Abstract
Artificial intelligence (AI) algorithms evaluating [supine] chest radiographs ([S]CXRs) have remarkably increased in number recently. Since training and validation are often performed on subsets of the same overall dataset, external validation is mandatory to reproduce results and reveal potential training errors. We applied a multicohort benchmarking to the publicly accessible (S)CXR analyzing AI algorithm CheXNet, comprising three clinically relevant study cohorts which differ in patient positioning ([S]CXRs), the applied reference standards (CT-/[S]CXR-based) and the possibility to also compare algorithm classification with different medical experts’ reading performance. The study cohorts include [1] a cohort, characterized by 563 CXRs acquired in the emergency unit that were evaluated by 9 readers (radiologists and non-radiologists) in terms of 4 common pathologies, [2] a collection of 6,248 SCXRs annotated by radiologists in terms of pneumothorax presence, its size and presence of inserted thoracic tube material which allowed for subgroup and confounding bias analysis and [3] a cohort consisting of 166 patients with SCXRs that were evaluated by radiologists for underlying causes of basal lung opacities, all of those cases having been correlated to a timely acquired computed tomography scan (SCXR and CT within < 90 min). CheXNet non-significantly exceeded the radiology resident (RR) consensus in the detection of suspicious lung nodules (cohort [1], AUC AI/RR: 0.851/0.839, p = 0.793) and the radiological readers in the detection of basal pneumonia (cohort [3], AUC AI/reader consensus: 0.825/0.782, p = 0.390) and basal pleural effusion (cohort [3], AUC AI/reader consensus: 0.762/0.710, p = 0.336) in SCXR, partly with AUC values higher than originally published (“Nodule”: 0.780, “Infiltration”: 0.735, “Effusion”: 0.864). The classifier “Infiltration” turned out to be very dependent on patient positioning (best in CXR, worst in SCXR). The pneumothorax SCXR cohort [2] revealed poor algorithm performance in CXRs without inserted thoracic material and in the detection of small pneumothoraces, which can be explained by a known systematic confounding error in the algorithm training process. The benefit of clinically relevant external validation is demonstrated by the differences in algorithm performance as compared to the original publication. Our multi-cohort benchmarking finally enables the consideration of confounders, different reference standards and patient positioning as well as the AI performance comparison with differentially qualified medical readers.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
In primary diagnostics, [supine] chest radiography ([S]CXR), performed for common indications such as suspected pneumonia, pneumothorax, effusion, verification of catheter location, and/or detection of pulmonary nodules, remains one of the most frequently requested examinations worldwide, with significant public health implications1,2,3,4,5. Image interpretation is often aggravated by projection phenomena, requires a high level of experience and remains challenging for radiologists as well as a for non-radiologists6,7,8,9.
During the past years, clinical applications of artificial intelligence (AI) algorithms have been increasingly brought into scientific focus since several AI systems have already successfully mimicked healthcare specialists’ diagnostic performance levels10,11,12,13,14,15,16,17,18. A considerable number of CXR interpreting algorithms is trained on the basis of publicly available data sets with labels extracted from radiology reports using natural language processing (NLP)19,20, with algorithms are commonly validated on subgroups of these data sets. To identify potential confounders, external benchmarking is of exceptional importance to the algorithm training development process.
In the current paper, we present an external benchmarking pipeline that comprises three different (S)CXR cohorts. The cohorts differ in patient positioning during image acquisition (supine vs. upright), the underlying reference standards (radiologists’ [S]CXR vs. CT labelling) and the possibility to compare algorithm classification with different medical experts’ performances. By combining the cohorts, we try to cover a variety of different scenarios of daily clinical practice. Based on the cohorts, we characterize the performance of a well-established and publicly available implementation of the AI algorithm CheXNet21,22,23. By comparing the performance results with the results of the original publication, we demonstrate the necessity of extensive external algorithm validation including the analysis based on different cohort subgroups.
Materials and methods
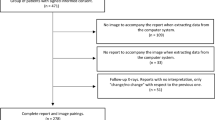
Approval of the institutional ethics commission (Ethics Committee of the Medical Faculty of Ludwig-Maximilians-University Munich) was obtained for this study (approval numbers 418-16, 18-399 and 19-541). Informed consent was waived due to the retrospective character of the study by the institutional ethics commission (Ethics Committee of the Medical Faculty of Ludwig-Maximilians-University Munich). All methods were performed in accordance with the relevant guidelines and regulations of Nature Research journals.
Patient cohorts (image selection and reading)
In the following paragraphs we display the three different (S)CXR cohorts.
Emergency unit chest radiograph cohort (CXR EU)
Cohort containing a total of 563 CXRs in upright position and posterior-anterior (PA) projection that were exclusively acquired in the emergency unit (EU) and were independently evaluated by 9 medical readers of different diagnostic expertise including radiologists (board-certified radiologists [BCRs], radiology residents [RRs]) and non-radiologists (non-radiology residents [NRRs]) (Fig. 1). CXRs contain a representative composition of common findings in the EU: Images without suspected pathologies, pleural effusions, pneumothoraces, consolidations suspicious for pneumonia and lung lesions. We defined the four target diseases as common, clinically important thoracic diseases of emergency radiology for which the primary diagnosis is usually made by chest radiography and for which rapid further therapy/diagnosis is required. Together, they cover a majority of the non-cardiac, non-traumatic causes of acute chest pain visible in the CXR24. With an estimated and/or approximated incidence of 1.5–14.0 (pneumonia)25, up to 322.7 (pleural effusion)26, 22.7 (pneumothorax)27 and 6.6–12.6 per 100,000 patients per year (pulmonary nodules)28, the four pathologies occur very frequently. In fact, pulmonary malignant neoplasms and pneumonia are among the top five respiratory diseases in terms of global burden29. A detailed cohort description is provided by Rudolph et al.30.
Reference standard and characteristics in the CXR EU cohort; (A) illustrates the definition of reference standards based on suspicion scores of the board-certified radiologists. According to the principle of a majority voting 4 reference standards (RFS) were built—RFS I being the most specific and RFS IV being the most sensitive one; (B) illustrates cohorts characteristics and pathology prevalence according to RFS I–IV.
Readers had to evaluate the images in terms of the mentioned findings on a five-point Likert scale: 0—no suspicion/1—unlikely/2—possible/3—likely/4—safe presence. BCR’s reading served as reference standard (RFS) exclusively. The consensus of all three BCR readers was converted into yes-or-no-call RFSs of different sensitivity/specificity as follows: Likert choices 0–3 have been pooled and considered as negative to build the most specific RFS I, choices 1–4 have been pooled and considered as positive representing the most sensitive RFS IV. The other RFSs (II/III) were built accordingly. The scheme is illustrated in Fig. 1A. The final RFSs were built by consensus (majority voting) based on the individual BCR’s yes-or-no-calls. The resulting pathology prevalences depending on the final RFSs are illustrated in Fig. 1B. Based on the above mentioned RFS, the performances of other readers (RRs and NNRs) have been compared with algorithm performance.
Supine chest radiograph unilateral pneumothorax cohort (SCXR PTX)
Cohort containing a total of 6,258 supine CXR (SCXR) cases in anterior–posterior view (AP) that were annotated in terms of a present unilateral pneumothorax (PTX) including the measure of dehiscence and the presence of a thoracic tube (Table 1). Detailed cohort information is described by Rueckel et al.31.
The identified images were annotated by two well-trained fourth-year medical students (the first approximately 50 cases were directly supervised by a radiology resident [RR]). Questionable cases (approximately 10–20%) were marked for review by a RR with 3 years of experience in thoracic imaging. Images have been annotated for PTX presence, PTX size (maximal dehiscence of visceral pleura from the thoracic wall, subgroups according to < 1 cm, 1–2 cm, > 2 cm) and inserted thoracic tubes. A total of 1476 cases with unilateral PTX and 4782 PTX negative control cases were identified, see Table 1.
Supine chest radiograph basal lung opacities in critically ill patients (SCXR BLO)
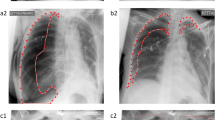
Cohort with a total of 166 patients who received both, an SCXR image in AP view and a CT scan (at least including the basal lung zones) within 90 min without any intervention in between. The cohort is used to differentiate basal lung opacities on SCXR, which are usually difficult to interpret for human readers. Due to the short time interval between SCXR and CT imaging with appropriate clinical indications for reapplication of radiation, there is a shift in this data set to critically ill patients, which predicts pathologies considered in the context of common causes of critical airway disease that are difficult to detect with SCXR alone. Detailed cohort characteristics are described in Kunz et al.32 and Rueckel et al.14.
SCXR images were evaluated by two radiological readers (1 BCR and 1 RR with 6 months of experience in thoracic imaging interpretation) regarding suspected pneumonia. Suspicion was side-separately quantified based on a three-point Likert scale: 0—no pneumonia, 1—possible pneumonia and 2—highly suspected pneumonia. The readers were blinded to the CT data. A consensus of both reading results was formed (in case no consensus could be reached BCR’s decision was considered). In a second reading process, another BCR (also blinded to CT data) evaluated the SCXRs images side-separately for the presence of 0—no pleural effusion, 1—possible pleural effusion and 2—highly suspected pleural effusion. To get a binary decision output for further statistical analysis, suspicion scores were pooled as follows: 1 and 2 were pooled as positive for pneumonia/pleural effusion, representing a sensitive reading; 0 and 1 were pooled and considered to be negative, representing a specific reading. CT scans served as RFS to distinguish consolidations suspicious for pneumonia and pleural effusions from other reasons for basal lung opacities (CT readers were blinded to all clinical information and SCXR results). Quantities of positive cases for pneumonia and/or pleural effusion are shown in Table 2.
Artificial intelligence algorithm
Benchmarking was performed on the convolutional neural network CheXNet (“AI_CheXNet”) that aims to mimic or outperform radiologist’s performance levels, was trained and validated on ChestX-ray14 dataset 20 and originally introduced by Rajpurkar et al.21,22. We used the open-to-public Python implementation by arnoweng from GitHub.com23. As required by the algorithm, DICOM files were converted into PNG format using the Python Library “cv2” (version 4.5.1). All DICOMs were controlled to be saved in negative mode (“bones white”) before conversion to PNG format using “skimage” (version 0.18.1). For those images with DICOM tags “WindowWidth” and “WindowCenter” available, intensities in the range of WindowCenter ± WindowsCenter/2 were compressed to 8-bit and scaled to the range 0 to 255 using “rescale_intensity” from skimage. For all other images, intensities were rescaled from the range of maximum/minimum intensity to 8-bit.
Results quantification and statistical analysis
AI algorithm and reading performance was quantified using receiver operator characteristics (ROC) analysis and calculation of the area under the ROC curve (AUC). Optimized ROC operating points were approximated to the maximum sum of sensitivity and specificity (Youden’s J Statistics) and marked with dots in the corresponding ROC-curves. Diagnostic metrics for the optimized operating points (accuracy [acc], sensitivity [sens], specificity [spec], positive [ppv] and negative predictive values [npv], false positive rate [fpr], false negative rate [fnr]) were calculated and tabularly illustrated. All statistics and graphic illustrations have been performed using open-source programming language R33.
Results
Pneumothorax (PTX)
Our benchmark pipeline can test the AI pneumothorax detection rate in the CXR EU cohort (Fig. 2—[1]) and the SCXR PTX cohort (Fig. 2—[2]).
Benchmarking of pneumothorax detection; [1] shows the results of the CXR EU cohort for all reference standards (RFS I–IV, (A)–(D)) pooled in subgroups of Radiology Residents (RR), Non-Radiology Residents (NRR) and CheXNet classifier “Pneumothorax” – CheXNet classifier “Pneumothorax” was statistically significant outperformed by RR consensus (green curve) and performed worse than NRR consensus (black curve) in all four RFS. Dotted green and black lines show individual reader’s performance levels. Accuracy (acc), sensitivity (sens), specificity (spec), positive predictive value (ppv), negative predictive value (npv), false positive rate (fpr), false negative rate (fnr), close top left (ctl) and area under the curve (AUC; with 95%-confidence intervals) are given for all consensus and CheXNet AI ROCs; [2] shows benchmarking results of the SCXR PTX cohort pooled by unilateral PTX size. (A) Considering PTX positive cases without inserted thoracic tubes CheXNet performed poorly in all subgroups. (B/C) Performance increases notably if the proportion of cases with inserted thoracic tube increases (B) or if PTX positive cases with inserted thoracic tubes are considered exclusively (C).
The CXR EU cohort allows for a direct performance comparison to radiology residents and non-radiology residents on PA CXR that are free of any foreign material (e. g. thoracic tubes). ROC curves are based on the four different RFSs (I–IV, see “Materials and methods” section). In Fig. 2—[1] the red line represents the AI algorithm, the green line the RR consensus (sum of the three individual RR readers) and the black line the NRR consensus (sum of the three individual NRR readers)—individual reader performance is illustrated by the dotted lines. Since pneumothorax detection was basically a yes-or-no-call for the readers, intermediate reading scores were disproportionally underrepresented (reading scores 1, 2 and 3 made up only 0.71% of all answers). The four different ROC curves (Fig. 2—[1], (A)–(D)) therefore show no major differences. For the clinically most relevant RFS IV (most sensitive) CheXNet AI AUC was 0.719, compared to RR consensus AUC 0.964 and NRR consensus AUC 0.837, which means that the AI algorithm performance was exceeded by NRRs (p < 0.05) and outperformed by RRs reader consensus for pneumothorax detection (p < 0.001).
Our SCXR PTX cohort allows for a further evaluation of PTXs depending on size and the presence of thoracic tubes and can additionally give a hint towards performance in SCXR which are more difficult to analyze. Note that corresponding CheXNet performances have already been published 31, but the key results are now uniformly illustrated according to our multi-cohort benchmarking: CheXNet showed major loss of performance in distinguishing PTX positive cases without thoracic tubes (TT) from PTX negative cases with inserted TT (Fig. 2—[2] (A); maximum AUC: 0.593), but had a better performance in detecting PTX positive cases with inserted thoracic tubes among PTX negative cases without thoracic tubes inserted (Fig. 2—[2] (C); maximum AUC: 0.875). This demonstrates the strong effect of confounding thoracic tubes on algorithm performance. Considering all cases, performance was best for bigger PTXs (Fig. 2—[2] (B); PTX > 2 cm AUC: 0.818), but the detection accuracy even for the largest PTX sizes was completely eliminated by the TT-related confounding bias represented by AUCs not significantly exceeding 0.5 (Fig. 2—[2] (A); PTX < 1 cm AUC: 0.593 (95% confidence interval [CI]: 0.542 – 0.644), PTX 1 – 2 cm AUC: 0.483 (CI 0.418–0.548), PTX > 2 cm AUC: 0.550 (CI 0.495–0.605)). For comparison with the performance results of CheXNet as mentioned in the original publication (AUCs) see Table 3.
Pleural effusion and consolidations suspicious of pneumonia
Pleural effusion and consolidation benchmarking is realized with our CXR EU cohort (Fig. 3—[1] and [2]) and our SCXR BLO cohort (Fig. 3—[3]) allowing for an evaluation on both CXRs and SCXRs with BCRs’ CXR assessment (CXR EU cohort) and CT (SCXR BLO cohort) as reference standards.
Benchmarking of pleural effusion and pulmonary infection detection [1]; CheXNet’s performance in pleural effusion detection (classifier “Effusion”) in the CXR EU cohort is displayed for all four RFS (RFS I-IV, (A)–(D)). CheXNet tended to perform better than NRR consensus but worse than RR consensus; [2] Pulmonary infection detection rate in the CXR EU cohort is displayed for all four RFS (A–D). Besides RR and NRR performance, CheXNet classifiers “Consolidation”, “Infiltration” and “Pneumonia” are pooled and displayed. Classifiers “Max” and “Sum” represent the combination of the three individual CheXNet classifiers (maximum output and sum of the outputs). In the most clinically relevant RFS IV classifiers “Consolidation” and “Pneumonia” performed on the level of NRR consensus. Classifier “Infiltration” was statistically significant outperformed by NRR and RR consensus. The combined classifiers did not outperform the individual ones; [3] Performance in the SCXR BLO cohort—(A) In pulmonary infection detection classifiers “Infiltration” and “Pneumonia” tended to exceed reader consensus’ performance (black line). Classifier “Consolidation” performed (not statistically significant) worse than the reader consensus. The combination of the three classifiers did not outperform the reader consensus—(B) Classifier “Effusion” performed slightly better than the reader in pleural effusion detection (not statistically significant).
Performance presentation in the CXR EU cohort is analog to PTX above. In case of pleural effusion and the clinically relevant, most sensitive RFS IV (Fig. 3—[1] (D)) CheXNet classifier “Effusion” (AUC: 0.897) performed better than the NRR consensus (AUC: 0.823, p < 0.01) but performed under the level of RR consensus (AUC: 0.965, p < 0.001). Considering the consolidations suspicious for pneumonia CheXNet (classifiers “Consolidation” and “Pneumonia”) mimicked NRR consensus in RFS IV (Fig. 3—[2](D); CheXNet classifier “Consolidation” AUC: 0.873 (p = 0.756), CheXNet classifier “Pneumonia” AUC: 0.876 (p = 0.663), NRR consensus AUC: 0.865) but was exceeded by RR consensus performance (AUC: 0.939 – comparison to “best” classifier “Pneumonia”: p < 0.01). CheXNet classifier “Infiltration” underperformed with an AUC of 0.737 (comparison to NRR consensus: p < 0.001). The combination of the three CheXNet classifiers did not outperform the individual classifiers (“Maximum” AUC: 0.798, “Sum” AUC: 0.857).
In the SCXR BLO cohort CheXNet classifiers “Infiltration” (p = 0.390) and “Pneumonia” (p = 0.710) non-significantly exceeded the radiology reader consensus for pneumonia suspicious consolidations (Fig. 3—[3] (A); CheXNet classifier “Infiltration” AUC: 0.825; classifier “Pneumonia” AUC: 0.801; reader consensus AUC: 0.782). CheXNet classifier “Consolidation” underperformed with an AUC of 0.673 and was outperformed by the classifier “Infiltration” (p < 0.01). Combining the three classifiers led to performances in between the individual CheXNet classifiers (“Maximum” AUC: 0.704, “Sum” AUC: 0.780). In pleural effusion detection CheXNet classifier “Effusion” non-significantly exceeded the reader’s (BCR) performance (Fig. 3—[3] (B); CheXNet classifier “Effusion” AUC: 0.762; Reader AUC: 0.710, p = 0.336). For comparison with originally published performance results see Table 3.
Pulmonary lesions
Our CXR EU cohort enables benchmarking of pulmonary nodule detection and further grading with respect to potential malignancy (Fig. 4).
Benchmarking of (suspicious) pulmonary lesion detection; Performance results in CXR EU cohort for all four RFS (RFS I–IV) are displayed for pulmonary lesion detection in general (A1–A4) and for suspicious pulmonary lesions when CT was recommended by CXR readers (B1–B4). RR, NRR and classifiers “Nodule” and “Mass” performance is displayed as ROC curves. Classifiers “Max” and “Sum” represent the combination of two CheXNet classifiers (maximum output and sum of the outputs). In the clinically most relevant RFS IV classifier “Nodule” performed slightly better than NRR consensus (A4) and could even beat (not statistically significant) RR consensus AUC in the detection of the potentially suspicious pulmonary lesions (B4). Classifier “Mass” performed slightly better than the NRR consensus detecting potentially suspicious lesions (B4) but slightly underperformed NRR consensus in general lesion detection (A4). The combination of the two classifiers did not outperform the better performing classifier “Nodule” in general lesion detection (A4). Classifier “Maximum” performed slightly better than classifier “Nodule” in the potentially suspicious lesions (B4).
Regarding pulmonary lesions in general, CheXNet classifier “Nodule” mimicked NRR consensus in the most sensitive RFS IV (Fig. 4 [A4]; CheXNet classifier “Nodule” AUC: 0.785; NRR consensus AUC: 0.760, p = 0.562) but was non-significantly exceeded by RR reader consensus performance (AUC: 0.836, p = 0.201). CheXNet classifier “Mass” performed under the level of NRR (p = 0.808) /RR (p < 0.05) consensus and classifier “Nodule” (p = 0.356) with an AUC of 0.750. Combining the two classifiers by considering the maximum values (“Maximum”) and the sum (“Sum”), in both cases led to an AUC of 0.782 which was lower than the AUC of the better performing CheXNet classifier “Nodule”.
Concerning the clinically most relevant potentially malignant nodules (BCR recommended a follow-up computed tomography), CheXNet classifier “Nodule” tended to perform better than the RR (p = 0.793) and NRR (p < 0.05) consensus in RFS IV (Fig. 4 [B4]; CheXNet classifier “Nodule” AUC: 0.851; RR consensus AUC: 0.839; NRR consensus AUC: 0.747). Classifier “Mass” (AUC: 0.800) showed a tendency towards better performance than the NRR consensus (p = 0.285), but (non-significantly) performed under the level of the RR consensus (p = 0.399). The combination of both classifiers resulted in AUCs of 0.854 (“Maximum”) and 0.845 (“Sum”). The “Maximum” therefore performed slightly better (not significantly significant) than individual classifier “Nodule”. For comparison with the results of the original publication see Table 3.
Discussion
CheXNet demonstrated good performance results in the detection of suspicious pulmonary nodules with a tendency to exceed RR and NRR consensus (sum) performance (which might have a relevant clinical impact in early diagnostics), whilst exceeding the AUC of the original publication 21. Solid performance could be shown in the detection of pleural effusions and consolidations suspicious of pneumonia: The algorithm (non-significantly) outperformed the readers in both pathologies in the SCXR BLO cohort, showed a tendency to exceed NRR consensus for pleural effusions in the CXR EU cohort and mimicked NRR consensus in the detection of consolidations suspicious of pneumonia in the CXR EU cohort. Interestingly and potentially of a beneficial clinical impact is the good performance of the algorithm in the detection of basal pneumonia and pleural effusions in the SCXR BLO cohort which is known to be very challenging for human readers. Here, the CheXNet classifier “Infiltration” showed the most promising results. Notably, the same classifier underperformed in the CXR EU cohort in which only CXR in upright positioning were considered. This phenomenon might be explained by the annotation in the training dataset, where it was found to be often associated with atelectasis and effusions20. At this point, the training dataset might have used an unfavorable terminology, which has been controversially discussed34,35. Solid performance results throughout both cohorts were reached by the CheXNet classifier “Pneumonia” which showed better AUCs than in the original publication21. In pneumothorax detection, CheXNet performance showed insufficient performance results in both tested cohorts (CXR EU and SCXR PTX cohort) with smaller calculated AUCs for classifier “Pneumothorax” than originally published21. In the subgroup analysis of our SCXR PTX cohort, we could furthermore observe that the performance correlates positively with the proportion of inserted thoracic tubes in PTX positive images and negatively with the proportion of thoracic tubes inserted in PTX negative control images. We can therefore infer that the underlying publicly available training data for pneumothoraces was insufficient and could partially lead to a misdirected algorithm training for thoracic tubes whilst further annotations are missing. These effects have been previously presented and discussed by Rueckel et al.31,36.
The main strength of our study design with different benchmarking cohorts is the variability of testing different clinically relevant scenarios. The tested algorithm can run several benchmarks one after the other in a sort of benchmarking pipeline. Thus, detection rates of the different pathologies tested are not simply reported as AUC values but can be further differentiated with respect to different subgroups: Depending on patient positioning, applied reference standards, the expression of the pathology and in comparison to differently qualified radiological readers. In the following, we will highlight the advantages and disadvantages of each cohort:
The Emergency Unit Chest Radiograph Cohort (CXR EU) is a powerful cohort that compares AI performance for all the four investigated pathologies with RR and NRR reading performance using the BCR consensus as the reference standard. It is particularly distinguished by its selection exclusively of images from the emergency department, which gives it a very clinically relevant character, as these patients are usually seen for the first time. Since non-radiologists (NRR) are also involved in primary diagnostics, their performance is given special importance as a benchmarking level. Further strengths of the cohort include the high number of cases (563 images) and readers (9 readers), the strong reference standard (BCR readers experienced with up to of 17 years in thoracic imaging) and a statistical workup with different reference standards which also takes general uncertainty and different confidence levels supposedly depending on pathology extent into account. Limitations include: a single-centered reading design with RRs being trained by BCRs, preselection of the cases by an RR (potential small selection bias—clear findings might be overrepresented), case number too low to quantify possible effects of pathology co-occurrences, reader AUC can be influenced by interpolated ROC-parts (result of the rough-staged suspicion scores) and the limitation to the mentioned four pathologies.
The Supine Chest Radiograph Unilateral Pneumothorax Cohort (SCXR PTX) allows testing for weaknesses in algorithm training concerning pneumothorax detection. Its key strength is the subgroup analysis with consideration of the presence of thoracic tube and the size extent of the pneumothorax. If an algorithm was trained solely based on NLP-extracted pathology related image labels (without catheter-/tube-based image labels or in-image annotations), there is a risk that the tube (which is obviously much more prevalent in PTX positive images) is detected rather than the pleural dehiscence line itself 31. In a recent study, Rueckel et al.36 could show that these systematic errors can be partially suppressed and overall performance significantly improved if the AI system was trained with in-image annotations related to the PTX shape. Another noteworthy strength is the cohort size with a total of 6258 cases and numerous cases in every subgroup (see Table 1). Limitations of the cohort include: the single-center study design (only locally used thoracic tubes), other potential imaging confounders are not considered (e. g. other types of catheters such as central venous lines, electrocardiogram-electrodes or other nonannotated or noncontrolled image features), only supine CXR have been included (detection rates might differ in upright PA CXRs).
The Supine Chest Radiograph Basal Lung Opacities (SCXR BLO) cohort is a benchmarking cohort that addresses differentiation of basal consolidations on SCXR images, which is considered very difficult by radiologists with detection accuracies of pneumonia on SCXR being usually lower than in autopsy, bronchoalveolar lavage or CT scans37,38,39,40,41. The main strength of the cohort is that all CXR images were correlated with very timely computed tomography scans (within 90 min) which results in a high-quality reference standard. The cohort consists of a clinically very important group of mainly critically ill patients that are under continuous surveillance. Since morbidity and mortality of hospital-acquired pneumonia is very high42,43,44, early detection of consolidations suspicious for pneumonia can be of extraordinary importance. Limitations of the cohort include the small number of readers (small consensus, no detailed interrater reliability calculation possible), the small number of suspicion scores (AUC calculation of readers is influenced by the interpolation of ROC curves) and the limitation to the findings of pulmonary infection and pleural effusion.
The three cohorts have so far been limited to the detection of four relevant pathologies. Future studies need to broaden the spectrum to also evaluate the accuracy of other parameters of (S)CXR interpretation algorithms. As in this study, the focus should reflect clinical reality (e. g. different projections, different reading settings/reference standards and comparison to different reader groups).
Conclusion
As an example of CXR interpreting AI algorithms, CheXNet shows that the primary published performance results may well differ from the results of an external validation. With our versatile multi-cohort benchmarking, we investigated multiple clinically relevant aspects that might influence algorithm performance, considering different patient positioning, different reference standards and comparison to different medical experts’ performances.
Abbreviations
- (S)CXR:
-
(Supine) chest radiography
- (S)CXRs:
-
(Supine) chest radiographs
- AI:
-
Artificial intelligence
- NLP:
-
Natural language processing
- PA:
-
Posterior-anterior projection
- EU:
-
Emergency unit
- BCR:
-
Board-certified radiologist
- RR:
-
Radiology resident
- NRR:
-
Non-radiology resident
- RFS:
-
Reference standard
- AP:
-
Anterior–posterior projection
- PTX:
-
Pneumothorax
- ROC:
-
Receiver-operating characteristics
- AUC:
-
Area under ROC curve
- ICU:
-
Intensive care unit
- Max:
-
Maximum
- acc:
-
Accuracy
- sens:
-
Sensitivity
- spec:
-
Specificity
- ppv:
-
Positive predictive value
- npv:
-
Negative predictive value
- fpr:
-
False positive rate
- fnr:
-
False negative rate
- ctl:
-
Closest top left
- TT:
-
Thoracic tube
- CI:
-
95% Confidence interval
References
Raoof, S. et al. Interpretation of plain chest roentgenogram. Chest 141, 545–558 (2012).
Gurney, J. W. Why chest radiography became routine. Radiology 195, 245–246 (1995).
Speets, A. M. et al. Chest radiography in general practice: Indications, diagnostic yield and consequences for patient management. Br. J. Gen. Pract. 56, 574–578 (2006).
Martindale, J. L. et al. Diagnosing acute heart failure in the emergency department: A systematic review and meta-analysis. Acad. Emerg. Med. 23, 223–242 (2016).
Hunton, R. Updated concepts in the diagnosis and management of community-acquired pneumonia. JAAPA 32, 18–23 (2019).
Ablordeppey, E. A. et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically Ill patients: A systematic review and meta-analysis. Crit. Care Med. 45, 715–724 (2017).
Levinsky, Y., Mimouni, F. B., Fisher, D. & Ehrlichman, M. Chest radiography of acute paediatric lower respiratory infections: Experience versus interobserver variation. Acta Paediatr. 102, e310-314 (2013).
Eisenhuber, E., Schaefer-Prokop, C. M., Prosch, H. & Schima, W. Bedside chest radiography. Respir. Care 57, 427–443 (2012).
Potchen, E. J. et al. Measuring performance in chest radiography. Radiology 217, 456–459 (2000).
Lakhani, P. & Sundaram, B. Deep learning at chest radiography: Automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 284, 574–582 (2017).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316, 2402–2410 (2016).
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577, 89–94 (2020).
Rueckel, J. et al. Artificial intelligence algorithm detecting lung infection in supine chest radiographs of critically Ill patients with a diagnostic accuracy similar to board-certified radiologists. Crit. Care Med. 48, e574–e583 (2020).
Chassagnon, G., Vakalopoulou, M., Paragios, N. & Revel, M.-P. Artificial intelligence applications for thoracic imaging. Eur. J. Radiol. 123, 108774 (2020).
Fontanellaz, M. et al. A deep-learning diagnostic support system for the detection of COVID-19 using chest radiographs: A multireader validation study. Invest. Radiol. 56, 348–356 (2021).
Christe, A. et al. Computer-aided diagnosis of pulmonary fibrosis using deep learning and CT images. Invest. Radiol. 54, 627–632 (2019).
Rudolph, J. et al. Artificial intelligence in chest radiography reporting accuracy: Added clinical value in the emergency unit setting without 24/7 radiology coverage. Invest. Radiol. 57, 90–98 (2022).
Irvin, J. et al. CheXpert: A Large Chest Radiograph Dataset with Uncertainty Labels and Expert Comparison. arXiv:1901.07031 [cs, eess] (2019).
Wang, X. et al. ChestX-ray8: Hospital-scale Chest X-ray Database and Benchmarks on Weakly-Supervised Classification and Localization of Common Thorax Diseases. 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 3462–3471 (2017) https://doi.org/10.1109/CVPR.2017.369.
Rajpurkar, P. et al. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv:1711.05225 [cs, stat] (2017).
Rajpurkar, P. et al. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 15, e1002686 (2018).
Arnoweng. A pytorch reimplementation of CheXNet.
Kienzl, D., Prosch, H., Töpker, M. & Herold, C. Imaging of non-cardiac, non-traumatic causes of acute chest pain. Eur. J. Radiol. 81, 3669–3674 (2012).
Regunath, H. & Oba, Y. Community-Acquired Pneumonia. in StatPearls (StatPearls Publishing, 2022).
Marel, M., Zrůstová, M., Stasný, B. & Light, R. W. The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest 104, 1486–1489 (1993).
Bobbio, A. et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax 70, 653–658 (2015).
Loverdos, K., Fotiadis, A., Kontogianni, C., Iliopoulou, M. & Gaga, M. Lung nodules: A comprehensive review on current approach and management. Ann. Thorac. Med. 14, 226–238 (2019).
Ferkol, T. & Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 11, 404–406 (2014).
Rudolph, J. et al. Interpretation of thoracic radiography shows large discrepancies depending on the qualification of the physician-quantitative evaluation of interobserver agreement in a representative Emergency Department Scenario. Diagnostics (Basel) 11, 1868 (2021).
Rueckel, J. et al. Impact of confounding thoracic tubes and pleural dehiscence extent on artificial intelligence pneumothorax detection in chest radiographs. Invest. Radiol. 55, 792–798 (2020).
Kunz, W. G. et al. The value of supine chest X-ray in the diagnosis of pneumonia in the Basal Lung Zones. Acad. Radiol. 25, 1252–1256 (2018).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2020).
Hall, F. M. Fleischner Society glossary of terms: Infiltrates. Radiology 248, 1083 (2008).
Hansell, D. M. et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 246, 697–722 (2008).
Rueckel, J. et al. Pneumothorax detection in chest radiographs: Optimizing artificial intelligence system for accuracy and confounding bias reduction using in-image annotations in algorithm training. Eur. Radiol. https://doi.org/10.1007/s00330-021-07833-w (2021).
Barloon, T. J., Galvin, J. R., Mori, M., Stanford, W. & Gingrich, R. D. High-resolution ultrafast chest CT in the clinical management of febrile bone marrow transplant patients with normal or nonspecific chest roentgenograms. Chest 99, 928–933 (1991).
Fàbregas, N. et al. Clinical diagnosis of ventilator associated pneumonia revisited: Comparative validation using immediate post-mortem lung biopsies. Thorax 54, 867–873 (1999).
Lefcoe, M. S., Fox, G. A., Leasa, D. J., Sparrow, R. K. & McCormack, D. G. Accuracy of portable chest radiography in the critical care setting. Diagnosis of pneumonia based on quantitative cultures obtained from protected brush catheter. Chest 105, 885–887 (1994).
Wunderink, R. G. et al. The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest 101, 458–463 (1992).
Weber, C. et al. Importance of digital thoracic radiography in the diagnosis of pulmonary infiltrates in patients with bone marrow transplantation during aplasia. Rofo 171, 294–301 (1999).
Roquilly, A. et al. Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir. Med. 7, 710–720 (2019).
Saleem, Z. et al. Point prevalence surveys of health-care-associated infections: a systematic review. Pathog. Glob. Health 113, 191–205 (2019).
Ceccato, A. et al. Lymphocytopenia as a predictor of mortality in patients with ICU-acquired pneumonia. J. Clin. Med. 8, E843 (2019).
Funding
Open Access funding enabled and organized by Projekt DEAL. Department of Radiology, University Hospital, LMU Munich received funding (research cooperation) from Siemens Healthcare GmbH.
Author information
Authors and Affiliations
Contributions
J.Rud., B.O.S. and J.Rue. developed the study design. B.O.S. and J.Ri. supervised the study. J.Rud., J.Rue., W.G.K. and L.T. identified image data. B.S. and M.I. assisted with the technical implementation of the AI solution. B.O.S., J.D., W.G.K., V.K., N.F., V.S., S.G., M.F., N.B. and M.J. radiologically assessed the image data. J.Rud. and J.Rue. statistically analyzed and graphically illustrated the results. J.Rud. wrote the initial manuscript draft. J.Rue. and B.S. assisted with manuscript preparation. B.S., N.F., V.K., V.S., S.G., L.T., B.F.H., N.M., M.F., N.B., M.J., J.D., W.G.K., J.Ri., M.I., B.O.S. and J.Rue. critically reviewed the manuscript. All co-authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
B. O. Sabel and J. Rueckel received compensation by Siemens Healthcare GmbH for lectures at conferences. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rudolph, J., Schachtner, B., Fink, N. et al. Clinically focused multi-cohort benchmarking as a tool for external validation of artificial intelligence algorithm performance in basic chest radiography analysis. Sci Rep 12, 12764 (2022). https://doi.org/10.1038/s41598-022-16514-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16514-7
- Springer Nature Limited