Abstract
This study aimed to evaluate the association between non-alcoholic fatty liver disease (NAFLD) and cognitive impairment and explore the effect modification by the inflammatory status. A total of 4400 community-based participants aged 50–64 years from the Cardiovascular and Metabolic Disease Etiology Research Center were included in this cross-sectional study. NAFLD was identified as the Fatty Liver Index 30 or higher in the absence of excessive alcohol consumption. Cognitive impairment was defined as the total score of the Mini-Mental State Examination (cutoff 24). The inflammatory status was evaluated using white blood cell (WBC) and high-sensitivity C-reactive protein (hsCRP). Multivariate logistic regression analyses were performed. Stratified analyses by the WBC count (the highest quartile) and the hsCRP level (≥ 1.0 mg/dL vs. < 1.0 mg/dL) were conducted. Participants with NAFLD showed an increased prevalence of cognitive impairment (odds ratio [OR] = 1.26; 95% confidence interval [CI] = 1.04–1.52) compared with the non-NAFLD population. In women, this association was significantly stronger in the highest quartile WBC group than in lower WBC group (OR = 1.81; 95% CI = 1.19–2.74 vs. OR = 1.02; 95% CI = 0.78–1.33, p-interaction = 0.05). NAFLD was positively associated with a higher proportion of cognitive impairment, and this association was stronger in women with higher inflammatory status.
Similar content being viewed by others
Introduction
Dementia is a burdensome disease that affects 43.8 million people worldwide1 and globally costs 1 trillion US dollars annually2. As dementia has a deteriorative and irreversible course, preventive interventions are indispensable to lower this burden. It has been suggested that modifying potential risk factors for dementia, such as less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, low social contact, excessive alcohol consumption, traumatic brain injury, and air pollution, might prevent or delay 40% of the dementias3. Besides diabetes4,5 and obesity6,7, metabolic risk factors including hyperlipidemia8,9 are known to affect cognitive impairment and play a role in the development of dementias. Metabolic diseases not only cause vascular dementia through cerebrovascular disease but also affects Alzheimer’s disease through insulin resistance10 and neuroinflammation11.
Non-alcoholic fatty liver disease (NAFLD) is a common metabolic disease, with a global prevalence of 25% in a meta-analysis of studies of populations aged ≥ 18 years in 22 countries12. NAFLD is known to be associated not only with liver cirrhosis and cardiovascular disease13, but also with psychiatric complications, such as depression, anxiety, and cognitive impairment14. NAFLD can be prevented or treated through lifestyle modifications, such as exercise or dietary changes15. With this high rate and modifiability of NAFLD, an association with cognitive impairment can have substantial public health implications.
Recently, several studies16,17,18,19 have investigated the association between NAFLD and impaired cognitive function in the general population, but positive results and negative results coexist. In the United States, a cross-sectional study of 4472 participants in the National Health and Nutritional Examination Survey (NHANES)16 showed that NAFLD was associated with lower cognitive performance on the Serial Digit Learning Test. However, in a cross-sectional study of the Framingham Study19, participants with NAFLD did not show lower performance on multiple cognitive function tests. To clarify this inconsistency, studies examining the association between NAFLD and cognitive function in a sufficiently large population would be needed. Moreover, to our knowledge, no studies have evaluated the association between NAFLD and cognitive function in the Asian general population. The prevalence of NAFLD in Asia is around 25%12, as high as in Western countries. Regarding the emerging importance of NAFLD in Asia, it would be beneficial to confirm the association with cognitive function in a representative Asian general population.
Meanwhile, the biological mechanism underlying the association between NAFLD and cognitive impairment is not yet elucidated. Inflammation is a pivotal mechanism in neuronal injury, the progression of Alzheimer’s disease11, and the development of NAFLD and its progression to non-alcoholic steatohepatitis20. Inflammation can also affect endothelial dysfunction, insulin resistance, and reactive oxidative stress, which are suggested as possible mechanisms linking NAFLD and cognitive impairment21. However, human studies investigating the role of inflammation in relation to the association between NAFLD and cognitive impairment are insufficient.
Therefore, we aimed to evaluate the association between NAFLD and cognitive impairment in a community-based population and investigate the effect modification of the inflammatory status in relation to the association between NAFLD and cognitive impairment.
Results
Of the 4400 participants included in this study, 1415 (32.2%) of them had Fatty Liver Index (FLI) ≥ 30, defined as NAFLD. 666 (15.1%) participants had Mini-Mental Status Examination (MMSE) < 24, defined as the cognitive impairment. Baseline characteristics according to the NAFLD are presented in Table 1. Participants with NAFLD were older, lesser female, less educated, more current smokers, and had higher proportions of a history of hypertension and diabetes. Participants with NAFLD were more likely to show cognitive impairment (NAFLD group: 233 [17.2%] vs. normal group: 422 [14.1%]; p < 0.001). In addition, participants with NAFLD had a higher level of white blood cell (WBC) and high-sensitivity C-reactive protein (hsCRP).
NAFLD was still significantly associated with cognitive impairment (odds ratio [OR] = 1.26; 95% confidence interval [CI] = 1.04–1.52) after fully adjusting for socio-demographics, lifestyle factors, and comorbidities (Table 2). For the stratification by sex, the association between NAFLD and cognitive impairment was slightly larger in males (OR = 1.39; 95% CI = 0.94–2.07) than in females (OR = 1.21; 95% CI = 0.98–1.51). With stepwise additions of covariates, the ORs for cognitive impairment were altered after adjusting education level, household income, and marital status (OR = 1.35; 95% CI = 1.13–1.62 in model 1 vs. OR = 1.27; 95% CI = 1.05–1.52 in model 2), and this pattern was replicated in sub-groups by sex. The positive association between NAFLD and cognitive impairment was also maintained in the sensitivity analyses, which tested the association between NAFLD and the continuous MMSE score (Supplementary Table 2), the association between the continuous FLI value and cognitive impairment (Supplementary Table 3), and the association between the continuous values of FLI and the MMSE score (Supplementary Table 4).
In the stratified analyses with inflammatory status, the association between NAFLD and cognitive impairment was significant in participants with a high inflammatory status (high WBC: OR = 1.70; 95% CI = 1.20–2.41, high hsCRP: OR = 1.54; 95% CI = 1.10–2.15), but not in those with a low inflammatory status (low WBC: OR = 1.09; 95% CI = 0.86–1.37, low hsCRP: OR = 1.25; 95% CI = 0.98–1.59) (Fig. 1). The interaction on the association of the WBC strata was statistically significant in women (high WBC: OR = 1.81; 95% CI = 1.19–2.74 vs. low WBC: OR = 1.02; 95% CI = 0.78–1.33, interaction-p = 0.05), but almost absent in men (high WBC: OR = 1.38, 95% CI = 0.73–2.67 vs. low WBC: OR = 1.42, 95% CI = 0.85–2.37, interaction-p = 0.97). The associations in high hsCRP strata were seemed to be stronger than in low hsCRP strata in both men (high hsCRP: OR = 1.74; 95% CI = 0.89–3.51 vs. low hsCRP: OR = 1.23; 95% CI = 0.75–2.02, interaction-p = 0.70) and women (high hsCRP: OR = 1.47; 95% CI = 0.99–2.17 vs. low hsCRP: OR = 1.26; 95% CI = 0.95–1.66, interaction-p = 0.90), but there were no statistical effect modifications.
Association between NAFLD and cognitive impairment stratified by the inflammatory status. NAFLD was defined as FLI ≥ 30, and cognitive impairment was defined as MMSE < 24. Each model was adjusted for sex, age, household income, marital status, current drinking status, current smoking status, diabetes, and hypertension. Low WBC: WBC < 75th %tile (6300/μL), High WBC: WBC ≥ 75th %tile (6300/μL). Low hsCRP: hsCRP < 1·0 mg/L, high hsCRP: hsCRP ≥ 1·0 mg/L. NAFLD, non-alcoholic fatty liver disease; WBC, white blood cell; hsCRP, high-sensitivity C-reactive protein; OR, odds ratio; CI, confidence interval; FLI, fatty liver index; MMSE, Mini-Mental State Examination.
Discussion
In this cross-sectional study of the general population aged 50–64 years, participants with NAFLD showed a significant association with cognitive impairment independent of various potential confounders, including socio-demographic factors, lifestyle factors, and comorbidities. The association between NAFLD and cognitive impairment remained significant only in participants with high inflammatory status, not in those with a low inflammatory status.
Using MMSE, this study confirmed the association between NAFLD and cognitive impairment that has been examined in previous studies. In a case–control study conducted in Turkey17, the Montreal Cognitive Assessment(MoCA), a screening test for the overall cognitive function, was used to examine the association between NAFLD and cognitive impairment. In this study, 70 hospital-based patients with NAFLD showed 2.99 times higher odds of overall cognitive impairment compared with 73 controls, which was higher than the OR of 1.26 in our study. It is possible that the size of the association in this study was inflated because the control group in this study included hospital staff as well as patients from outpatient clinics. Meanwhile, this difference suggested that NAFLD is more strongly associated with specific cognitive domains. Executive functioning is especially associated with metabolic diseases, such as obesity22 and diabetes23. Since the MoCA in this study has more emphasis on tasks of frontal functioning, such as executive function and visuospatial function, than the MMSE in our study, there is the possibility that the MoCA was more sensitive to detect cognitive impairment due to NAFLD than the MMSE. Indeed, in this study, NAFLD was only associated with low performance of visuospatial and executive function domains among subdomains of the MoCA. A cross-sectional study16 of 4472 adults in the NHANES showed that NAFLD was associated with low performance in the Serial Digit Learning Test but not the other two tests for other cognitive domains. In addition, in a cross-sectional study of 1287 participants in the Framingham Study19, there was no association between the presence of NAFLD and cognitive function, but the advanced fibrosis in patients with NAFLD was significantly related to executive function and abstract reasoning among various cognitive function tests. In a cross-sectional study of 1102 participants in the NHANES18, participants with both NAFLD and diabetes were associated with lower performance only on Digit Symbol Substitution Test, which evaluates processing speed, sustained attention, and working memory, compared to healthy controls. Taken together, there is the possibility that the cognitive impairment in NAFLD observed in our study might be focused on specific cognitive domains, particularly cognitive functions related to the frontal lobe. Further comprehensive studies on the various cognitive domains affected by NAFLD would be needed.
However, there have been studies18,19 demonstrating that NAFLD is not independently associated with cognitive impairment. In these studies, the mean ages of the study population were 69 years18 and 61 years19, respectively. Compared with our study, in which the maximum age of the study population was 64 years, these negative studies might include a larger proportion of the elderly population. As age increases, the effects of other metabolic and vascular risk factors on dementia tend to weaken24. Similarly, the association between NAFLD and cognitive impairment may vary with age. In the study that examined the association between NAFLD and lower performance on the Serial Digit Learning Test16, the mean ages of the study population were 37.3 years. Likewise, in the case–control study in Turkey17, the mean ages of the case group and the control group were 46.9 years and 43.9 years, respectively. Thus, the association between NAFLD and cognitive function tended to be stronger in the younger population. NAFLD in the middle-aged population may have important implications as a preventive risk factor for dementia.
As intrahepatic fat accumulation progresses, damaged hepatocytes of patients with NAFLD secrete excess pro-inflammatory cytokines25. Activated peripheral inflammatory cytokines can activate microglia and increase the permeability of the blood–brain barrier (BBB)26, resulting in the transmission of immune cells and neurotoxic factors. These contribute to chronic neuroinflammation and the formation of beta-amyloid plaques27, resulting in cognitive impairment through neuronal cell injury or death. The results of our study showed that the association between NAFLD and cognitive impairment remained significant only in the high inflammatory status. This implies the possibility that participants with higher severity of NAFLD were included in the high inflammatory strata due to enhanced systemic inflammation by the liver with steatosis, as well as the possibility that the mechanism of NAFLD causing neuroinflammation was accelerated by the existing inflammation due to other causes. Further studies considering the severity of NAFLD are needed to evaluate the role of inflammation in the association between NAFLD and dementia.
In this study, the effect modification of WBC on the association between NAFLD and cognitive impairment was more pronounced in women. There is no study yet on the sex-related differences in the relationship between NAFLD and cognitive impairment. In women, estrogen deficiency after menopause is suggested as one of the mechanisms of cognitive decline with age28,29. Estrogen may have a protective effect on the BBB30, suggesting the possibility that the BBB of postmenopausal women is relatively vulnerable to systemic inflammation. In other words, postmenopausal women in the high inflammatory status might be more susceptible to neurotoxic effects of NAFLD transmitted through the BBB. In our study, since most of the women included were postmenopausal, the effect of NAFLD on cognitive impairment in women can be strengthened in the high inflammatory strata. However, further comprehensive studies are needed to investigate the interplay between sex and inflammation.
This study has several strengths. We could utilize a variety of covariate information to build our final model to control for potential confounding factors. In addition, 4400 community participants were analyzed, which is similar to the sample size of the largest of the previous studies16. We believe our study utilized the largest sample in the Asian population, evaluating the relationship between NAFLD and cognitive function. With such an adequate sample size, it was possible to demonstrate the association between NAFLD and cognitive impairment in several sensitivity analyses. In addition, this is the first study to investigate the role of inflammation in the association between NAFLD and cognitive impairment.
However, this study has several limitations. First, the definition of NAFLD in this study was based on the FLI, and there was no imaging or histological confirmation. However, as mentioned above, FLI is a widely used surrogate marker of NAFLD and is well validated. Second, the MMSE cannot examine specific domains of cognition which has been associated with NAFLD in a previous study16. Further studies using a cognition test battery that comprehensively evaluates multiple cognitive domains would explore the NAFLD-specific cognitive domain. On the other hand, since the MMSE is a screening test that examines the overall cognitive function, it is possible to extend the results of our study to general health care, such as a health examination. Third, since this was a cross-sectional study, it was challenging to guarantee the causality of cognitive impairment due to NAFLD. However, the biological mechanism by which impaired cognitive function can cause NAFLD is rare; therefore, the causality of cognitive impairment caused by NAFLD can be considered. Fourth, participants aged 50–64 years were included in this study; therefore, these results may not be generalizable to the whole geriatric population. Fifth, unmeasured or residual confounding factors may exist, although various potential confounders were adjusted. Sixth, the sample size of the subgroups by sex and inflammatory status was not sufficient to guarantee the statistical power of subgroup analyses. Further studies with a larger sample size would be needed.
Conclusion
Our results confirmed the association between NAFLD and cognitive impairment independent of potential confounders in a large Asian general population aged 50–64 years. In addition, this association was noticeable with high inflammatory status. Overall, our study highlights the potential promise of NAFLD as an independent risk factor for dementia in the geriatric population, especially in women with high inflammatory status. Further studies with a longitudinal study design in other large populations are needed to replicate our results with causal relationships.
Methods
Data source and selection of participants
Data were obtained from the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) cohort. The CMERC cohort is a multicenter cohort study aimed at identifying novel risk factors and developing prevention strategies for cardiovascular diseases in Korea31. Accordingly, participants who had not been diagnosed with myocardial infarction, stroke, and heart failure during their lifetime were recruited from a community near Seoul, the capital of Korea. From 2013 to 2018, baseline information on the socio-demographic characteristics, medical history, lifestyle factors including alcohol consumption, and psychiatric health of the participants were collected by trained interviewers. In addition, physical examination and blood analysis were conducted on the same day as the interview. Blood samples were collected in the morning after 8 h fasting. A total of 8097 community-based participants aged 30–64 years were recruited at Yonsei University College of Medicine in Seoul, Korea and Ajou University School of Medicine in Suwon, Korea. Among them, 5280 people aged ≥ 50 years underwent the MMSE.
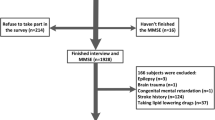
We excluded nine participants with missing information in the MMSE. In addition, gamma-glutamyl transferase (γ-GTP) was used to calculate the Fatty Liver Index (FLI); therefore, three participants without information on γ-GTP were excluded. To rule out alcoholic fatty liver disease, a total of 871 participants with a history of excessive alcohol consumption were excluded. Excessive alcohol consumption was defined as ≥ 14 drinks per week for men or ≥ 7 drinks per week for women32 based on self-reports of average alcohol consumption over the past year. The excluded population had different characteristics from the included population by excluding heavy drinkers. (Supplementary Table 1) Included participants were mostly female, older, less educated, and had lower incomes. There were lesser current drinkers and smokers and higher proportions of those with a history of hypertension and diabetes. Finally, 4400 participants aged 50–64 years were included in the analysis (Fig. 2).
Measurement of the exposure variables and defining NAFLD
NAFLD was defined in this study using the FLI33. FLI comprises body mass index (BMI, kg/m2), waist circumference (cm), and serum triglyceride (mg/dL) and γ-GTP (IU/L) levels. In this study, height and body weight were measured using stadiometers and a digital weight scale, respectively. Waist circumference was measured using a plastic measuring tape at the midpoint between the lower border of the rib cage and the upper border of the iliac crest during exhalation. Triglycerides and γ-GTP were measured enzymatically using the ADVIA 1800 AutoAnalyzer (Siemens Medical Sol., USA). The formula of the FLI was as follows: FLI = 100 × exp(0.953 × loge(triglycerides) + 0.139 × BMI + 0.718 × loge(γ-GTP) + 0.053 × waist circumference − 15.745)/[1 + exp(0.953 × loge(triglycerides) + 0.139 × BMI + 0.718 × loge(γ-GTP) + 0.053 × waist circumference − 15.745)]33. According to the formula, FLI can theoretically have a value between 0 and 100. In addition, the application of FLI did not differ by sex.
From the initial utilization of FLI as a criterion for defining NAFLD, FLI has been validated for the diagnosis of NAFLD in a large population, not only in Western countries34,35 but also in Asian countries36,37. In these validation studies of the Asian population, the area under the receiver operator characteristic curve (AUROC) was higher than 0.8. FLI has been widely used to define NAFLD in the general population of East Asia38,39,40, as is the case of other regions41,42,43. In the European Clinical Practice Guidelines44, FLI was suggested as an acceptable alternative for the diagnosis of hepatic steatosis for epidemiologic studies.
Furthermore, we conducted a receiver operator characteristic analysis for FLI on the subgroup of participants to support the validity of FLI. Among 5280 participants aged ≥ 50 years, 2640 people underwent quantitative computed tomography measuring adiposity of liver and spleen. The average value of Hounsfield units (HU) of liver and spleen was measured using a Somatom Definition AS + 128 channel CT (Siemens Healthcare, Forchheim, Germany), a Somatom sensation 64 channel CT (Siemens Healthcare), or a GE Lightspeed VCT apparatus (General Electric Medical System, Milwaukee, WI, USA). With the liver to spleen HU ratio < 145 as the standard diagnosis for fatty liver, the AUROC of FLI was 0.802. (Supplementary Fig. 1).
Although Bedogni et al. suggested FLI ≥ 60 as the cut-off value for NAFLD, validation studies36,37,46 in East Asia argued that a lower cut-off value is required, considering the difference in BMI and waist circumference according to ethnicity. The validation study36 of the Chinese population aged over 40 years suggested FLI ≥ 30 as an optimal cut-off value; therefore, NAFLD was defined as FLI ≥ 30 in this study.
Defining cognitive impairment as an outcome variable
MMSE is routinely used as a screening tool in clinical settings to evaluate the global cognitive status47. In the CMERC cohort, MMSE was conducted by trained interviewers at baseline. The MMSE comprises 30 items evaluating orientation, attention, memory, language, and visual-spatial functions. Each item was counted as 1 point, and the total MMSE score ranged from 0 to 30. The MMSE was validated in the Korean population, and a cut-off value of 23/24 was suggested for cognitive impairment48. Participants with an MMSE score of < 24 were classified as having “cognitive impairment” in this study, based on this cut-off value.
Measuring the inflammatory status as an effect modifier variable
The WBC count and the hsCRP level were measured and used as a marker of the systemic inflammatory status. The WBC count was determined with flow cytometry using the ADIVIA 2120i (Siemens Medical Sol., USA). Using the serum separated from the collected blood sample, the turbidimetry method using the ADVIA 1800 AutoAnalyzer (Siemens Medical Sol., USA) was used to detect the hsCRP level. We stratified the association between NAFLD and cognitive impairment by the WBC count and the hsCRP level. The WBC count was categorized as high (the highest quartile) and low (other quartiles), and the hsCRP level was categorized as high (≥ 1.0 mg/L) and low (< 1.0 mg/L)49.
Covariates
As mentioned below, structured questionnaires for covariates were queried by trained interviewers in the CMERC baseline survey. Years of education were classified based on the final graduation school: not educated, elementary school, or middle school (education attainment years ≤ 9 years), high school (10–12 years), and university or higher education (> 12 years of education). Participants were also asked about their average annual household income, which was classified into quartiles according to the distribution of the population. Diabetes status was defined as a participant satisfying one of the following three conditions50: fasting plasma glucose ≥ 126 mg/dL, glycosylated hemoglobin (HbA1c) ≥ 6.5%, self-reported diabetes diagnosis, or the use of anti-diabetic medications. Plasma glucose and HbA1c levels were measured using the colorimetric method (ADVIA 1800 AutoAnalyzer, Siemens Medical Sol., USA) and high-performance liquid chromatography (Variant II Turbo, Bio-Rad Laboratories, USA), respectively. Hypertension was defined as participants who satisfied one of the following three conditions51: systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, self-reported diagnosis of hypertension, or the use of antihypertensive medications. Blood pressure was measured in the participant’s right arm using an automated oscillometric device (HEM-7080, Omron Health, Japan) by trained investigators. Systolic and diastolic blood pressures were measured thrice at 2-min intervals, and the mean of the second and third measurements was adopted in this study.
Statistical analyses
Baseline characteristics by the cognitive impairment status were compared using the t-test and chi-square test. The association between NAFLD and cognitive impairment was evaluated using a multiple logistic regression model. Models were additionally adjusted for (1) age and sex, (2) socio-demographic factors (education level, household income, and marital status), (3) lifestyle factors (current smoking and current drinking), and (4) comorbidities (diabetes and hypertension). A stratification analysis was conducted with cut-off values (highest quartile and 1.0 mg/L, respectively) of the WBC count and the hsCRP level to examine if the association between NAFLD and cognitive impairment differed by the inflammatory status. Since NAFLD and cognitive impairment were defined based on a single cut-off value in the main analyses, sensitivity analyses were performed to consolidate the association between NAFLD and cognitive impairment. The FLI value as a continuous variable was used as an independent variable in the regression analysis. Similarly, a continuous MMSE score was used as a dependent variable in the multiple linear regression. All analyses were conducted using the R software V.4.0.4.52.
Ethics
The protocol of this study was approved by the Institutional Review Board of Yonsei University (YUIRB-4-2013-0661), and written informed consent was obtained from all participants. All procedures in this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and the Helsinki Declaration of 1975.
Data availability
The data of the CMERC cohort study can be obtained by submitting an application to the National Biobank of Korea (NBK) and receiving approval from the NBK Access and Sharing Committee. Detailed information is available on the NBK website (http://nih.go.kr/biobank/cmm/main/engMainPage.do). However, we are not permitted to share the data.
References
Nichols, E. et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 88–106 (2019).
Patterson, C. World alzheimer report 2018 (2018).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Luchsinger, J. A. et al. Relation of diabetes to mild cognitive impairment. Arch. Neurol. 64, 570–575 (2007).
Cheng, G., Huang, C., Deng, H. & Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 42, 484–491 (2012).
Whitmer, R. A., Gunderson, E. P., Barrett-Connor, E., Quesenberry, C. P. & Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 330, 1360 (2005).
Pedditizi, E., Peters, R. & Beckett, N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 45, 14–21 (2016).
Solomon, A., Kivipelto, M., Wolozin, B., Zhou, J. & Whitmer, R. A. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord. 28, 75–80 (2009).
Anstey, K. J., Ashby-Mitchell, K. & Peters, R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: Review and meta-analysis. J. Alzheimers Dis. 56, 215–228 (2017).
Talbot, K. et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 122, 1316–1338 (2012).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Colognesi, M., Gabbia, D. & De Martin, S. Depression and cognitive impairment—extrahepatic manifestations of NAFLD and NASH. Biomedicines 8, 229 (2020).
Romero-Gómez, M., Zelber-Sagi, S. & Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 67, 829–846 (2017).
Seo, S. W. et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 86, 1136–1142 (2016).
Celikbilek, A., Celikbilek, M. & Bozkurt, G. Cognitive assessment of patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 30, 944–950 (2018).
Weinstein, A. A. et al. Cognitive performance in individuals with non-alcoholic fatty liver disease and/or type 2 diabetes mellitus. Psychosomatics 59, 567–574 (2018).
Weinstein, G. et al. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int. 39, 1713–1721 (2019).
Arrese, M., Cabrera, D., Kalergis, A. M. & Feldstein, A. E. Innate immunity and inflammation in NAFLD/NASH. Dig. Dis. Sci. 61, 1294–1303 (2016).
Gehrke, N. & Schattenberg, J. M. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 158, 1929–1947. e1926 (2020).
Fitzpatrick, S., Gilbert, S. & Serpell, L. Systematic review: Are overweight and obese individuals impaired on behavioural tasks of executive functioning?. Neuropsychol. Rev. 23, 138–156 (2013).
Van Den Berg, E. et al. Cognitive functioning in elderly persons with type 2 diabetes and metabolic syndrome: The Hoorn study. Dement. Geriatr. Cogn. Disord. 26, 261–269 (2008).
Legdeur, N. et al. The association of vascular disorders with incident dementia in different age groups. Alzheimer’s Res. Ther. 11, 1–8 (2019).
Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910 (2014).
Elahy, M. et al. Blood–brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 12, 1–9 (2015).
Wang, H. et al. Targeted inhibition of RAGE reduces amyloid-β influx across the blood-brain barrier and improves cognitive deficits in db/db mice. Neuropharmacology 131, 143–153 (2018).
Foster, T. C. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 22, 656–669 (2012).
Sherwin, B. B. Estrogen and cognitive functioning in women: Lessons we have learned. Behav. Neurosci. 126, 123 (2012).
Maggioli, E. et al. Estrogen protects the blood–brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav. Immun. 51, 212–222 (2016).
Shim, J.-S. et al. Cohort profile: The cardiovascular and metabolic diseases etiology research center cohort in Korea. Yonsei Med. J. 60, 804 (2019).
National Institute on Alcohol Abuse and Alcoholism Drinking Levels Defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
Bedogni, G. et al. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 1–7 (2006).
Koehler, E. M. et al. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin. Gastroenterol. Hepatol. 11, 1201–1204 (2013).
Foschi, F. G. et al. External validation of surrogate indices of fatty liver in the general population: The Bagnacavallo study. J. Clin. Med. 10, 520 (2021).
Huang, X. et al. Validation of the Fatty Liver Index for Nonalcoholic Fatty Liver Disease in Middle-Aged and Elderly Chinese. Medicine (Baltimore) 94, e1682. https://doi.org/10.1097/md.0000000000001682 (2015).
Yang, B.-L. et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE 10, e0120443 (2015).
Park, J.-H. et al. Association between non-alcoholic fatty liver disease and the risk of biliary tract cancers: A South Korean nationwide cohort study. Eur. J. Cancer 150, 73–82 (2021).
Roh, J. H. et al. Association between non-alcoholic fatty liver disease and risk of new-onset atrial fibrillation in healthy adults. Liver Int. 40, 338–346 (2020).
Hsing, J. C. et al. Associations between body fat, muscle mass, and nonalcoholic fatty liver disease: A population-based study. Hepatol. Commun. 3, 1061–1072 (2019).
Kunutsor, S. K. et al. Circulating total bilirubin and risk of non-alcoholic fatty liver disease in the PREVEND study: Observational findings and a Mendelian randomization study. Eur. J. Epidemiol. 35, 123–137 (2020).
Christensen, K., Lawler, T. & Mares, J. Dietary carotenoids and non-alcoholic fatty liver disease among US adults, NHANES 2003–2014. Nutrients 11, 1101 (2019).
Croci, I. et al. Non-alcoholic fatty liver disease: Prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT Study. Progress Cardiovasc. Dis. 62, 127–134 (2019).
European Association for the Study of The Liver & European Association for the Study of Diabetes EASL-EASDEASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obes. facts. 9, 65–90 (2016).
Zeb, I. et al. Computed tomography scans in the evaluation of fatty liver disease in a population based study: The multi-ethnic study of atherosclerosis. Acad. Radiol. 19, 811–818 (2012).
Kim, J. H., Kwon, S. Y., Lee, S. W. & Lee, C. H. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 31, 1600–1601 (2011).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Park, J.-H. & Kwon, Y. C. Standardization of Korean version of the Mini-Mental State Examination (MMSE-K) for use in the elderly. Part II. Diagnostic validity. J. Korean Neuropsychiatr. Assoc. 28, 508–513 (1989).
Pearson, T. A. et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511 (2003).
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 40, S11–S24 (2017).
Chobanian, A. V. et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42, 1206–1252 (2003).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2021). https://www.R-project.org/.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (Grant number 2020R1C1C1003502).
Author information
Authors and Affiliations
Contributions
Study design and concept: S.K. and S.J.J. Acquisition, analysis, or interpretation of data: D.J.K., H.C.K., and S.J.J. Drafting of the manuscript: S.K. Critical revision of the manuscript for important intellectual content: E.K., H.C., D.J.K., H.C.K. and S.J.J. Statistical analysis: S.K. Obtained funding: H.C.K. and S.J.J. Study supervision: S.J.J. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, S., Kim, E., Cho, H. et al. Associations between non-alcoholic fatty liver disease and cognitive impairment and the effect modification of inflammation. Sci Rep 12, 12614 (2022). https://doi.org/10.1038/s41598-022-16788-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16788-x
- Springer Nature Limited
This article is cited by
-
Impact of insulin resistance on mild cognitive impairment in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease
Diabetology & Metabolic Syndrome (2023)