Abstract
Anti-programmed death-1 (PD-1) blockade is a standard treatment for advanced non-small-cell lung cancer (NSCLC). However, no appropriate modality exists for monitoring its therapeutic response immediately after initiation. Therefore, we aimed to elucidate the clinical relevance of 18F-FDG PET/CT versus CT in predicting the response to PD-1 blockade in the early phase. This prospective study included a total of 54 NSCLC patients. 18F-FDG PET/CT was performed at 4 weeks and 9 weeks after PD-1 blockade monotherapy. Maximum standardized uptake values (SULmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were evaluated. Among all patients, partial metabolic response and progressive metabolic disease after PD-1 blockade were observed in 35.2% and 11.1% on SULmax, 22.2% and 51.8% on MTV, and 27.8% and 46.3% on TLG, respectively, whereas a partial response (PR) and progressive disease (PD), respectively, based on RECIST v1.1 were recognized in 35.2% and 35.2%, respectively. The predictive probability of PR (MTV: 57.9% vs. 21.1%, p = 0.044; TLG: 63.2% vs. 21.1%, p = 0.020) and PD (MTV: 78.9% vs. 47.3%, p = 0.002; TLG: 73.7% vs. 21.1%, p = 0.007) detected based on RECIST at 4 weeks after PD-1 blockade initiation was significantly higher using MTV or TLG on 18F-FDG uptake than on CT. Multivariate analysis revealed that metabolic response by MTV or TLG at 4 weeks was an independent factor for response to PD-1 blockade treatment. Metabolic assessment by MTV or TLG was superior to morphological changes on CT for predicting the therapeutic response and survival at 4 weeks after PD-1 blockade.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1)/PD ligand-1 (PD-L1) are widely used to treat human neoplasms. Recent studies on a limited population have shown that the therapeutic efficacy of ICIs was closely associated with long-term survival1,2. In particular, approximately 20% of patients with stage IV non-small-cell lung cancer (NSCLC) survived for > 5 years after PD-1 blockade monotherapy1,2. Approximately 80% of NSCLC patients exhibit a partial (PR) or complete (CR) response in the relatively early phase after PD-1 blockade initiation1,2. To establish the effectiveness of PD-1 blockade, its response should be detected as early as possible; however, it is challenging to distinguish responders from non-responders within approximately 9 weeks of treatment using computed tomography (CT) for morphological assessment3,4.
2-Deoxy-2-[fluorine-18]-fluoro-d-glucose (18F-FDG) positron emission tomography (PET)/ CT is useful for distinguishing benign from malignant lesions, and could predict early response to targeting agent in advanced NSCLC patients5,6F-FDG uptake within tumor cells is determined by the presence of increased glucose metabolism and hypoxia5, whereas upregulation of PD-L1 expression is partially regulated by tumor hypoxia7,8. Several studies have demonstrated that the accumulation level of 18F-FDG is closely associated with PD-L1 expression in NSCLC patients9,10,11,12.
Recently, it was proposed that 18F-FDG PET could predict the response to PD-1 blockade at the early phase within 2 or 4 weeks, whereas CT failed to detect early response4,13. However, previous reports were preliminary and could not confirm the therapeutic significance of 18F-FDG PET for early monitoring of ICI treatment. To date, no established biomarkers for predicting the efficacy of PD-1 blockade exist, although PD-L1 expression and tumor mutation burden (TMB) weakly correlate with the therapeutic efficacy of ICIs14. Although several devices, such as CT, have been used to evaluate tumor shrinkage, 18F-FDG PET has been identified as the best modality to reflect tumor metabolic changes in the early phase4. As the assessment metrics of 18F-FDG uptake, maximum standardized uptake values (SULmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are frequently used, with MTV or TLG suggested as better biomarkers reflecting the tumor burden than SULmax15.
Here, we aimed to elucidate the possibility of therapeutic monitoring using 18F-FDG PET at 4 and 9 weeks after PD-1 blockade in advanced NSCLC patients. Through a multi-institutional study using different PET scanners, we attempted to confirm whether 18F-FDG PET could be useful for detecting responders or non-responders in the early phase after ICI treatment.
Results
Patient characteristics
Fifty-eight patients were initially enrolled, but four were excluded because of inadequate PET study and data unavailability. A total of 54 patients (38 men, 26 women; median age, 73 years; age range, 42–84 years) were analyzed. Their demographics are presented in Table 1. Forty-two (77.8%) patients had a smoking history, and PS was 0, 1, and 2 in 12 (22.2%), 31 (57.4%), and 11 (20.4%) patients, respectively. Regarding the histological type, adenocarcinoma (AD) was observed in 29 (53.7%), squamous cell carcinoma (SCC) in 15 (27.8%), and other in 10 (18.5%). As treatment lines, 22 (40.7%) patients received pembrolizumab in first-line and 32 (59.3%) in second or more lines (nivolumab in 25 patients, pembrolizumab in 6, and atezolizumab in 1). Among the 54 patients, 19, 16, and 19 exhibited PR, stable disease (SD), and progressive disease (PD), respectively. The objective response was 35.2% (50.0% in first-line; 25.0% in second or more lines).
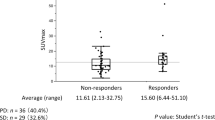
PET study and response evaluation
There were 100 assessable target lesions, including 47 pulmonary, 37 metastatic lymph nodes, 6 pleural, 8 metastatic bone, 1 adrenal metastasis, and 1 liver metastasis. Median MTV, TLG, SULpeak, and SULmax values before PD-1 blockade treatment were 19.2 (range, 2.8–95.2) cm3, 85.2 (range, 8.2–972.2) gcm3/mL, 8.3 (range, 3.9–17.0), and 8.6 (range, 2.5–22.7), respectively. The assessable lesion for SUL was defined as that with the highest 18F-FDG uptake. Baseline SULmax values were significantly correlated with MTV (r = 0.357, p = 0.008) and TLG (r = 0.441, p < 0.001) values. The median period from baseline 18F-FDG PET/CT to PD-1 blockade monotherapy initiation was 9 (range, 1–28) days and that from PD-1 blockade monotherapy to first (4 weeks) and second (9 weeks) performance of 18F-FDG PET/CT was 28 and 63 days, respectively. Fifty-four patients underwent 18F-FDG PET/CT at 4 weeks; however, 6 did not receive 18F-FDG PET/CT at 9 weeks because of PD-1 blockade cessation due to PD and/or severe adverse events.
Among the 54 patients, PR, SD, and PD detected by early CT (4 weeks) were noted in 19 (35.2%), 16 (29.6%), and 19 (35.2%) patients, respectively. PMR, SMD, and PMD detected by early PET (4 weeks) were observed in 35.2% (19/54), 53.7% (29/54), and 11.1% (6/54), respectively, on SULmax; 27.8% (15/54), 62.9% (34/54), and 9.2% (5/54), respectively, on SULpeak; 22.2% (12/54), 25.9% (14/54), and 51.8% (28/54) on MTV; and 27.8% (15/54), 25.9% (14/54), and 46.3% (25/54) on TLG. On early CT scan, PR, SD, and PD were 9.3% (5/54), 70.3% (38/54), and 20.4% (11/54), respectively.
The concordance rate of tumor response between confirmed overall objective response (OOR) by RECIST and that at 4 and 9 weeks after PD-1 blockade treatment are shown in Fig. 1. The concordance rate of PR (MTV: 57.9% vs. 21.1%, p = 0.044, TLG; 63.2% vs. 21.1%, p = 0.020) and PD (MTV: 78.9% vs. 47.3%, p = 0.002; TLG: 73.7% vs. 21.1%, p = 0.007) detected based on RECIST at 4 weeks after PD-1 blockade initiation was significantly higher in MTV or TLG than in CT (Fig. 1A). SULmax and SULpeak were significantly superior to CT in the concordance rate of PR at 4 weeks (63.2% vs. 21.1%, p = 0.020) whereas they were inferior to CT in the concordance rate of PD at 4 weeks (10.5% vs. 47.3%, p = 0.029) (Fig. 1A). Although the concordance rate of PR (94.7% vs. 73.6%, p = 0.179) and PD (92.8% vs. 71.4%, p = 0.325) confirmed based on RECIST at 9 weeks was higher in MTV or TLG than in CT, it was not statistically significant (Fig. 1B). Next, concordance rates according to treatment lines (first-line and second or more line settings) and histological type were also examined (Fig. C, online only). The concordance rate of PR and PD in the 18F-FDG uptake (SULmax, MTV and TLG) at 4 weeks tended to be high in patients with non-AC compared to those with AC, without statistical significance (Figs. C1, C3). Moreover, the concordance rate of PR in the 18F-FDG uptake (SULmax, MTV and TLG) at 4 weeks exhibited a significantly higher in first-line setting than in second line or more (Figs. C5, C7).
Concordance rate between Response by RECIST and tumor response at 4 weeks (A) and 9 weeks (B) after PD-1 blockade. (A) Among the 19 patients with PR based on RECIST, PMR at 4 weeks after PD-1 blockade by SULmax, SULpeak, MTV, and TLG was observed in 12 (63.2%), 12 (63.2%), 11 (57.9%), and 12 (63.2%), respectively, and CT at 4 weeks confirmed PR in 4 of 19 patients (21.1%). In the 19 patients with PD based on RECIST, PMD at 4 weeks by SULmax, SULpeak, MTV, and TLG was noted in 2 (10.5%), 2 (10.5%), 15 (78.9%), and 14 (73.7%), respectively, whereas CT at 4 weeks identified PD in 9 of 19 patients (47.3%). The predictive probability of PR and PD according to RECIST at 4 weeks after PD-1 blockade administration was significantly higher in MTV and TLG than in CT, whereas, the predictive probability of SD after its treatment was significantly higher in CT than in MTV and TLG. (B) Moreover, PMR at 9 weeks by SULmax, SULpeak, MTV, and TLG was observed in 15 (78.9%), 13 (68.4%), 18 (94.7%), and 18 (94.7%) of 19 patients with PR according to RECIST, respectively, and CT at 9 weeks confirmed PR in 10 of 14 patients (73.6%). Among the 19 patients with PD according to RECIST, PMD at 9 weeks by SULmax, SULpeak, MTV, and TLG was identified in 7 (50.0%), 6 (42.8%), 13 (92.8%), and 13 (92.8%), respectively, and CT at 9 weeks displayed PD in 10 of 14 patients (71.4%). The predictive probability of PR and PD according to RECIST at 9 weeks after PD-1 blockade administration was significantly higher in CT and SULmax than in MTV and TLG. *Statistically significant difference.
Out of 54 patients, 2 (3.7%) patients experienced pseudoprogression. One patient with confirmed PR based on RECIST, had PMD by MTV and TLG, PD by CT scan, and SMD by SULmax and SULpeak at 4 weeks after first-line pembrolizumab, because of markedly increased primary site. However, PMR was observed by MTV, TLG, SUV, and SUV on 18F-FDG PET at 9 weeks, similar to PR by CT scan. Although the other patient with confirmed PR based on RECIST also experienced psuedoprogression within 4 weeks after nivolumab initiation as second line setting, the objective response by CT at 4 and 9 weeks exhibited SD, that by MTV and TLG showed SMD at 4 weeks and PMR at 9 weeks, and that by SULmax and SULpeak depicted PMR at 4 and 9 weeks.
Survival analysis according to 18F-FDG uptake
The median follow-up period for all patients was 296 days (range 75–741). Forty patients experienced disease recurrence, and 21 died. The median PFS and OS were 174 days and not reached, respectively. Kaplan–Meier curves of PFS and OS according to CT and 18F-FDG uptake at 4 and 9 weeks after PD-1 blockade among all patients are shown in Fig. 2. A significant difference in PFS and OS was identified between PMD and non-PMD defined according to 18F-FDG uptake by MTV and TLG at 4 and 9 weeks, but not at 4 weeks but 9 weeks on PET by SULmax (Fig. 2). Next, the outcome of 38 patients with SD on CT scan at 4 weeks after PD-1 blockade initiation were analyzed according to metabolic response by 18F-FDG uptake (PMD vs. non-PMD) (Fig. 3). A significant difference in PFS and OS was identified between PMD and non-PMD based on MTV (Fig. 3A) and TLG (Fig. 3B) at 4 and 9 weeks, but not on PET by SULmax (Fig. 3C). Results of the univariate and multivariate analyses are presented in Table 2. In multivariate analysis, MTV and TLG on 18F-FDG uptake at 4 weeks after PD-1 blockade were confirmed as independent predictive factors.
Kaplan–Meier curve of PFS and OS according to CT and 18F-FDG uptake at 4 and 9 weeks after PD-1 blockade initiation in all patients (n = 54). A significant difference in PFS, but not in OS, was noted between PD and non-PD defined according to CT at 4 and 9 weeks (A). A significant difference in PFS and OS was identified between PMD and non-PMD defined according to the 18F-FDG uptake by SULmax (B) and SULpeak (C) at 9 weeks, but not at 4 weeks. A significant difference in PFS and OS was identified between PMD and non-PMD defined according to 18F-FDG uptake by MTV (D) and TLG (E) at 4 and 9 weeks.
Kaplan–Meier curve of PFS and OS according to 18F-FDG uptake at 4 and 9 weeks in 38 patients with SD on CT scan at 4 weeks after PD-1 blockade initiation. A significant difference in PFS and OS was noted between PMD and non-PMD based on MTV (A) and TLG (B) at 4 and 9 weeks, but not SULmax at 4 and 9 weeks (C). A significant difference in the PFS and OS according to SULpeak at 9 weeks was observed between PMD and non-PMD, but no at 4 weeks (D).
Discussion
This prospective study compared CT from PET for the assessment of early response after PD-1 blockade monotherapy in advanced NSCLC patients. The concordance rate of PR and PD using RECIST at 4 weeks after PD-1 blockade was significantly higher using 18F-FDG uptake by MTV or TLG than by using morphological changes on CT. In addition, PMD by MTV or TLG at 4 weeks could significantly predict worse survival after PD-1 blockade administration. At least 9 weeks after its administration, 18F-FDG uptake by MTV or TLG accurately predicted the tumor response confirmed based on RECIST and survival after PD-1 blockade, compared to the morphological assessment by CT. In this study, we also found that the concordance rate of PR and PD detected based on RECIST at early phase after PD-1 blockade tended to be higher in patients with non-AC or first-line setting. In particular, metabolic response by MTV and TLG could differentiate responder from non-responder in 38 patients with SD on CT at 4 weeks after PD-1 blockade administration.
Recently, Park et al.16 retrospectively evaluated early response assessment after immunotherapy using 18F-FDG PET/CT in 24 advanced NSCLC patients. They presented the case of 5 patients with CMR or PMR who had a clinical benefit after two or three cycles of ICI treatment, whereas none of the 14 patients with PMD experienced any clinical benefit16. It was speculated that 5 patients with SMD needed meticulous follow-up because of varying clinical benefits16. Castello et al.17 prospectively compared morphological and metabolic responses at 8 or 9 weeks after PD-1 blockade using 18F-FDG PET/CT in 35 NSCLC patients. Although they assessed the metabolic response by the SUV value, 3 (75%) of 4 patients with PR had PMR or CMR, 14 (87%) of 16 patients with PD exhibited PMD, and SMD was observed in 4 (26%) of 15 patients with SD17. In patients with SD on CT, metabolic response by PET could discriminate those with longer survival17. 18F-FDG uptake based on PERCIST or immunotherapy-modified PERCIST accurately reflects the overall metabolic response and survival after PD-1 immunotherapy in NSCLC patients4,14,18.
Currently, PD-L1 is considered a rough biomarker for the therapeutic prediction of PD-1 blockade, and promising markers such as TMB or tumor-infiltrating lymphocytes fail to identify the progression to ICI treatment. Thus, the presence of responders and their progression should be identified as early as possible after PD-1 blockade to predict long-term responders. In our study, 18F-FDG uptake on PET yielded a higher predictive value to achieve PR at 4 weeks in first-line setting or histology with non-AC compared to CT. However, it remains unclear about its detailed mechanisms, thus, further investigation is warranted to elucidate the results of our study by using large –sample size.
Rossi et al.19 compared between 18F-FDG and CT-based criteria as response assessment at 8 weeks after nivolumab in 48 NSCLC patients and reported a low overall concordance between CT-based and PET-based responses, but PMR assessed by PET predicted longer OS than CT-based PR. In their study, neither MTV nor TLG but SUVpeak was used to assess the metabolic response, and early response was not investigated, as we did in this study. Our results indicated that the concordance rate by SULmax was apparently inferior to that by MTV or TLG, and there was no significant difference between metabolic response by SULmax and morphological response by CT. However, MTV or TLG was identified as a significant marker to predict the tumor response and survival in the early phase, such as 4 weeks after PD-1 blockade, compared to CT. Thus, SULmax on PET may not be suitable for early detection of ICI response. The present study includes the patients receiving PD-1 blockade as first-line and second-line or more setting, however, previous investigations discussed the therapeutic monitoring of 18F-FDG PET after immunotherapy in patients with previously treated NSCLC4,13,16. The added value of our study demonstrated that the tumor response detected by early PET is useful for the therapeutic prediction of first-line pembrolizumab, moreover, the therapeutic significance of monitoring by early PET is also different according to the histological types of NSCLC. Nowadays, PD-1 blockade is generally established treatment as first-line setting against the advanced NSCLC without any drive gene mutations. Compared to previous studies reporting the monitoring by PET after ICIs administration, the data of first-line pembrolizumab in our study is helpful for our clinical practice. Moreover, our analysis was on a per patient basis and not on a per lesion basis. As several lesions in the same patients may depict different 18F-FDG uptake, the therapeutic monitoring by 18F-FDG uptake based on a per patient basis would be helpful for evaluating the response and survival of ICIs treatment.
Although several investigations demonstrated that baseline 18F-FDG PET could predict the outcome of PD-1 immunotherapy in NSCLC patients, it is difficult to discriminate responders from non-responders or PD from non-PD patients by baseline 18F-FDG uptake20,21,22. Because 18F-FDG uptake was assessed according to different PET machines in individual institutions, it may not be consistent at baseline. Therefore, we did not explore the efficacy and survival of PD-1 blockade based on baseline 18F-FDG uptake.
Recently, Lopci et al.23 described the new guidelines of 18F-FDG PET imaging during immunotherapy treatment in patients with solid tumors. To identify pseudoprogressive patients after immunotherapy, the refinement of standard response evaluation guidelines is needed, thus, immune-related response criteria (irRC), immune RECIST (iRECIST) and immune-modified RECIST (irRECIST) for solid tumors were proposed23. However, there are no established immunotherapy guidelines to categorize the therapeutic response of patients with pseudoprogressive disease (PPD). The interpretation of 18F-FDG PET should take into account PPD during ICIs treatment. In the current study, we found two patients with PPD (3.7%; 2 of 54 patients). SULmax and SULpeak were useful to detect the exact response at 4 weeks after ICIs administration in one of 2 patients with PPD, whereas, it was difficult to identify the correct response at early phase by MTV and TLG on PET. In these two patients, 18F-FDG PET imaging could detect the true response based on RECIST at 9 weeks after PD-1 blockade administration. However, it remains unclear whether 18F-FDG uptake on PET could be helpful for the detection of PPD. In our study, the response evaluation by PET was investigated based on the RECIST, however, there are several new immunotherapy guidelines such as irRC, iRECIST and irRECIST. Although we tried to analyze our data using iRECIST, the results of response evaluation after immunotherapy were not different between RECIST and iRECIST (data not shown). Therefore, further investigation is warranted to establish a new response guideline during immunotherapy aside from the current guidelines.
There are several limitations in our studies. In this study, firstly, the SULs were not harmonized between the PET scanners. However, as these were devices of almost the same generation of the same manufacturer, we believe that changes before and after treatment of SULs, MTV, and TLG were captured to some extent in each facility. Our results warrant for large-scale multi-center research on harmonizing SULs of each PET scanner. Second, our sample includes heterogeneous populations such as AC and non-AC or first-line and second-line settings. As the concordance rate of objective response was different according to histology and treatment lines, further study should be focused on the selected patients. In the present study, 6 (20.6%) of 29 patients with AC yielded positive epidermal growth factor (EGFR) mutation and one patients had anaplastic lymphoma kinase (ALK)-echinoderm microtubule-associated protein-like 4 (EML4) fusion gene. Five of 7 patients with these driver mutations were confirmed as PD in second-line setting (data not shown). The AC patients harboring EGFR mutations are low sensitive to ICs and were identified as lower 18F-FDG uptake on PET than wild-type.24 This may bias the different concordance rate between AC and non-AC. Finally, it is unknown if morphological assessment with RECIST is suitable modality to evaluate response to ICI therapy4,13. The aim of our study is to compare PET with CT in the assessment of tumor response at early phase after PD-1 blockade initiation. However, we believe that metabolic tumor response by PET may be better to predict the response and outcome of ICIs compared to CT. Moreover, the optimal detection by CT scan in PPD after ICIs initiation is difficult, but, the therapeutic monitoring by 18F-FDG uptake on PET also seemed to yield some limitations for the early detection of PPD. Further study is warranted to develop the modality to confirm the presence of PPD at early phase after ICIs initiation.
In conclusion, metabolic assessment by MTV or TLG was useful in predicting an early therapeutic response and survival after PD-1 blockade, compared to morphological changes on CT, specially, in patients with non-AC or first-line setting. 18F-FDG uptake may be a promising biomarker for predicting the therapeutic efficacy of ICIs; thus, it may contribute to individualized treatment planning in clinical practice.
Methods
Patients
This prospective study enrolled advanced NSCLC patients who received PD-1 blockade monotherapy at multiple institutions between January 2019 and October 2020. The inclusion criteria were (a) pathologically confirmed NSCLC; (b) candidate for PD-1 blockade monotherapy such as nivolumab, pembrolizumab or atezolizumab in first-, second- or more lines; (c) performance status (PS) on the Eastern Cooperative Oncology Group of 0–2; (d) 18F-FDG PET/CT imaging scheduled within 4 weeks before the first cycle of PD-1 blockade monotherapy, and (e) possessing adequate organ functions. The exclusion criteria were (a) evidence of concurrent cancer, (b) uncontrolled diabetes mellitus, (c) interstitial pneumonia or pulmonary fibrosis, and (d) active infection requiring antibiotic therapy. Baseline 18F-FDG PET/CT was performed as part of the disease evaluation workup before initiating PD-1 blockade monotherapy. Post-treatment PET/CT was needed at 4 and 9 weeks after the first cycle of PD-1 blockade. The protocol required that both pre- and post-treatment PET-CT be performed using the same scanner (Fig. A, online only).
This study was approved by the institutional review board (Saitama Medical University) and conducted according to the Declaration of Helsinki. All patients provided written informed consent before participation and were able to withdraw from the study at any time. This trial was registered in Japan Registry of Clinical Trials (jRCTs031180036) on 01/11/2018.
PET imaging and data analysis
Patients fasted for at least 6 h before 18F-FDG administration for PET, performed using a PET/CT scanner. Three-dimensional data acquisition was initiated 60.0 ± 8.1 min after FDG injection. Attenuation-corrected transverse images obtained with 18F-FDG were reconstructed with the conditions of clinical settings at the institutions. Imaging parameter for PET/CT scanner at different institutions are listed in Table A1 (online only).
For semiquantitative analysis, the standardized uptake value (SUV) in lean body mass (LBM) corrected for the Japanese population was obtained based on the injected dosage of 18F-FDG, patient’s bodyweight, and cross-calibration factor between PET and the dose calibrator. SUV correction by LBM was based on the literature25. The SUL and Japanese-corrected LBM (JLBM) are defined as follows:
CT for initial staging was performed with an intravenous contrast medium, and board-certified radiologists interpreted the images. We used RAVAT software (Nihon Medi-physics Co. Ltd., Japan) on a Windows workstation to semi-automatically calculate the maximum SUL (SULmax), SULpeak, MTV, and TLG, defined as MTV multiplied by SULmean, of each lesion using SUL thresholds obtained by the SUL in the liver VOI. Each threshold was defined as the average of 1.5 × SUL (SULmean) plus 2 × SD of SUL in the liver26,27. These SUL thresholds were the optimum values for generating a 3D VOI in which the whole tumor mass was completely enclosed in all cases using the CT image as the reference. Regions of activity other than tumors, including myocardium, gastro-intestinal tracts, kidneys, and urinary tracts, were eliminated manually according to the orientation provided by the board-certified nuclear medicine physician.
In this study, SULs between facilities and between devices were not harmonized.
Efficacy assessment
The confirmed tumor response was assessed according to the findings in CT imaging at 9 weeks interpreted using the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v. 1.1). PET-based tumor response was defined according to the PET Evaluation Criteria in Solid Tumors (PERCIST) guidelines27: complete metabolic response (CMR), complete resolution of 18F-FDG uptake within the target lesion; partial metabolic response (PMR), ≥ 30% decrease in 18F-FDG uptake in the target tumor; progressive metabolic disease (PMD), ≥ 30% increase in 18F-FDG uptake in the target tumor or the advent of new 18F-FDG avid lesions; stable metabolic disease (SMD), neither CMR, PMR, nor PMD.
Statistical analysis
Statistical analyses were performed using Student’s t-test and χ2 test for continuous and categorical variables, respectively. Statistical significance was set at p < 0.05. Correlations between SULmax, MTV, and TLG on 18F-FDG uptake were analyzed using Pearson’s rank test. Univariate and multivariate analyses of the relationship between scoring by 18F-FDG uptake and different variables were performed using logistic regression analysis. Progression-free survival (PFS) was defined as the time from initial immunotherapy to disease progression or death; OS was defined as the time from initial immunotherapy to death from any cause. The Kaplan–Meier method was used to estimate survival as a function of time, and survival differences were analyzed using log-rank test. The progression of disease for survival analysis was defined as progression in imaging.
Metabolic responses at 4 and 9 weeks after the injection of PD-1 blockade were evaluated according to the response on PET25. All statistical analyses were performed using GraphPad Prism software (v.8.0; GraphPad Software, San Diego, CA, USA) and JMP 14.0 (SAS Institute Inc., Cary, North Carolina, USA).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Gettinger, S. et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J. Clin. Oncol. 36, 1675–1684 (2018).
Garon, E. B. et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 37, 2518–2527 (2019).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Kaira, K. et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 45, 56–66 (2018).
Kaira, K. et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J. Clin. Oncol. 28, 3746–3753 (2010).
Sunaga, N. et al. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer 59, 203–210 (2008).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Chang, Y. L. et al. High co-expression of PD-L1 and HIF-1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur. J. Cancer 60, 125–135 (2016).
Takada, K. et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 6, 2552–2561 (2017).
Kasahara, N. et al. Correlation of tumor-related immunity with 18F-FDG-PET in pulmonary squamous-cell carcinoma. Lung Cancer 119, 71–77 (2018).
Kaira, K. et al. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur. J. Cancer 101, 181–190 (2018).
Zhang, M. et al. Prognostic significance of PD-L1 expression and 18F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget 8, 51630–51640 (2017).
Umeda, Y. et al. Predictive value of integrated 18F-FDG PET/MRI in the early response to nivolumab in patients with previously treated non-small cell lung cancer. J. Immunother. Cancer 8, e000349 (2020).
Ready, N. et al. First-line Nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): Outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J. Clin. Oncol. 37, 992–1000 (2019).
Im, H. J. et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: A meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 42, 241–251 (2015).
Park, S. et al. Response evaluation after immunotherapy in NSCLC: Early response assessment using FDG PET/CT. Medicine 99, e23815 (2020).
Castello, A. et al. Comparison of metabolic and morphological response criteria for early prediction of response and survival in NSCLC patients treated with anti-PD-1/PD-L1. Front. Oncol. 10, 1090 (2020).
Ayati, N. et al. Response evaluation and survival prediction following PD–1 immunotherapy in patients with non-small-cell lung cancer: comparison of assessment methods. J. Nucl. Med. 62, 926–933 (2021).
Rossi, G. et al. Comparison between 18F-FDG PET-based and CT-based criteria in non-small cell lung cancer patients treated with nivolumab. J. Nucl. Med. 61, 990–998 (2020).
Takada, K. et al. 18F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci. Rep. 9, 13362 (2019).
Hashimoto, K. et al. Potential of FDG-PET as prognostic significance after anti-PD-1 antibody against patients with previously treated non-small cell lung cancer. J. Clin. Med. 9, 725 (2020).
Yamaguchi, O. et al. Tumor metabolic volume by 18F-FDG-PET as a prognostic predictor of first-line pembrolizumab for NSCLC patients with PD-L1 ≥ 50. Sci. Rep. 10, 14990 (2020).
Lopci, E. et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended used of [18F] FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 49, 2323–2341 (2022).
Ly, Z. et al. Value of F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: a retrospective analysis of 849 Chines patients. Eur. J. Nucl. Med. Mol. Imaging 45, 735–750 (2018).
Zasadny, K. R. et al. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: Variations with body weight and a method for correction. Radiology 189, 847–850 (1993).
Fencl, P. et al. The analysis of factors affecting the threshold on repeated 18F-FDG-PET/CT investigations measured by the PERCIST protocol in patients with esophageal carcinoma. Nucl. Med. Commun. 33, 1188–1194 (2012).
Wahl, R. L. et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1), 122S-S150 (2009).
Acknowledgements
The authors thank the patients, their families, the coordinators, and study investigators. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study is funded by Nihon Medi-Physics Co.,Ltd. The study drug, 18F-FDG, is provided by Nihon Medi-Physics Co.,Ltd. The design, management, analysis, reporting and publication of the study are entirely independent of Nihon Medi-Physics Company.
Author information
Authors and Affiliations
Contributions
O.Y., K.K., I.K. and N.S.: study conception and manuscript preparation. I.N., Y.U., T.H., S.W., K.I., K.T., N.K., K.H., S.S., A.S., A.M., H.I., K.I., T.I. and K.M.: patient management. K.M., I.K., T.H. and S.S.: statistical analysis and patient data collection. O.Y., K.K., I.N., Y.U., T.H., H.K. and N.S.: manuscript revision. All authors contributed and agreed with the content of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
KK has received research grants and a speaker honorarium from Ono Pharmaceutical Company, Boehringer Ingelheim, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan, Nihon Medi-Physics Co.,Ltd and AstraZeneca. OY have received a speaker honorarium from Eli Lilly, Taiho Pharmaceutical, Pfizer, Chugai Pharmaceutical, and AstraZeneca. AM have received a speaker honorarium from Ono Pharmaceutical Company, Taiho Pharmaceutical, Pfizer, Daiichi Sankyo Company, Merck Biopharma Company, Novartis Pharma, Thermo Fisher Scientific, Chugai Pharmaceutical, and AstraZeneca. HK has received research grants and a speaker honorarium from Ono Pharmaceutical Company, Bristol-Myers Company, Boehringer Ingelheim, MSD, Daiichi Sankyo Company, Chugai Pharmaceutical, Taiho Pharmaceutical, Merck Biopharma Company, Eli Lilly Japan, and AstraZeneca. SW has received grants and a speaker honorarium from AstraZeneca, Eli Lilly and Boehringer Ingelheim, a speaker honorarium from Chugai Pharma, Ono Pharmaceutical, Bristol-Myers, MSD, Taiho Pharmaceutical, Pfizer, Novartis and Daiichi Sankyo. KI received research grants and a speaker honorarium from AstraZeneca, Chugai Pharma, Bristol-Myers, Boehringer Ingelheim, Ono Pharmaceutical, Taiho Pharmaceutical, Novartis International AG, Daiichi Sankyo Company. NS has received research grants and a speaker honorarium from Ono Pharmaceutical Company, Bristol-Myers Company, MSD, AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Pfizer Japan, Eli Lilly Japan, Taiho Pharmaceutical, Daiichi Sankyo Company, and Merck Biopharma Company. IK has received research grants from FUJIFILM Toyama Chemical Co., Ltd. and Nihon Medi-Physics Co.,Ltd. All other authors declare no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamaguchi, O., Kaira, K., Naruse, I. et al. Prospective assessment using 18F-FDG PET/CT as a novel predictor for early response to PD-1 blockade in non-small-cell lung cancer. Sci Rep 12, 11832 (2022). https://doi.org/10.1038/s41598-022-15964-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15964-3
- Springer Nature Limited
This article is cited by
-
Baseline and early 18F-FDG PET/CT evaluations as predictors of progression-free survival in metastatic breast cancer patients treated with targeted anti-CDK therapy
Cancer Imaging (2024)
-
Comparative analysis of different response criteria at early phase after PD-1 blockade in non-small lung cancer
Cancer Imaging (2023)