relationship between occupational IR exposure (mean external brain dose: not available) and brain cancer among the cohort of Mayak Production Association workers in Russia first employed in 1948–1982 and followed up until 2008 (ERR per Gy = < 0.00; 95% CI: < − 0.10, 0.32; ndeaths = 66)30. Zablotska et al. also found a non-significant negative dose–response relationship between IR exposure (cumulative person-time weighted lung dose: 21.64 mSv, max: 678.78 mSv) and brain/CNS cancers among a cohort of 45,316 Canadian nuclear workers (ERR per Sv = − 1.45; 95% CI: < − 1.47, 5.83; ndeaths = 22)31. Furthermore, non-significant negative dose–response relationship was reported among 53,698 US nuclear power plant industry workers between IR exposure (mean cumulative dose: 25.7 mSv) and brain/CNS cancers (ERR per Sv = − 2.50; 95% CI: < − 2.51, 27.1; ndeaths = 23)29.
Similar results were found in uranium miners and processing worker studies: non-significant negative dose–response relationship was found among 2,514 Mallinckrodt uranium processing workers exposed to a combination of X-ray, uranium and radium (mean brain dose from all sources of external and internal radiation combined: 37.2 mGy; max: 750 mGy) and brain/CNS cancers (ERR at 100 mGy = − 0.13; 95% CI: − 0.55, 0.29; ndeaths = 22)32; a non-significant negative dose–response relationship was reported between radon exposure (cumulative radon exposure: 35.1 WLM) and brain/CNS cancers among 5,400 French uranium miners followed up from 1946 to 2007 (ERR per 100 WLM = − 0.12; 95% CI: NA, NA; ndeaths = 28)33.
Flight attendants
Yong et al. reported a significant relationship between cumulative dose of galactic cosmic radiation (mean: 28 mSv, max: 71 mSv) and brain/CNS cancers mortality among 5,964 former US commercial cockpit crew (pilots and engineers) (HR per 10 mSv = 2.37; 95% CI: 1.01, 6.12; ndeaths = 32 under the hypothesis of a 10-year lag in cumulative dose)34. Whereas, Dreger et al. found no evidence of a dose–response relationship between cumulative effective dose (overall median: 34.2 mSv, max: 116 mSv) and brain/CNS cancers risk among a cohort of 26,846 aircrew personnel35.
Medical workers
No statistically significant association was reported between cumulative radiation exposure (mean badge dose: 7.2 mSv, max: >20 mSv) and brain/CNS cancers among diagnostic medical radiation workers in South Korea (ERR per 100 mGy = − 0.29; 95% CI: − 3.14, 2.55; ndeaths = 43)36. Similarly, no significant association was observed between cumulative occupational radiation exposure to the brain (cumulative mean absorbed brain dose: 12 mGy, max: 290 mGy) and mortality from malignant brain/CNS cancers in the US Radiologic Technologists (ERR per 100 mGy: 0.1; 95% CI: < − 0.30, 1.50; ndeaths = 193)17. Finally, the recent study of medical radiation workers in the United States found that cumulative absorbed dose to the brain (mean: 18.9 mGy, max: 1.08 Gy) was not significantly associated with brain cancers (ERR at 100 mGy = 0.20; 95% CI: − 0.30, 0.71; ndeaths = 165)37.
Military using nuclear materials
Friedman-Jimenez et al. found a non-significant dose–response relationship between IR exposure (mean cumulative IR badge dose: 5.7 mSv, max: 242 mSv) and brain/CNS cancers (ERR per 10 mSv = 0.025; 95% CI: − 0.33, 0.39; ndeaths = 43) among 85,033 enlisted men who had served on nuclear-powered submarines in the United States Navy between 1969 and 198238. Boice et al. found a significant negative association between radiation dose (mean NuTRUS film badge gamma radiation dose: 6 mSv, max: 908 mSv) and brain/CNS cancers (ERR at 100 mGy = − 1.40; 95% CI: − 2.50, − 0.32; ndeaths = 495) among US military participants to atmospheric tests in Nevada and Pacific from 1945 to 196239. Gillies et al. found similar mortality and incidence relative risks in 21,357 males UK participants followed between 1952 and 2017 in the UK’s atmospheric nuclear weapons tests and experimental programs, of whom 8% had non-zero recorded radiation dose from gamma radiation (mean: 9.9 mSv), compared to a group of 22,312 males controls (RR = 0.99; 95% CI: 0.80, 1.22 and RR = 1.05; 95% CI: 0.87, 1.27 respectively)40.
Chernobyl cleanup workers
A significantly increased incidence of malignant brain tumors was observed in clean-up workers from the Baltic countries who worked in the Chernobyl area in 1986 and stayed onsite for more than 90 days, in comparison with the males of the general population of each country (PIR = 2.08; 95% CI: 1.07, 3.63; ndeaths = 12), but there was no trend when PIR were given by dose category41. However, the accuracy of the diagnosis and the representativeness of the unexposed cohort are an issue in this study.
Atomic bombing and environmental studies
Atomic bombing survivors
While a significant linear dose–response relationship was found for all CNS tumors (glioma, meningioma, schwannoma, and other/not otherwise specified tumors) in the entire population of the LSS study of atomic bomb survivors from Hiroshima and Nagasaki, the ERR at 1 Gy was no longer statistically significant when age at exposure was restricted to the age of 20 and more (ERR per Gy = 1.10; 95% CI: − 0.02, 2.97; ndeaths and diseases = 92 in the group > 25 years old at exposure)14.
Environmental radiation
A statistically significant association between residential radon and brain tumors (including benign) incidence (IRR = 1.96; 95% CI: 1.07, 3.58; ndiseases = 121), accounting for a 10-year latency period, was shown in a Danish cohort comprising 57,053 individuals recruited between 1993 and 1997 exposed to a median estimated radon dose of 40.5 Bq/m342.
Meta-analyses
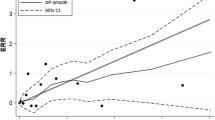
A pooled ERR at 100 mGy was calculated using 12 studies14,17,18,28,29,30,31,32,36,37,38,39, showing no dose-risk association (ERRpooled = − 0.01; 95% CI: − 0.05, 0.04) and no heterogeneity (Q = 11.62, p = 0.39 and I2 = 5.35%), nor publication bias (p = 0.56). (Fig. 2).
Supplementary analyses were carried out for mortality and incidence rates in cohorts compared to those in the general populations (supplementary material, Table S3).
Discussion
Brain/CNS tumors risks after adult and adolescent older than 16 years exposure to low-to-moderate doses of IR was analyzed based on 18 studies. Our results suggest no dose–response relationship between IR exposure and brain/CNS tumors death using ERR.
Our results contrast with the previously increased risk of CNS tumors after childhood exposure to low-to-high doses of radiation, as reported in the UNSCEAR 2006 Report2, and more specifically in studies of survivors of pediatric primary tumors4 and in children irradiated for benign medical conditions5,6. Since children have a higher radiosensitivity, this difference in results does not appear surprising because of the focus on adulthood exposure in our study. The rate of cell proliferation during neurogenesis in the adult brain is much lower compared to that in the developing brain of children and to other adult tissues, such as bone marrow, intestine and lung43. Consequently, brain cancer in adults accounts only for a small fraction of all cancers (~ 1%), whereas in children, brain tumors are the most common solid tumors44. It is possible that the association between IR exposure and CNS tumors may mostly be driven by the pediatric subjects. Overall, it seems that although our results reveal a lack of correlation between exposure to low-to-moderate doses of IR in adults and the risk of CNS tumors incidence and mortality (corroborated by comparisons of mortality and incidence rates with those of the general population, Figs. S1 and S2), there are still many factors that need to be considered in future studies.
Among them, several environmental risk factors are currently studied and debated: non-ionizing radiation (e.g., radiofrequency, electromagnetic fields)45, pesticides, heavy metals (e.g., lead, mercury), nitro compounds, certain viral infections (e.g., SV40) and cigarette smoking46,47,48. Other factors that affect the incidence of brain cancer include previous history of allergy (reducing the risk)49, regular use of certain medication50, diet and lifestyle51,52 and others. Accounting for these factors in IR-exposed populations would help delineate the link between IR and the risk of brain tumors. Furthermore, exposure to IR in the occupational setting is often accompanied by co-exposure to other health risk factors (e.g., chemical substances, pesticides, heavy metals, nitro compounds, non-ionizing radiations, air pollution, tobacco use, etc.) and may confound and/or modify the relationship between IR exposure and a health outcome. While research on health effects of co-exposures to two or more risk factors (exposome) is a very dynamic area of research and there are examples of synergies or antagonisms following co-exposure to different environmental agents53,54, overall, the interaction of various factors and associated health outcomes are poorly characterized to date. It is also estimated that 5% of brain tumors are related to hereditary factors55, with even smaller percentage in adult brain cancers patients.
The most informative way to assess causality is to estimate dose-risk relationships. Accordingly, studies which presented only SMRs or SIRs were included only in supplementary material for information. Of the 18 studies included in this work, 12 reported dose–response relationships and were included in the meta-analysis. The pooled ERR was non-significant, leading to the conclusion that there was no association between adult IR exposure and brain/CNS tumors risk. However, uncertainties in dosimetry assessment can obscure the relationship. We can note a lack of accurate dosimetry reconstruction for several studies, as those focusing on medical workers, and encourage future research to improve dose assessment in studies, including as much as possible homogenization of the dosimetric units used. Indeed, nine out of the twelve studies with dose–response analyses considered absorbed doses to the brain (in Gy)14,17,18,28,30,32,36,37,39, whereas the three other studies29,31,38 used equivalent whole-body doses (in Sv) from external photons based on individual monitoring records . However, we calculated a pooled ERR/Gy using the numerical values of the estimated ERR/Sv as they are reported in the studies, assuming that the brain dose from external photons correlates with the whole-body equivalent dose, yet being aware that the absorbed dose to the brain is certainly lower than the equivalent whole-body dose18,56.
Among the studies included in the meta-analysis, conditions of exposure to IR were heterogeneous: uranium miners are repeatedly exposed internally and externally to a mix of radon gas and its progenies, external gamma rays, and uranium dust57; nuclear workers are predominantly exposed to external gamma rays, possibly combined with tritium, uranium, plutonium, or neutrons depending on their activity30,56; medical radiation workers are predominantly exposed to X-rays58; the atomic bomb survivors were acutely exposed to external irradiation59. Some inconsistency in the results of the included studies may be explained by the different conditions of radiation exposure. In the meta-analysis, four out of twelve studies focused on radiation workers or uranium miners possibly exposed to internal contamination by radionuclides18 such as uranium or plutonium30,32 or by tritium31, whereas only the external exposure has been considered, except for the study on Mallinckrodt uranium processing workers32. The dose to the brain due to radionuclides intakes was not taken into account in the dose-risk analyses but this should not induce a significant bias because only a small proportion of workers are concerned. Although it is generally assumed that radionuclides may deposit only in small proportion in the brain leading to a possibly limited impact on CNS tumors risk, it is currently suspected that improvements in dose estimation for internal emitters are needed to better characterize their impact on the brain60.
A common limit of the considered studies is the lack of information on the histologic brain tumor subtypes, the most common being glioma, meningioma, and schwannoma. Race/ethnicity affects the incidence of different histologies of brain neoplasia61, further complicating identification of exposure-associated risk factors. Although it has been hypothesized that the risk of CNS tumors after IR exposure may vary by histologic subtype3, only Brenner et al.14 reported risks by histologic subtypes of CNS tumors among the atomic bomb survivors. Other studies analyzing mortality linked to brain tumors have no histologic information. Besides, the definition of this outcome was heterogeneous across studies (e.g., either limited to brain tumors or including tumors of the nervous system (not only central), either including benign tumors or limited to malignant tumors, etc.) which made it difficult to pool the studies together. However, most of them were based on national death registers on which the causes of death were coded according to the International Classification of Diseases, which ensures a certain reliability in the classification of deaths.
One major limitation of the considered studies was related to the over-representation of men in the different occupational cohorts, while biological responses to IR have been shown to differ by gender62. It has been suggested that susceptibility to IR-induced cancer is higher in women than in men62. In addition, incidence of CNS tumors in adults varies by sex, with a complex relationship depending on tumor biology (malignant vs. benign) and subtype61. Several studies included in our work were limited to men only32,33,34,38,39,40,41, while the others included both men and women. Only six studies carried out analyses for men and women separately14,17,28,35,36,37. However, women groups were much smaller compared to men groups, resulting in a low number of cancer cases and thus preventing robust results and conclusions. Furthermore, annual occupational radiation doses in women were shown to be substantially lower than those in men18, thus reinforcing the need to account for gender in the analyses. Nevertheless, studies of occupationally exposed medical workers17,36,37, as well as studies of cohorts exposed environmentally42, provide an opportunity to include a higher proportion of women in the analyses.
Despite the limitations described above related to specific study designs, the meta-analysis conformed to the PRISMA guidelines, ensuring a systematic and objective data analysis. The quality score between 7 and 9 on the Newcastle Ottawa Scale for all studies included in this review allowed to obtain a good homogeneity between the studies considered, confirmed by statistical tests. While, the NOS scale is the most accepted and used tool in systematic review work for quality studies assessment, some other scales have been proposed specifically in the field of radiation epidemiology to take into account the quality of the dose reconstruction63. However, as we exclude studies without dose assessment, the NOS scale seems appropriate to evaluate the quality of the studies.
Furthermore, to increase the robustness of our analysis and to account for unavoidable inherent heterogeneity between studies (mostly related to differences in populations, various types of radiation exposure, chronically or acute exposure), we used random effects models to calculate our estimates. Sensitivity analyses in which each pooled estimate (ERR) was calculated excluding each study one at a time and each group (e.g., flight attendants, nuclear workers or uranium miners, medical workers, etc.) revealed no substantial alteration of the overall heterogeneity. Then, our results can be considered as a good summary of the available literature on the risk of brain/CNS tumors after adult exposure to IR. The review of the studies also provided a better assessment of key points for future research in this area.
Conclusion
This systematic review examined the relationship between low-to-moderate doses of IR when exposure occurred during adulthood/young adulthood and the risk of CNS tumors. There is no evidence of a dose–response association between IR exposure and risk of CNS tumors. Limitations of the studies include the lack of histological information on CNS tumors and large uncertainties in dose assessment. Further studies, ideally large-scale studies with adequate dosimetry and available information on potential confounding factors, will be essential to expand our knowledge on the effects of low-to-moderate doses of IR in adulthood/young adulthood on the CNS.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
UNSCEAR. Sources and effects of ionizing radiation, United nations scientific committee on the effects of atomic radiation (UNSCEAR) 2008 report, Vol. I. United Nations Available on: https://www.un-ilibrary.org/content/books/9789210582520 (2010).
UNSCEAR. Effects of ionizing radiation, United nations scientific committee on the effects of atomic radiation (UNSCEAR) 2006 report, Vol. I United Nations. Available on: https://www.un-ilibrary.org/content/books/9789210582513 (2008).
Braganza, M. Z. et al. Ionizing radiation and the risk of brain and central nervous system tumors: A systematic review. Neuro Oncol. 14(11), 1316–1324 (2012).
Taylor, A. J. et al. Population-based risks of CNS tumors in survivors of childhood cancer: The British childhood cancer survivor study. J. Clin. Oncol. 28(36), 5287–5293 (2010).
Ron, E. et al. Tumors of the brain and nervous system after radiotherapy in childhood. N. Engl. J. Med. 319(16), 1033–1039 (1988).
Yeh, H. C., Matanoski, G. M., Wang, N. Y., Sandler, D. P. & Comstock, G. W. Cancer incidence after childhood nasopharyngeal radium irradiation: A follow-up study in washington county Maryland. Am. J. Epidemiol. 153(8), 749–756 (2001).
Mathews, J. D. et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 346, 7910 (2013).
Meulepas, J. M. et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J. Natl. Cancer Inst. 111(3), 256–263 (2019).
Pearce, M. S. et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 380(9840), 499–505 (2012).
Abalo, K. D., Rage, E., Leuraud, K., Richardson, D. B., Le Pointe, H. D., Laurier, D et al. Early life ionizing radiation exposure and cancer risks: systematic review and meta-analysis. Pediatr. Radiol. Available on: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85090790496&doi=10.1007%2fs00247-020-04803-0&partnerID=40&md5=815341163f942849cb2a3e6288d8b092 (2020).
Wakeford, R. & Bithell, J. F. A review of the types of childhood cancer associated with a medical X-ray examination of the pregnant mother. Int. J. Radiat. Biol. 97(5), 571–592 (2021).
Shore, R. E. et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J. Radiol. Prot. 38(3), 1217–1233 (2018).
UNSCEAR. Effects of radiation exposure of children UNSCEAR report, sources, effects and risks of ionizing radiation (Scientific Annex B) (New York: United Nations Scientific Committee on the Effects of Ionizing Radiation). [Accessed on 20 May 2022]. Available on: //www.unscear.org/unscear/en/publications/2013_2.html (2013).
Brenner, A. V. et al. Radiation risk of central nervous system tumors in the Life Span Study of atomic bomb survivors, 1958–2009. Eur J Epidemiol. 35(6), 591–600 (2020).
Preston, D. L. et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J. Natl. Cancer Inst. 94(20), 1555–1563 (2002).
Cardis, E. et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: Estimates of radiation-related cancer risks. Radiat. Res. 167(4), 396–416 (2007).
Kitahara, C. M. et al. Occupational radiation exposure and deaths from malignant intracranial neoplasms of the brain and CNS in US radiologic technologists, 1983–2012. Am. J. Roentgenol. 208(6), 1371–1377 (2017).
Richardson, D. B. et al. Site-specific solid cancer mortality after exposure to ionizing radiation: A cohort study of workers (INWORKS). Epidemiology 29(1), 31–40 (2018).
Roguin, A., Goldstein, J., Bar, O. & Goldstein, J. A. Brain and neck tumors among physicians performing interventional procedures. Am. J. Cardiol. 111(9), 1368–1372 (2013).
Smilowitz, N. R., Balter, S. & Weisz, G. Occupational hazards of interventional cardiology. Cardiovasc. Revascularization Med. 14(4), 223–228 (2013).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Lopes, J. et al. Risk of developing non-cancerous central nervous system diseases due to ionizing radiation exposure during adulthood: Systematic review and meta-analyses. Brain Sci. 12(8), 984 (2022).
Linet, M. S. et al. Historical review of occupational exposures and cancer risks in medical radiation workers. Radiat. Res. 174(6), 793–808 (2010).
World Health Organization. International classification of diseases : [9th] Ninth revision, basic tabulation list with alphabetic index. World Health Organization (1978).
World Health Organization. ICD-10 : International statistical classification of diseases and related health problems: Tenth Revision. World Health Organization (2004).
Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [Accessed on 18 May 2022]. Available on: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2013).
Richardson, D. B. et al. Meta-analysis of published excess relative risk estimates. Radiat. Environ. Biophys. 59(4), 631–641 (2020).
Boice, J. D. et al. Mortality among workers at the los alamos national laboratory, 1943–2017. Int. J. Radiat. Biol. 0, 1–28 (2021).
Howe, G. R., Zablotska, L. B., Fix, J. J., Egel, J. & Buchanan, J. Analysis of the mortality experience amongst U.S. Nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat. Res. 162(5), 517–526 (2004).
Sokolnikov, M., Preston, D., Gilbert, E., Schonfeld, S. & Koshurnikova, N. Radiation effects on mortality from solid cancers other than lung, liver, and bone cancer in the mayak worker cohort: 1948–2008. PLoS ONE 10(2), e0117784 (2015).
Zablotska, L. B., Lane, R. S. D. & Thompson, P. A. A reanalysis of cancer mortality in canadian nuclear workers (1956–1994) based on revised exposure and cohort data. Br. J. Cancer 110(1), 214–223 (2014).
Golden, A. P. et al. Updated mortality analysis of the Mallinckrodt uranium processing workers, 1942–2012. Int. J. Radiat. Biol. 98(4), 701–721 (2022).
Rage, E., Caër-Lorho, S. & Laurier, D. Low radon exposure and mortality among Jouac uranium miners: An update of the French cohort (1946–2007). J. Radiol. Prot. Déc. 38(1), 92–108 (2017).
Yong, L. C., Pinkerton, L. E., Yiin, J. H., Anderson, J. L. & Deddens, J. A. Mortality among a cohort of U.S. Commercial airline cockpit crew. Am. J. Ind. Med. 57(8), 906–914 (2014).
Dreger, S. et al. Cohort study of occupational cosmic radiation dose and cancer mortality in German aircrew, 1960–2014. Occup. Environ. Med. 77(5), 285–291 (2020).
Lee, W. J. et al. Occupational radiation exposure and cancer incidence in a cohort of diagnostic medical radiation workers in South Korea. Occup. Environ. Med. 78, 876–883 (2021).
Boice, J. D. et al. Mortality among medical radiation workers in the united states, 1965–2016. Int. J. Radiat. Biol. 1–63 (2021).
Friedman-Jimenez, G., Kato, I., Factor-Litvak, P. & Shore, R. Low-dose ionizing radiation and cancer mortality among enlisted men stationed on nuclear-powered submarines in the United States Navy. Int. J. Radiat. Biol. 0, 1–9 (2022).
Boice, J. D. et al. Mortality among U.S. military participants at eight aboveground nuclear weapons test series. Int. J. Radiat. Biol. 1, 1–22 (2020).
Gillies, M., Haylock, R. Mortality and cancer incidence 1952-2017 in United Kingdom participants in the United Kingdom’s atmospheric nuclear weapon tests and experimental programmes. J. Radiol. Prot. [Internet]. [Accessed on 19 May 2022]; Available on: https://doi.org/10.1088/1361-6498/ac52b4 (2022).
Rahu, K. et al. Site-specific cancer risk in the baltic cohort of chernobyl cleanup workers, 1986–2007. Eur. J. Cancer 49(13), 2926–2933 (2013).
Bräuner, E. et al. Residential radon and brain tumour incidence in a danish cohort. PLoS ONE 8, e74435 (2013).
Denoth-Lippuner, A. & Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 22(4), 223–236 (2021).
Johnson, K. J. et al. Childhood brain tumor epidemiology: A brain tumor epidemiology consortium review. Cancer Epidemiol. Biomark. Prev. 23(12), 2716–2736 (2014).
IARC. List of classifications by cancer sites with sufficient or limited evidence in humans, Vol. 1 to 132. Lyon, France (IARC Monographs). Int Agency Res. Cancer (2021).
Cardis, E. et al. Risk of brain tumours in relation to estimated RF dose from mobile phones: results from five Interphone countries. Occup. Environ. Med. 68(9), 631–640 (2011).
Navas-Acién, A., Pollán, M., Gustavsson, P. & Plato, N. Occupation, exposure to chemicals and risk of gliomas and meningiomas in Sweden. Am. J. Ind. Med. 42(3), 214–227 (2002).
Provost, D. et al. Brain tumours and exposure to pesticides: A case–control study in southwestern France. Occup. Environ. Med. 64(8), 509–514 (2007).
Amirian, E. S. et al. Approaching a scientific consensus on the association between allergies and glioma risk: A report from the glioma international case-control study. Cancer Epidemiol. Biomark. Prev. 25(2), 282–290 (2016).
Seliger, C. et al. Statin use and risk of glioma: Population-based case–control analysis. Eur J Epidemiol. 31(9), 947–952 (2016).
Kuan, A. S. et al. Diet and risk of glioma: Combined analysis of 3 large prospective studies in the UK and USA. Neuro. Oncol. 21(7), 944–952 (2019).
Allès, B. et al. Dietary and alcohol intake and central nervous system tumors in adults: Results of the CERENAT multicenter case-control study. NED. 47(3–4), 145–154 (2016).
Lin, H. et al. Ambient PM2.5 and O3 and their combined effects on prevalence of presbyopia among the elderly: A cross-sectional study in six low- and middle-income countries. Sci. Total Environ. 655, 168–173 (2019).
Pirani, M. et al. Analysing the health effects of simultaneous exposure to physical and chemical properties of airborne particles. Environ. Int. 79, 56–64 (2015).
Hemminki, K., Li, X. & Collins, V. P. Parental cancer as a risk factor for brain tumors (Sweden). Cancer Causes Control. 12(3), 195–199 (2001).
Thierry-Chef, I. et al. Dose estimation for a study of nuclear workers in France, the United Kingdom and the United States of America: Methods for the international nuclear workers study (INWORKS). Radiat. Res. 183(6), 632–642 (2015).
Duport, P. Is the radon risk overestimated? Neglected doses in the estimation of the risk of lung cancer in uranium underground miners. Radiat. Prot. Dosim. 98(3), 329–338 (2002).
Linet, M. S. et al. Mortality in U.S. Physicians likely to perform fluoroscopy-guided interventional procedures compared with psychiatrists, 1979 to 2008. Radiology 284(2), 482–494 (2017).
Cullings, H. et al. DS02R1: Improvements to atomic bomb survivors’ input data and implementation of dosimetry system 2002 (ds02) and resulting changes in estimated doses. Health Phys. 112, 56–97 (2017).
Leggett, R. W., Tolmachev, S. Y. & Boice, J. D. Potential improvements in brain dose estimates for internal emitters. Int. J. Radiat. Biol. 98(4), 644–656 (2022).
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 20(4), iv1–iv86 (2018).
Narendran, N., Luzhna, L., Kovalchuk, O. Sex difference of radiation response in occupational and accidental exposure. Front. Genet. [Internet]. [Accessed on 7 July 2021];10. Available on: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6509159/ (2019).
National Council on Radiation Protection and Measurements (NCRP). Radiation exposure in space and the potential for central nervous system effects: Phase II. Bethesda, Maryland (2019). Report No. 183.
Acknowledgements
The authors are grateful to all the authors of the studies included in this work, and to Eric Blanchardon and Cécile Challenton de Vathaire, Institute for Radiological Protection and Nuclear Safety (IRSN), for their contribution with the meta-analyses.
Author information
Authors and Affiliations
Contributions
J.L., C.B. and M.-O.B. designed the study; J.L. and C.B. made the systematic research and independently performed the selection of studies according to the PRISMA guidelines; disagreements have been discussed and resolved by M.-O.B.; J.L. performed the qualitative and quantitative syntheses of the studies; J.L., C.B., K.L., D.K. and M.-O.B. drafted the initial report, they take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopes, J., Baudin, C., Leuraud, K. et al. Ionizing radiation exposure during adulthood and risk of developing central nervous system tumors: systematic review and meta-analysis. Sci Rep 12, 16209 (2022). https://doi.org/10.1038/s41598-022-20462-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20462-7
- Springer Nature Limited