Abstract
We aimed to describe the clinical features of lymphangioleiomyomatosis (LAM) in Korean patients and identify factors associated with progressive disease (PD). Clinical features of 54 patients with definite or probable LAM from 2005 to 2018 were retrospectively analysed. Common features were pneumothorax (66.7%) and abdominal lymphadenopathy (50.0%). Twenty-three (42.6%) patients were initially treated with mechanistic target of rapamycin (mTOR) inhibitors. Lung transplantation (LT) was performed in 13 (24.1%) patients. Grouped based on the annual decline in forced expiratory volume in 1 s (FEV1) from baseline and LT, 36 (66.7%) patients exhibited stable disease (SD). All six deaths (11.1%) occurred in PD. Proportion of SD was higher in those treated initially with mTOR inhibitors than in those under observation (p = 0.043). Univariate analysis revealed sirolimus use, and baseline forced vital capacity, FEV1, and diffusing capacity of the lungs for carbon monoxide are associated with PD. Multivariate analysis showed that only sirolimus use (odds ratio 0.141, 95% confidence interval 0.021–0.949, p = 0.044) reduced PD. Kaplan–Meier analysis estimates overall survival of 92.0% and 74.7% at 5 and 10 years, respectively. A considerable proportion of LAM patients remain clinically stable without treatment. LT is an increasingly viable option for patients with severe lung function decline.
Similar content being viewed by others
Introduction
Lymphangioleiomyomatosis (LAM) is a rare systemic disease that primarily affects women of reproductive age1. The disease, characterized by proliferation of smooth-muscle-like LAM cells in lungs and lymphatic system, results in cystic lung destruction, chylothorax, renal angiomyolipoma, and formation of lymphangioleiomyomas. LAM can occur sporadically or in association with tuberous sclerosis complex (TSC). Clinical manifestations of LAM include exertional dyspnoea, haemoptysis, and pneumothorax. The disease progresses with a continuous decline in lung function, eventually resulting in clinically important respiratory impairment.
Because previous studies included a relatively small number of severe cases, LAM was considered a fatal disease affecting women primarily of child-bearing age2. Recent studies describe LAM as a chronic disease with a highly diverse clinical phenotype and a relatively long life expectancy3,4,5. Increased detection of asymptomatic or mild cases through regular health screenings and more frequent high-resolution computed tomography (HRCT) scans might account for this change. There is no definite evidence of racial or ethnic differences in incidence or clinical course of LAM2.
The clinical experience of LAM in Korea is in line with the above trend. A study of 21 patients in 1999 revealed that, despite hormonal treatment, the disease progressed in 80% of the patients and resulted in the death of 30% of the patients followed up for more than 12 months6. A more recent nationwide survey of 63 patients found an increase in the incidence of LAM since 2004, with a higher 5-year survival of 84%7. The study further showed that patients diagnosed before 2004 had higher rates of dyspnoea, lower baseline forced expiratory volume in 1 s (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO).
Therapeutic options for LAM are limited. In the Multicentre International Lymphangioleiomyomatosis Efficacy and Safety of sirolimus (MILES) trial, sirolimus was found to stabilize the decline of lung function8. Subsequent studies found that sirolimus not only stabilizes lung function9,10, but also improves other clinical manifestations, such as chylous effusions and lymphangioleiomyomas11. Hormonal therapy has only shown limited efficacy in post-menopausal women12. In the advanced stages, lung transplantation (LT) remains as a definitive treatment option13,14.
Selecting the optimal treatment require a better understanding of LAM, especially the factors that affect disease progression. Some clinical factors, such as baseline lung function3,15,16, age at diagnosis3,17,18, and menopausal status3,17, were consistently thought to affect the natural course of LAM. Despite these recent findings, the causes of disease progression remain unclear. The aim of this study was to describe the clinical features and clinical course of LAM, and to identify factors that may be associated with rapid disease progression of LAM.
Results
Baseline characteristics
A total of 59 patients with LAM diagnosis on electronic medical records was identified from 2005 to 2018 at Severance Hospital, South Korea. All diagnoses of LAM have been reviewed based on the European Respiratory Society (ERS) guidelines1.
Among these patients, 5 patients who were excluded were diagnosed with ‘possible LAM’ based on the ERS guidelines. These 5 patients did not show any respiratory symptoms, clinical features indicative of LAM, or decline in lung function in spirometry, but only exhibited the typical features of LAM on HRCT. Two of these patients received video-assisted thoracic surgical biopsy, but with no evidences of LAM in the final pathology report.
The final analysis was performed with a total of 54 patients diagnosed with definite (52/54, 96.3%) or probable (2/54, 3.7%) LAM. Regardless of the use of mechanistic target of rapamycin (mTOR) inhibitors, the study population was divided into two groups based on their disease course: namely, stable and progressive disease. Stable disease (SD) course was defined as annual decline of less than 10% in the percentage of the predicted FEV1 and not receiving LT; progressive disease (PD) course was defined as either annual decline of greater than or equal to 10% in the percentage of the predicted FEV1 or undergoing LT (Fig. 1). The annual decline in the predicted FEV1 was calculated in each patient by dividing the percent difference between the final and baseline predicted FEV1 by the time between the two pulmonary function test (PFT) measurements.
Flowchart of patient selection and study groups. Patients included in the final analysis are diagnosed with definite or probable LAM. These patients were divided by whether mTOR inhibitor was used as definitive treatment. Then, patients were subsequently divided by the disease course, according to lung transplantation status and ΔFEV1, defined as annual decline in the percentage of the predicted FEV1 compared to baseline. *Diagnosis based on ERS guidelines (2010, ERJ) on LAM. ǂThree patients treated with everolimus had tuberous sclerosis complex-associated LAM and angiomyolipoma. Figure was created using Microsoft PowerPoint version 16.0 and Microsoft Paint version 11.2201. LAM, lymphangioleiomyomatosis; mTOR, mechanistic target of rapamycin; FEV1, forced expiratory volume in 1 s.
Table 1 summarizes the baseline characteristics of the study population. All patients except one (53/54, 98.1%) were female, with a median age at diagnosis of 36.5 years. Only four (9.5%) patients were post-menopausal at the time of diagnosis. Six patients (11.1%) had TSC-associated LAM (TSC-LAM), one of whom was the only male patient in the study. In 36 (66.7%) patients, diagnosis of LAM was established with biopsy (Fig. 2). All patients showed characteristic patterns of LAM on HRCT. Pneumothorax was observed in 36 (66.7%) patients, abdominal lymphadenopathy in 27 (50.0%), angiomyolipoma in 18 (33.3%), and chylothorax in six (11.1%). LT was performed in 13 (24.1%) patients. mTOR inhibitors were used as first-line treatment in 23 (42.6%) patients. Sirolimus was administered in 20 (37.0%) patients; everolimus in three (5.6%) patients, all of whom had TSC-LAM.
Lung biopsy from a patient diagnosed with lymphangioleiomyomatosis. Lung biopsy specimen from a patient diagnosed with lymphangioleiomyomatosis. Hematoxylin and eosin staining reveals (a) multiple cysts of varying sizes at low magnification (10X) and (b) spindle cells at high magnification (100X). Immunohistochemical staining shows positive stains for (c) smooth muscle actin, (d) HMB-45, and (e) progesterone receptor. Figure was created by arranging the panel images with Microsoft Paint version 11.2201.
In the cohort, 36 (66.7%) patients showed SD course, 18 (33.3%) showed PD course. All 13 patients who received LT showed PD course. No significant differences in clinical features at diagnosis at were observed between two groups. However, the treatment methods and baseline PFT findings were significantly different between the groups. The proportion of patients exhibiting SD was higher in those initially treated with mTOR inhibitors than in whose who were under medical observation (mTOR inhibitor 19/23, 82.6% vs. medical observation 17/31, 54.8%, p = 0.043; Table 1, Fig. 3). All PFT parameters at baseline, except forced vital capacity (FVC), were significantly worse in patients with PD (p < 0.001 for FEV1, p < 0.001 for FEV1/FVC ratio, p = 0.001 for DLCO). Changes in predicted FEV1 values with respect to time are shown in Supplementary Figure S1.
Distribution of annual FEV1 change and follow-up duration of pulmonary function test by patient groups. The annual decline in the percentage of the predicted FEV1 compared to baseline and the duration of follow-up of PFT for each patient is plotted, using SPSS software version 23 (www.ibm.com/analytics/spss-statistics-software) and Microsoft Paint version 11.2201. Patients are divided into four groups, according to mTOR inhibitor use and disease course. mTOR, mechanistic target of rapamycin; PFT, pulmonary function test; FEV1, forced expiratory volume in 1 s.
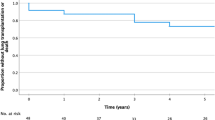
Six (11.1%) patients died during the follow-up period; all of them had undergone LT. Overall survival rates using the Kaplan–Meier analysis were 92.0% and 74.7% at 5 years and 10 years, respectively (Fig. 4a). Transplant-free survival rates were 84.1% and 59.6% at 5 years and 10 years, respectively (Fig. 4b).
Kaplan–Meier analyses for survival. Kaplan–Meier analyses of (a) overall survival for all patients, (b) transplant-free survival for all patients, and (c) overall survival for lung transplantation. Figure was created using SPSS software version 23 (www.ibm.com/analytics/spss-statistics-software) and Microsoft Paint version 11.2201.
Factors associated with progressive disease
Logistic regression analyses were performed to determine factors associated with PD. The following clinical parameters were considered: age at diagnosis, menopause at the time of diagnosis, TSC-LAM, use of sirolimus, baseline PFT parameters, pneumothorax, abdominal lymphadenopathy, angiomyolipoma, and chylothorax (Table 2). Age at diagnosis (cut-off at 35 years) and baseline PFT parameters (cut-off at 70% of predicted value for all parameters) were analysed as categorical variables. Univariate analysis identified poor baseline PFT (odds ratio (OR) 8.667, 95% confidence interval (CI) 2.230–33.682, p = 0.002 for baseline FEV1) and medical observation (OR for use of sirolimus 0.224, 95% CI 0.055–0.908, p = 0.036) to be associated with PD. Multivariate analysis, performed with sirolimus use, baseline FVC, baseline FEV1, baseline DLCO, abdominal lymphadenopathy, and angiomyolipoma in the model, showed that only the use of sirolimus (OR 0.141, 95% CI 0.021–0.949, p = 0.044) reduces the risk of PD (Table 2). In an additional multivariate analysis performed with sirolimus use, baseline FEV1 (cut-off at 80% of predicted value), abdominal lymphadenopathy, and angiomyolipoma, both sirolimus use (OR 0.118, 95% CI 0.023–0.602, p = 0.010) and poor baseline FEV1 (OR 5.026, 95% CI 1.021–24.741, p = 0.047) were significantly associated with PD course (Supplementary Table S1, Model 2).
Patients who underwent lung transplantation
Table 3 summarizes the clinical features of all patients who underwent LT. All patients were females diagnosed with sporadic LAM, with a median age of 37 years at disease onset. The most common feature observed was pneumothorax (n = 10, 76.9%) followed by abdominal lymphadenopathy (n = 9, 69.2%), angiomyolipoma (n = 2, 15.4%), and chylothorax (n = 1, 7.7%). All patients underwent bilateral transplantation, except one who underwent two unilateral transplantations. Recurrence of LAM, observed in two (15.4%) patients, was treated with sirolimus. As mentioned earlier, six patients (46.2% of LT cases) died: three due to pneumonia, one due to infection other than pneumonia, one due to cerebral infarction, and one due to an unknown cause. Kaplan–Meier analysis showed a median survival of 98.4 months (95% CI 0.0–217.1) and estimated 5-year survival of 60.6% (Fig. 4c). A higher proportion of patients who underwent LT after 2011 (5 out of 7, 71.4%) survived than those who underwent LT before 2011 (1 out of 5, 20.0%).
Discussion
Our study described the long-term clinical course of 54 patients diagnosed with definite or probable LAM. Patients with SD were more likely to have better baseline PFT and to be treated with mTOR inhibitors. Multivariate analysis identified medical observation as the only factor increasing the risk of PD. As a tertiary referral centre, we included a high proportion of patients with LT, compared to previous studies5,19. Interestingly, we found that one-third of all patients (n = 17) in the study showed SD without definitive treatment, possibly suggestive of a phenotypic subtype of LAM.
In our study, sirolimus use was a significant predictor of SD course. Consensus statements recommend considering the use of sirolimus in patients showing rapid progression; however, the definition of rapid progression is not clear1,20. The efficacy of sirolimus has been demonstrated in patients with predicted FEV1 value of 70% or less8, and with symptomatic chylothorax11. Other studies have suggested that patients with rapidly declining FEV1, reduced DLCO, exercise-induced or resting hypoxemia, angiomyolipomas or lymphangioleiomyomas might benefit from sirolimus21,22.
Despite growing evidence of benefit of sirolimus treatment, our study shows a high proportion of patients who have not received sirolimus treatment. Its use in the treatment of LAM was approved for reimbursement by the Korean National Health Insurance starting early 2016, five years after results from the MILES trial were available. This temporal discrepancy, with a lack of clear consensus on the proper conditions for sirolimus use, might have led to a conservative use of sirolimus in this study. While we observe that the changes in predicted FEV1 for patients treated with sirolimus are variable among patients (Supplementary Figure S2), multiple models of regression analysis for predicting PD course suggests that sirolimus treatment reduces the risk for exhibiting PD course (Table 2, Supplementary Table S1). Additionally, we found that the proportion of patients exhibiting SD was higher in those initially treated with mTOR inhibitors than in those who were under medical observation. Sirolimus treatment should be strongly considered in patients exhibiting high-risk features, such as low baseline FEV1 and/or DLCO, symptomatic chylothorax, and hypoxemia.
A significant proportion (n = 14, 60.9%) of patients on mTOR inhibitors experienced adverse events (Supplementary Table S2), most common of which was oral mucositis or ulcer (n = 8, 34.8%), followed by infection (n = 4, 17.4%). Any event of drug discontinuation was documented in 12 (52.2%) patients, in seven of whom the drug was discontinued permanently. Rates of adverse events were comparable to those reported in other studies, although sirolimus drug level was maintained higher in our study9,23. These studies concluded that a lower dose of sirolimus was still effective in slowing FEV1 decline. As adverse events might eventually lead to treatment discontinuation, further studies are warranted to determine the optimal sirolimus dosage in LAM.
We also found that better baseline lung function is associated with stable course (Table 2). A study on mTOR inhibitor-naïve patients in Japan revealed that faster FEV1 decline was observed in the patient group with a baseline predicted FEV1 value of 70% or less15. The group with faster FEV1 decline not only had poor overall baseline PFT parameters, but also showed faster decline in FVC. In a large retrospective review of 275 patients, initial FEV1 and DLCO, in addition to age, were found to be predictors of lung function decline16. A longitudinal analysis of a LAM cohort identifies baseline PFT (FEV1 and DLCO) as most predictive factors of mortality3. In the MILES study, which only enrolled patients with FEV1 of 70% or less of the predicted value, faster FEV1 decline was seen in patients with bronchodilator response in spirometry17.
Consistent with many previous findings, our regression analysis shows that baseline predicted FVC, FEV1, and DLCO of 70% or less are independently associated with PD course. While these PFT parameters were not found to be significant in multivariate analysis presented in Table 2, using higher cut-offs of 80% or less of predicted value for PFT parameters show that FEV1 is a significant predictor in PD course. In a multivariate regression analysis model containing sirolimus use, baseline FEV1 (cut-off at 80% of predicted value), abdominal lymphadenopathy, and angiomyolipoma, baseline FEV1 was found to be a significant predictor of PD course (Supplementary Table S1, Model 2). Subsequent Kaplan–Meier analyses of patients at risk for death and/or LT found a significantly higher frequency of death or LT in patients with poor initial FEV1, while no association was seen with initial DLCO (Supplementary Figure S3 and S4). These findings indicate that FEV1 is more significant than other PFT parameters in determining the disease status.
Past studies have suggested different prognostic predictors for LAM. In addition to PFT abnormalities, older age at diagnosis3,17,18, menopausal status3,17, presence of pneumothorax15, chylous effusion11, and angiomyolipoma18, and use of supplemental oxygen and hormonal agents18 have been identified as clinical features associated with the disease progression and/or mortality due to LAM. However, our study found no other clinical features correlated with severity of the disease, likely due to the differences in study design. Another possible reason for this discrepancy is the relatively small size of the study population.
We observe a high proportion of patients with stable lung function in this cohort. Of the 31 patients in this cohort initially under medical observation, 17 exhibited SD. These patients, comprising nearly one-third of the study population, were associated with good baseline lung function, menopause at diagnosis, and absence of abdominal lymphadenopathy (Supplementary Table S3). Clinical manifestations of LAM are highly heterogeneous21. As seen in our study population (Supplementary Fig. 1a), lung function changes in patients with LAM have also been reported to vary widely, with one study reporting 42% of patients showing normal findings on spirometry24. Because the reasons for this variability are poorly understood, future studies are warranted to elucidate the benefit of sirolimus in patients with stable LAM phenotype.
Our study included 13 patients (24.1%) who underwent LT, which is higher than that in other observational studies5,19. All patients were female and diagnosed with sporadic LAM. Most of these patients had rapidly declining FEV1 (Fig. 3). LAM recurred in two patients, both of whom were treated with sirolimus. Similar to the observation in another study14, patients who underwent transplantation after 2011 in our study showed better survival outcomes than those who underwent transplantation before 2011 (Table 3). This was most likely due to the increased experience of transplantation team. LT remains a viable treatment option in LAM patients with significant decline in lung function.
Although LAM cohorts in earlier studies showed poor survival, recent studies suggest a more favourable prognosis for LAM. Survival rates at 10 years are reported to be 86%, 76%, and 79% across patients with LAM in the United States, Japan, and France, respectively5,18,24. Median transplant-free survival is over 20 years according to a recent LAM registry in the United States3. Consistent with these findings, the survival rate of 74.7% at 10 years in our study shows that LAM has a rather favourable prognosis.
There are some limitations to this retrospective single-centre study. Patients show a significant variation in the PFT values at baseline (Table 1, Supplementary Figure S1a), which may underestimate the effect of poor baseline PFT on subsequent disease course. However, to adjust for this issue, we add LT as another criterion for defining disease course. Of the 15 patients with severe airflow limitations (predicted FEV1 of less than 50%) at baseline, 12 of them received LT. In addition, our study defines disease course based on the changes in predicted FEV1 over time, because it is a quantifiable measure of changes in lung function that is readily applicable to clinical decision making1. There were irregularities in the frequency and interval of spirometry measurements among patients, which might explain some differences in our findings compared to those in other studies.
As mentioned above, the decision to use sirolimus seem discrepant from the recent clinical guidelines, due to a number of reasons. The late inclusion of sirolimus in the treatment of LAM for reimbursement by the Korean National Health Insurance may have contributed to the observed discrepancy. In addition, our study enrols patients diagnosed with LAM as early as 2005, when the benefit of mTOR inhibitors was not as clear as the current clinical recommendations.
Being a tertiary referral centre, we see a higher number of cases with severe disease, often resulting in LT. But the inclusion of a considerable proportion of LT cases also enables a more comprehensive overview of the clinical landscape of LAM. Moreover, the measurement of vascular endothelial growth factor-D, a potential biomarker for diagnosis and treatment response of LAM, were not available in our institution until recently (Supplementary Table S4).
In conclusion, sirolimus treatment predicts stable disease course in LAM patients. Better baseline function is also associated with stable disease course in LAM. A significant proportion of patients remain clinically stable even without treatment, highlighting the need for future studies identifying the clinical characteristics of this phenotype. We included a considerable proportion of patients undergoing LT, with better survival outcomes in more recent cases. Although highly heterogeneous in clinical presentation, LAM has a favourable overall prognosis. Survival rates in our study similar to reports in more recent studies.
Methods
This study retrospectively reviewed the electronic medical records of patients diagnosed with LAM from 2005 to 2018 at Severance Hospital, South Korea. The anonymised data on clinical history, lab studies, PFTs, and imaging studies of the patients have been obtained for analysis. This study was approved by the Institutional Review Board (IRB) of Severance Hospital (IRB number: 4-2012-0685), which waived the requirement of obtaining written informed consent due to the retrospective nature of this study. The present study was conducted in accordance with the Declaration of Helsinki.
The diagnosis of LAM was confirmed according to the 2010 ERS criteria1. The diagnosis of TSC-LAM was made in patients with definite TSC, as defined by the TSC diagnostic criteria update in 201225.
Statistical analysis was performed using SPSS software version 23 (www.ibm.com/analytics/spss-statistics-software). Mann–Whitney U test was used to compare median values between the groups. Fisher’s exact test and chi-square tests were performed to compare proportions between the groups. Logistic regression analysis was used to assess the impact of clinical features on the disease course. Variables included in the multivariate analyses either show significance level of p < 0.10 in univariate analysis, or is a PFT parameter, which reflects disease status in LAM. For all analyses, a two-tailed significance level of 0.05 was used. Kaplan–Meier analysis was performed for survival analysis.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Johnson, S. R. et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur. Respir. J. 35, 14–26. https://doi.org/10.1183/09031936.00076209 (2010).
Taveira-DaSilva, A. M., Pacheco-Rodriguez, G. & Moss, J. The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis. Lymphat. Res. Biol. 8, 9–19. https://doi.org/10.1089/lrb.2009.0024 (2010).
Gupta, N. et al. The NHLBI LAM registry: prognostic physiologic and radiologic biomarkers emerge from a 15-year prospective longitudinal analysis. Chest 155, 288–296. https://doi.org/10.1016/j.chest.2018.06.016 (2019).
Johnson, S. R., Whale, C. I., Hubbard, R. B., Lewis, S. A. & Tattersfield, A. E. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax 59, 800–803. https://doi.org/10.1136/thx.2004.023283 (2004).
Hayashida, M. et al. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology 12, 523–530. https://doi.org/10.1111/j.1440-1843.2007.01101.x (2007).
Oh, Y. M. et al. Pulmonary lymphangioleiomyomatosis in Korea. Thorax 54, 618–621. https://doi.org/10.1136/thx.54.7.618 (1999).
Park, H. Y. et al. A nationwide survey of lymphangioleiomyomatosis in Korea: recent increase in newly diagnosed patients. J. Korean Med. Sci. 25, 1182–1186. https://doi.org/10.3346/jkms.2010.25.8.1182 (2010).
McCormack, F. X. et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 364, 1595–1606. https://doi.org/10.1056/NEJMoa1100391 (2011).
Bee, J., Fuller, S., Miller, S. & Johnson, S. R. Lung function response and side effects to rapamycin for lymphangioleiomyomatosis: a prospective national cohort study. Thorax 73, 369–375. https://doi.org/10.1136/thoraxjnl-2017-210872 (2018).
Takada, T. et al. Efficacy and Safety of Long-Term Sirolimus Therapy for Asian Patients with Lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 13, 1912–1922. https://doi.org/10.1513/AnnalsATS.201605-335OC (2016).
Taveira-DaSilva, A. M., Hathaway, O., Stylianou, M. & Moss, J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann. Intern. Med. 154, 797–805, W-292–793. https://doi.org/10.7326/0003-4819-154-12-201106210-00007 (2011).
Lu, C. et al. A phase II clinical trial of an aromatase inhibitor for postmenopausal women with lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 14, 919–928. https://doi.org/10.1513/AnnalsATS.201610-824OC (2017).
Ando, K. et al. Lung Transplantation for Lymphangioleiomyomatosis in Japan. PLoS ONE 11, e0146749. https://doi.org/10.1371/journal.pone.0146749 (2016).
Ussavarungsi, K. et al. Mayo clinic experience of lung transplantation in pulmonary lymphangioleiomyomatosis. Respir. Med. 109, 1354–1359. https://doi.org/10.1016/j.rmed.2015.08.014 (2015).
Hayashida, M. et al. Reductions in pulmonary function detected in patients with lymphangioleiomyomatosis: An analysis of the Japanese National Research Project on Intractable Diseases database. Respir. Investig. 54, 193–200. https://doi.org/10.1016/j.resinv.2015.11.003 (2016).
Taveira-DaSilva, A. M., Stylianou, M. P., Hedin, C. J., Hathaway, O. & Moss, J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest 126, 1867–1874. https://doi.org/10.1378/chest.126.6.1867 (2004).
Gupta, N. et al. Analysis of the MILES cohort reveals determinants of disease progression and treatment response in lymphangioleiomyomatosis. Eur. Respir. J. https://doi.org/10.1183/13993003.02066-2018 (2019).
Oprescu, N., McCormack, F. X., Byrnes, S. & Kinder, B. W. Clinical predictors of mortality and cause of death in lymphangioleiomyomatosis: a population-based registry. Lung 191, 35–42. https://doi.org/10.1007/s00408-012-9419-3 (2013).
Hasegawa, W. et al. Clinical features of 280 hospitalized patients with lymphangioleiomyomatosis in Japan. Respirology 20, 160–165. https://doi.org/10.1111/resp.12430 (2015).
McCormack, F. X. et al. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am. J. Respir. Crit. Care Med. 194, 748–761. https://doi.org/10.1164/rccm.201607-1384ST (2016).
O’Mahony, A. M., Lynn, E., Murphy, D. J., Fabre, A. & McCarthy, C. Lymphangioleiomyomatosis: a clinical review. Breathe (Sheff) 16, 200007. https://doi.org/10.1183/20734735.0007-2020 (2020).
Johnson, S. R., Taveira-DaSilva, A. M. & Moss, J. Lymphangioleiomyomatosis. Clin. Chest. Med. 37, 389–403. https://doi.org/10.1016/j.ccm.2016.04.002 (2016).
Yoon, H. Y., Hwang, J. J., Kim, D. S. & Song, J. W. Efficacy and safety of low-dose Sirolimus in Lymphangioleiomyomatosis. Orphanet J. Rare Dis. 13, 204. https://doi.org/10.1186/s13023-018-0946-8 (2018).
Urban, T. et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM"O"P). Medicine (Baltimore) 78, 321–337. https://doi.org/10.1097/00005792-199909000-00004 (1999).
Northrup, H., Krueger, D. A., International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 49, 243–254. https://doi.org/10.1016/j.pediatrneurol.2013.08.001 (2013).
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work: E.H.L. and M.S.P. Acquisition of data: analysis: J.S.C., A.Y.L., Su.H.L., Sang.H.L., S.Y.K., K.S.C., J.Y.J., Y.A.K., Y.S.K., J.G.L., H.C.P., H.S.S, E.H.L. and M.S.P. Interpretation of data: S.C. and E.H.L. Drafting the manuscript: S.C. and E.H.L. Revising the manuscript critically for important intellectual content: S.C., E.H.L. and M.S.P. Final approval of the version to be published: All authors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, S., Choi, J.S., Leem, A.Y. et al. Long-term clinical course and progression of lymphangioleiomyomatosis in a single lung transplant referral centre in Korea. Sci Rep 12, 8260 (2022). https://doi.org/10.1038/s41598-022-12314-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12314-1
- Springer Nature Limited