Abstract
River alterations for natural hazard mitigation and land reclamation result in habitat decline and fragmentation for riparian plant species. Extreme events such as floods are responsible for additional local species loss or population decline. Tributaries might provide refugia and subsequent source populations for the colonization of downstream sites in connected riverine networks with metapopulations of plant species. In this study, we analyzed the metapopulation structure of the endangered riparian shrub species Myricaria germanica along the river Isel, Austria, which is part of the Natura 2000 network, and its tributaries. The use of 22 microsatellite markers allowed us to assess the role of tributaries and single populations as well as gene flow up- and downstream. The analysis of 1307 individuals from 45 sites shows the influence of tributaries to the genetic diversity at Isel and no overall isolation by distance pattern. Ongoing bidirectional gene flow is revealed by the detection of first-generation migrants in populations of all tributaries as well as the river Isel, supporting upstream dispersal by wind (seeds) or animals (seeds and pollen). However, some populations display significant population declines and high inbreeding, and recent migration rates are non-significant or low. The genetic pattern at the mouth of river Schwarzach into Isel and shortly thereafter river Kalserbach supports the finding that geographically close populations remain connected and that tributaries can form important refugia for M. germanica in the dynamic riverine network. Conservation and mitigation measures should therefore focus on providing sufficient habitat along tributaries of various size allowing pioneer plants to cope with extreme events in the main channel, especially as they are expected to be more frequent under changing climate.
Similar content being viewed by others
Introduction
Riparian habitats along rivers are of major importance for biodiversity worldwide as they offer high species diversity1 and many ecosystem functions2. Centuries of river alterations for land reclamation have resulted in habitat reduction and fragmentation especially for sessile riparian plant species3,4. Extreme events such as large floods are often responsible for local extinction of plant populations5 and are likely to increase under changing climate6,7,8. Tributaries might provide refugia and subsequent source populations for the colonization of downstream sites in connected riverine networks5,9,10,11.
Connectivity between tributaries and downstream rivers is especially important for plant species inhabiting the ever-changing dynamic riverine zone, as local loss or population decline is frequent already at yearly returning floods12, despite plants being highly adapted to changing environmental conditions13. To counteract genetic diversity loss by reduced local density, functional metapopulation networks connecting populations up- and downstream of rivers are necessary14.
Studies on genetic diversity have shown the importance of upstream as well as downstream dispersal for riparian species15. Many plant species in habitats close to the waterline display seed morphologies suitable for wind and water dispersal16, as well as animal-mediated dispersal mechanisms17,18. Despite many means of propagation, riparian metapopulations are generally genetically highly structured and e.g. show isolation by distance patterns11,19,20,21. Water mediated dispersal (hydrochory) might enhance connectivity to distant populations downstream21,22, but gene flow along catchments can be highly impacted by barriers such as canyons or reservoirs23.
Neutral genetic markers allow to investigate if gene flow and therefore functional connectivity is still present along river networks24,25, if there is local population decline26, or ongoing migration27 despite fragmented habitat. This information is crucial to assess the importance of tributaries and the contribution of single populations to genetic diversity further up- or downstream28,29.
In this study, we investigate connectivity along the river Isel and its tributaries by analysing genetic diversity patterns for the characteristic and endangered riparian shrub species, the German tamarisk, Myricaria germanica13,30. This catchment offers a high number of populations of the pioneer plant of the dynamic riverine zon31 despite natural canyons, many hydro-morphological river alterations in the past (land use and flood protection) as well as barriers linked to hydropower. The analysis of 45 sites with both small and large (more than 30 individuals) populations within the catchment allows to assess the influence of tributaries on the genetic diversity as well as the connectivity between sites. If gene flow between populations persists, the tributaries and the main reach should reveal similar genotype compositions, and only low population differentiation.
As isolation by distance patterns and high genetic structure in riparian metapopulations indicate short distance wind-mediated upstream and downstream dispersal to have a higher impact than long-distance water-mediated dispersal24, the highly variable microsatellite markers are applied to reveal the primary mechanism of dispersal and if unidirectional or bidirectional gene flow along the river network is more frequent. Moreover, we determine the presence of migrants and migration rates, as ongoing gene flow would support intact functional connectivity along Isel and its tributaries.
Results
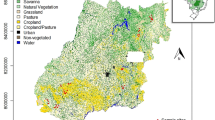
A total of 1307 individuals were analyzed from 45 sites (Fig. 1). Sites and populations including age structure were described in detail in the project documentation (see Supplementary Information Table 1 and in32,33). Populations showed high numbers of polymorphic loci out of the 22 loci analyzed (Table 1). Private alleles were not found for any population, and genetic diversity analyzed as expected heterozygosity estimates were low for most populations with a maximum of 0.34 for one site at Isel (I-05). Inbreeding coefficients are high for several sites along Isel (I-06: 0.63; I-01: 0.86), Schwarzach (S-08: 0.72; S-04: 0.76, see Table 1). Contrary to that, two sites, one at Isel and one at Schwarzach, have negative FIS-values indicating a proportion of outbred individuals (I-04 and S-07 see Table 1).
Austria (a) with the river Isel and its tributaries in East Tyrol, before they flow into the river Drau (b). The sites where Myricaria germanica has been sampled along the Tauernbach, Kleine Isel, Schwarzach, Kalserbach, and Daberbach as well as the populations along Isel are shown (c): Populations of different size were sampled (size of black dots representative of number of individuals). The river (blue) and riverscape morphology including canyons (light grey river course), dams and embankments (dark grey triangles), and the digital terrain model (DTM from Land Tirol, data.tirol.gv.at) are shown in the background.
FST-values revealed high values and significant differentiation between most populations (Supplementary Information, Table 2). Of the 44 populations analysed, 12 showed indications for heterozygote excess as analysed under various mutation models and applying different tests in bottleneck (Table 2). Most populations with indication for limited genetic diversity were found at Kleine Isel (4 out of 7 sites), followed by Isel (3 populations), Schwarzach (2 populations), Tauernbach (2 populations) and Kalserbach (1 population, see Table 2).
The results of the AMOVA analysis revealed that the lowest genetic diversity was found between rivers (8.14%, df = 7, Sum of squares = 570.676) and between populations within each river (13.99%, df = 38, Sum of squares = 600.508). Highest variance was within individuals (45.94%, df = 1307, Sum of squares = 1192.5) and within populations (31.93%, df = 1315, Sum of squares = 2748.048).
The analysis in Structure Harvester revealed that the most likely number of groups of genotypes could be assigned to K = 4 (Supplementary Inforamtion, Fig. 1). The resulting genotype assignment at the population level revealed some gradients, with considerable changes in genotype group assignment of Kleine Isel, Tauernbach and Kalserbach compared to Isel river (Fig. 2, details for values see also Supplementary Information Table 3), but no unique gene pool was found for any river. By comparing genetic differentiation (FST) and distances along rivers, no isolation by distance pattern was detected (correlation between pairwise FST and geographic distance, R2 = 0.0176, Mantel test, p = 0.6).
Genetic structure along Isel and its tributaries. For each sampled site, the percentage of genotypes assigned to each of the four groups is shown (displayed in red, orange, yellow and brown, sorted by site see Table 1). The tributaries Tauernbach (north, mainly yellow and red), Kleine Isel (north-west, mainly orange), Schwarzach (west, mainly red) and Kalsersbach (east, mainly brown) all contribute to the high diversity at Isel. The high genetic diversity in the area where Schwarzach and Kalserbach flow into Isel is subsequently lost further downstream, where a majority of genotypes is assigned to one group (red), before the inflow of Daberbach (contribution of yellow group).
A total of 80 individuals of the 1307 analyzed were more probable to originate from other populations than they were found in (p < 0.01). Of the 45 sites studied, 30 showed individuals which are most likely first-generation migrants from another population (Table 3, p < 0.01). Most migrants per site were detected in two population at Isel (I-08 and I-09), and potential sources were traced to upstream tributaries but also to other sites at Isel downstream. Similar to the findings for Isel, sources of migrants were assigned to both up- as well as downstream sites for all tributaries (Table 3 and Fig. 3). We detected mainly of non-significant recent migration rates (Supplementary Information, Table 4). The only significant values > 0.2 were obtained for geographically close populations at Kleine Isel (KI-02, KI-04, KI-05, KI-06, KI-07), as well as the close populations at the junction of Isel and Schwarzach (I-07, I-08, S-09, Supplementary Information, Table 4).
Genetic structure and number of migrants at the junction of Schwarzach (left), Kalserbach (right) and Isel (center) along the rivers (blue lines). The four genotype groups are represented in different colours (red, orange, yellow and brown) and number of immigrants from a source upstream (dark blue) and downstream (light blue) are shown in circles. Although two sites (one at Schwarzach and one at Kalserbach) are separated from the next source population upstream by canyons (grey blocks), they show immigrants from upstream. Geographically close populations next to the inlet of Kalserbach all show migrants from downstream sites.
Discussion
Genetic diversity of the Isel metapopulation
The German tamarisk displays many populations along the Isel catchment despite changes in river morphology and dynamics by humans (see Fig. 1 as well as in31). Our comprehensive study on both large and small populations shows that genetic diversity is especially high at the large populations at the Isel river where also patterns of population demography indicate ongoing rejuvenation.
Contrary to the general situation at the main Isel reach (downstream of Matrei i.O.), many populations of various size within the catchment show high inbreeding. As the German tamarisk is capable of selfing23, even large populations might display low genetic diversity due to few founders and genetic drift34, similar to other shrub species in dynamic environments35. This is apparent in a population at Isel, occurring after the junction with Schwarzach and Kalserbach, which has a long history of German tamarisk presence31,36. Despite a large population size, the central position in the riverine network and no barrier limiting dispersal, signs of inbreeding and a bottleneck were detected at this site. This stresses the importance of genetic analyses to assess the comprehensive diversity of a single population37, and the full impact of a site to a metapopulation network5,14.
Genetic diversity patterns are reflecting habitat fragmentation due to river morphology and connectivity38,39,40, providing valuable insights for conservation measures when linked to demographic and topographic data41. In the headwaters (Kleine Isel) and the tributary Tauernbach, detected reduced genetic diversity is reflecting limited connectivity due to topographical characteristics31,42,43. Similarly, the signs of bottlenecks at two sites close to the confluence with Isel of Schwarzach (S-09) and of Kalserbach (K-08) are most probably due to the location downstream of canyons, which are responsible for habitat fragmentation and non-functional connection to upstream populations by wind-mediated dispersal (see in23). However, the genetic diversity pattern at the catchment revealed that downstream transport via canyons (i.e. by floating seeds or vegetative dispersal by plant ramnets) has been possible through the Kalserbachklamm, the Defregger-Klamm and the Prosseggklamm, similar to findings for other riparian species44.
The role of tributaries on gene flow
Contrary to findings of high differentiation along rivers for other riparian plant species (by diaspore mimics45, studies on woody riparian guildes46, and studies on floodplain meadow species47), the tributaries of Isel do not display unique gene pools or clear isolation by distance patterns, as e.g. found for fish48. Still, some tributaries showed higher percentage of certain groups of genotypes (e.g. Tauernbach, see Fig. 2) as well as some gradients along the river (e.g. Schwarzach, west–east gradient following flow direction, see Fig. 2). Similar to findings for M. germanica populations in other countries23,49 as well as other riparian species20,21, the studied populations were highly differentiated in pairwise comparisons. This pattern reflects a typical metapopulation for a species with various dispersal mechanisms (see in24), which has been found for other riparian plant species in the dynamic riverine zone50.
Recent migrants were detected both between large as well as small populations, although contemporary migration rates were small. This indicates that individuals of tributaries might have been sources for long distance dispersal downstream (e.g. by vegetative dispersal) during extreme flood events24, despite being isolated under steady-state-conditions (e.g. Daberbach, see Fig. 2). First-generation immigrants (as detected at two sites) can lead to increased genetic diversity in future generations if the habitat is suitable for local species expansions51.
Especially interesting for metapopulation studies along rivers are junctions, as they form unique riparian habitats and allow to assess the impact of single inlets on the genetic diversity at the catchment52. The genetic structure of populations at junctions can further reveal functional connectivity up- and downstream at a small scale25. At the junction of Schwarzach and Kalserbach with Isel, the populations of the tributaries are more related to the populations at Isel downstream than to the other sites at the tributaries upstream, partly also due to the canyons (Fig. 3). With all populations showing at least one migrant in this geographically limited area, the junction is a hotspot of gene flow both up-and downstream, and therefore a focal point in the metapopulation network53.
Directional gene flow and importance of barriers to dispersal
First-generation migrants can reflect vegetative as well as propagule dispersal, as the German tamarisk is capable of both18. While vegetative dispersal is unidirectional by water flow, seed dispersal by wind is common for the German tamarisk54 and is playing an important role for functional connectivity for many riparian species15. The lack of an isolation by distance pattern and the detection of migrants both from upstream and downstream sources suggest bidirectional gene flow mainly by wind dispersal, similar to previous findings49. Hydrochory is less likely, given the high number of human-made barriers such as check dams along the Isel catchment, which likely inhibit water-mediated dispersal 4,23,55.
Long-distance wind dispersal seems to be rare (but see15), but for the German tamarisk it has even been previously detected even between catchments49. Additionally, gene flow over long distances is possible also by pollen mediated dispersal18, and insect pollinators can cross barriers to both water- and wind-mediated gene flow. The current genetic structure indicates that M. germanica can partially overcome both natural canyons and man-made check dams along Isel and its tributaries, although limitations in gene flow might only be detectable after a certain time26.
Unexpected re-colonization of isolated sites could be linked to human-mediated dispersal by gravel extraction or relocation during or after construction along rivers, as seen at a site within a series of sediment check dams at Ködnitzbach (a tributary to Kalserbach), where a large population was established at the small gravel bar within the highly impacted river stretch K-03, see Fig. 2 and32. This is similar to findings of another study after sediment check dam construction56. The fate of such populations remains unclear, as subsequent lack of sediment and hydrological dynamics can influence population persistence, e.g. by preventing rejuvenation57.
Management and conservation implications
Due to the lack of a long-term monitoring of the metapopulation at Isel36, indirect evidence for ongoing functional connectivity as provided by this study is necessary for management planning for the river stretches within the Natura 2000 area. Although habitat fragmentation is present (e.g. natural canyons, lateral embankments and check dams), the metapopulation network is functionally connected. Still, management strategies are necessary, as M. germanica populations are frequently found in dynamic riverine zones, which are subject to major loss during extreme events (such as HQ30 or HQ100 flood events). As populations with mainly young individuals show less genetic diversity than sites with older plants, the main focus of conservation strategies should be on protecting large populations with diverse age classes. As all tributaries contribute to the gene pool, sheltered and currently more isolated sites (e.g. Daberbach) might provide important refugia and sources for subsequent re-colonization after extreme events as exemplified by58.
For management planning, knowledge on species’ dispersal mechanisms is especially important for species inhabiting rivers and riverscapes59. Wind-mediated dispersal with a continuous supply of seeds during summer54 together with pollen mediated gene flow mainly allow for shorter dispersal distances60. Smaller populations or less persistent sites in highly dynamic riverine zones might provide important short-term nodes in the metapopulation network52, and currently unsuitable habitat should be restored to provide a better network. The overall habitat loss, e.g. due to hydro-morphological changes like channelization, is the major threat for M. germanica, and therefore, re-introduction projects are often initiated in revitalization projects such as river widenings e.g.61,62,63. If human mediated re-introductions of individual plants or seeds are considered, they should only use material originating from the closest populations along the tributary, given the genetic differentiation detected in this study. A future monitoring of sites and population structures as well as changes in genetic diversity would allow to assess the status and development of the German tamarisk along Isel based on the presented study.
Conclusions
Our study on the genetic diversity of M. germanica along the Isel and its tributaries provides a first comprehensive overview of the metapopulation, and highlights the importance of tributaries within the catchment for maintaining gene flow. Both large and small populations might play important roles in the metapopulation network, but are equally subject to population declines. Conservation management of the Isel and its tributaries should therefore focus on enabling habitat formation or restoring habitat for the German tamarisk, with a special focus on the migration hotspots at the junctions. This ensures the survival of M. germanica under expected more frequent and more intense extreme events such as floods due to changing climate despite fragmented habitat.
Methods
Study species
The German tamarisk, Myricaria germanica (L.) Desv., is the main indicator for the protected habitat “Alpine rivers and their ligneous vegetation with Myricaria germanica”, Natura 2000 code 3230 for a study on the habitat, e.g.64. In Tyrol, M. germanica is protected since 2005 (Tiroler Naturschutzgesetz, Article 23, attachment IV lit.b) and the manipulation of the plant as well as habitat changes resulting in loss of individuals is prohibited (Ordinance of Nature protection, Verordnung der Tiroler Landesregierung 2006, Article 1 and 2). Over the last decade, the species showed a severe decline along European rivers30,36. As a typical pioneer species on gravel banks and bars, the German tamarisk is threatened, if sediment and hydrological dynamics are restricted.
The shrubs can reproduce vegetative by re-rooting of branches, but also produce seeds that germinate within 48 h after seed landing65. Juvenile plants reach age of first flowering after 1–2 years see also16,64, and the sentenced flowers are pollinated by insects18. Additionally, the plant is reported to be capable of selfing23. As it provides nutrition for insects such as honey bees, recommendations for the cultivation of the shrub along rivers in order to improve the honey yield were promoted in Tyrol in the mid-twentieth century66. Seeds of the German tamarisk have a pappus, facilitating both wind- and water-mediated dispersal43,67. For wind-mediated dispersal, the majority of seeds were found close to the mother plant, but dispersal kernels show long tails54, with rare long-distance dispersal of several kilometers43. While water-mediated dispersal is known as long distance dispersal for many riparian plants68, dams and canyons are reported to inhibit water-mediated dispersal of M. germanica23.
Study site: Isel and tributaries
This study focuses on the river Isel (ca. 50 km) in East Tyrol, Austria, and its tributaries: North to South: Tauernbach (17 km), Schwarzach (43 km) and Kalserbach (17 km) as well as the small stream Daberbach (3 km, Fig. 1). The river Isel is usually referred to as “Kleine Isel” upstream of the junction with Tauernbach and thereafter as “Isel” further downstream. In 2015, the Isel and stretches along the tributaries Kalserbach and Schwarzach (“Osttiroler Gletscherflüsse Isel, Schwarzach und Kalserbach”) were designated as Natura 2000 area (see https://natura2000.eea.europa.eu/Natura2000, site code AT3314000).
In the Isel catchment mean annual temperatures of 0.5–1 °C and precipitation of 1400–1500 mm per year were recorded, with high amounts of snow and ice in Winter69. Along the tributaries of the Isel, many constructions to prevent natural hazards such as sediment check dams are established, and there are also some barriers related to hydropower (see Fig. 1). The Isel and its tributaries have been monitored in the past for the presence of M. germanica31,36 and mainly provide habitat for the study species where the shoreline of the river is not stabilized by lateral embankment or where the formation of gravel banks is possible due to wide river section31.
The Tauernbach mainly consists of deep canyons upstream and has artificial side banks along the downstream regions, but the stretch below the canyon Prosseggklamm (see Fig. 1) offers habitat to pioneer vegetation including the study species31. Similarly, large canyons in the lower course the Kalserbach (Kalserklamm) and the Schwarzach (Defregger Klamm) display German tamarisks upstream and downstream of the canyons (Fig. 1). However, long stretches of these tributaries are also stabilized by lateral embankments, reducing the habitat availability for the German tamarisk. Therefore, the study region covers both sites with large, long-term persistent populations but also more remote sites with few individuals.
Field work
Field work along Isel and its tributaries was performed from 2014 to 2018, and a total of 45 sites were sampled (Table 1). All individual plants were recorded using GPS (Garmin Oregon 700). To assess the population structure at each site, plants were assigned to four age categories as defined in previous studies69. For large populations with over 30 individuals, at least 30 samples per sites were collected, while all individuals were sampled at sites with few shrubs (see Table 1). For each sampled individual, plant leave material was collected, dried with Silica gel (Silica Gel Orange, ROTH, Nr. P077.1) and subsequently stored at − 20 °C. All methods were performed in accordance with relevant guidelines and regulations.
Genetic analysis
For each sample, 15 mg ± 3 mg leave material was lyophilized (BETA 1-8 L0 plus, Christ, at 40 bar and − 55 °C) prior to total DNA extraction (DNeasy®96 Plant Kit, Qiagen, Cat.No. 69181). Following the protocol of70 using Multiplex PCR Master Mix», 2x (Qiagen, No. 1066295), 22 microsatellite loci were analysed using PCR. All PCR products were diluted (1:2) with ultrapure water, and 1 µL of the mix was added to 9.5 µL HiDi-LIZ solution (Applied Biosystems, Lot. 1,401,295) and size standard mixture (concentration 15 µl/mL, GeneScanTM-500 LIZ®, Applied Biosystems, Lot. 1,401,359) for the analysis on a 3730xl DNA Analyzer (ABI, Applied Biosystems).
Data analysis
Using the software GeneMapper (Applied Biosystems, V5.0), fragment analysis of the 22 microsatellite loci was performed using scoring bin sets of previous studies for details see23,49,70. Fragment length raw data is available from the authors upon request. The resulting multilocus genotype data of each individual was formatted and analyzed using the packages “poppr” and “tidyr” in the program R71: polymorphic loci, private alleles, the expected heterozygosity as well as the inbreeding coefficient (FIS) were calculated to assess genetic diversity. Additionally, the program Arlequin 3.572 was used to calculate FST-values for population differentiation, and pairwise comparisons to detect significantly differentiated populations.
To identify if single populations underwent a drastic decrease in effective population size, we used the program Bottleneck73 for populations with at least 10 individuals. We performed all three tests available in Bottleneck, the sign test, standardized difference test73 and the one-tailed Wilcoxon sign-rank test74 to evaluate if the populations showed a heterozygosity excess or deficit. Expected heterozygosity was based on simulations for the genetic distribution for each populations under the assumption of two models, as the microsatellite motifs did not allow to make a prior choice on a single model70: the stepwise mutation model (SMM) and a two-phase model (TPM) allowing for a combination of 90% of SMM and 10% of infinite allele model.
Molecular variance (AMOVA) within and between populations using Isel and each of its tributaries as a predefined geographic structure (resulting in 6 groups) was performed in Arlequin 3.572. The genetic structure of the study site was assessed in the program structure 2.3.4, testing for K = 1–45 groups75, with 108 iterations and a burn-in of 104. The output of this Bayesian approach to identify the number of groups the multilocus genotypes could be assigned to was tested for statistical support in the program Structure Harvester76.
Limitations to gene flow in the study sites as seen in an isolation by distance pattern was tested by a Mantel test based on the comparison of genetic differentiation (FST) of populations with more than 10 individuals and geographic distances along rivers Manteltest in GenAIEx 6.50377. Distances along rivers between the chosen populations were determined using the package “riverdist” in R71. To assess if migration between sites is ongoing, we applied a Bayesian approach27 to identify migrants using the software GeneClass78. In this program, we estimated the likelihood of first-generation migrants by assessing the likelihood of an individual multilocus genotype to originate from the population it was sampled from compared to the likelihood that it is sampled from another population in the catchment see also in78. Probability computations (based on Monte-Carlo simulations) were done using the algorithm of Paetkau79 with 100′000 simulated individuals and a 0.01 type I error rate.
To analyze the extent of migration between populations, we used the program BayesAss 3.0.480 implementing Markov chain Monte Carlo techniques to simulate recent migration rates from allele frequencies of multilocus genotypes. Following the manual for BayesAss, we first identified suitable mixing parameters for migrations rates, allele frequencies and inbreeding coefficients to allow for acceptance rates between 20 and 40%, as suggested from empirical analyses81. We run simulations with 108 iterations and 104 million burn-in, and diagnosed for convergence of chains using the software Tracer 1.782. Runs were repeated with different random seeds and we then identified a suitable run calculating Bayesian Deviance using the R script as described in83. To identify significant migration rates, we checked if the 95% Confidence Intervals (CI) excluded 084.
Ethics approval
All sampling permits were issued by the Office of the Tyrolean Regional Government (Amt der Tiroler Landesregierung). Sampling in 2014 and 2016 was carried out on behalf of the Office of the Tyrolean Regional Government. Sampling in 2018 was carried out based on the permit (for sampling parts of protected plants) NSCH/N-269/6–2017 from 06.09.2017 (district authority Lienz) and the decision of the State Administrative Court of Tyrol LVwG-2017/41/2267–19 from 23.05.2018.
References
Sabo, J. et al. Riparian zones increase regional species richness by harbouring different, not more, species. Ecology 86, 56–62 (2005).
Lind, L., Hasselquist, E. & Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J. Environ. Manage. 249, 109391–109391 (2019).
Merritt, D., Nilsson, C. & Jansson, R. Consequences of propagule dispersal and river fragmentation for riparian plant community diversity and turnover. Ecol. Monogr. 80, 609–626 (2010).
Jansson, R., Nilsson, C. & Renöfält, B. Fragmentation of riparian floras in rivers with multiple dams. Ecology 81, 899–903 (2000).
Mari, L. et al. Metapopulation persistence and species spread in river networks. Ecol. Lett. 17, 426–434 (2014).
Blöschl, G. et al. Changing climate both increases and decreases European river floods. Nature 573, 108–111. https://doi.org/10.1038/s41586-019-1495-6 (2019).
Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 10, 13768. https://doi.org/10.1038/s41598-020-70816-2 (2020).
Wobus, C. et al. Climate change impacts on flood risk and asset damages within mapped 100-year floodplains of the contiguous United States. Nat. Hazards Earth Syst. Sci. 17, 2199–2211 (2017).
Meyer, J. L. et al. The contribution of headwater streams to biodiversity in river networks1. J. Am. Water Resour. Assoc. 43, 86–103. https://doi.org/10.1111/j.1752-1688.2007.00008.x (2007).
Van Looy, K. & Piffady, J. Metapopulation modelling of riparian tree species persistence in river networks under climate change. J. Environ. Manage. 202, 437–446 (2017).
Sochor, M. et al. Can gene flow among populations counteract the habitat loss of extremely fragile biotopes? An example from the population genetic structure in Salix daphnoides. Tree Genet. Genomes 9, 1193–1205 (2013).
Garssen, A. G. et al. Effects of increased flooding on riparian vegetation: Field experiments simulating climate change along five European lowland streams. Glob. Change Biol. 23, 3052–3063. https://doi.org/10.1111/gcb.13687 (2017).
Ellenberg, H. Vegetation Mitteleuropas mit den Alpen in Ökologischer, Dynamischer und historischer Sicht. 6., vollst. neu bearb. und stark erw. Aufl edn, (Ulmer, 2010).
Hanski, I. Metapopulation Biology: Ecology, Genetics, and Evolution (Academic Press, New York, 1997).
Wubs, E. R. J. et al. Going against the flow: A case for upstream dispersal and detection of uncommon dispersal events. Freshw. Biol. 61, 580–595 (2016).
Chen, F.-Q. & Xie, Z.-Q. Reproductive allocation, seed dispersal and germination of Myricaria laxiflora, an endangered species in the Three Gorges Reservoir area. Plant Ecol. 191, 67–75 (2007).
Bonn, S. Ausbreitungsbiologie der Pflanzen Mitteleuropas: Grundlagen und kulturhistorische Aspekte. (Quelle und Meyer Verlag, 1998).
Müller-Schneider, P. Verbreitungsbiologie der Blütenpflanzen Graubündens: Diasporology of the Spermatophytes of the Grisons. Vol. 85. (Switzerland) (1986).
Aradottir, A., Svavarsdottir, K. & Bau, A. Clonal variability of native willows (Salix pylicifofia and Salix lanata) in Iceland and implications for use in restoration. Icel. Agric. Sci. 20, 61–72 (2007).
Egelund, B., Pertoldi, C. & Barfod, A. S. Isolation and reduced gene flow among Faroese populations of tea-leaved willow (Salix phylicifolia, Salicaceae). N. J. Bot. J. Bot. Soc. B. Isles 2, 9–15 (2012).
Van Puyvelde, K. & Triest, L. ISSRs indicate isolation by distance and spatial structuring in Salix alba populations along Alpine upstream rivers (Alto Adige and Upper Rhine). Belg. J. Bot. 140, 100–108 (2007).
Ngeve, M. N., Van der Stocken, T., Sierens, T., Koedam, N. & Triest, L. Bidirectional gene flow on a mangrove river landscape and between-catchment dispersal of Rhizophora racemosa (Rhizophoraceae). Hydrobiologia 790, 93–108. https://doi.org/10.1007/s10750-016-3021-2 (2017).
Werth, S., Schoedl, M. & Scheidegger, C. Dams and canyons disrupt gene flow among populations of a threatened riparian plant. Freshw. Biol. 59, 2502–2515 (2014).
Pollux, B. J. A., Luteijn, A., Van-Groenendael, J. M., Ouborg, N. J. & Ouborg, N. J. Gene flow and genetic structure of the aquatic macrophyte Sparganium emersum in a linear unidirectional river. Freshw. Biol. 54, 64–76 (2009).
Davis, C., Epps, C., Flitcroft, R. & Banks, M. Refining and defining riverscape genetics: How rivers influence population genetic structure. Wiley Interdiscip. Rev. Water 5, e1269 (2018).
Vega-Retter, C. et al. Dammed river: Short- and long-term consequences for fish species inhabiting a river in a Mediterranean climate in central Chile. Aquat. Conserv.Mar. Freshw. Ecosyst. 30, 2254–2268. https://doi.org/10.1002/aqc.3425 (2020).
Rannala, B. & Mountain, J. L. Detecting immigration by using multilocus genotypes. Proc. Natl. Acad. Sci. U.S.A. 94, 9197–9201 (1997).
Altermatt, F., Alther, R. & Mächler, E. Spatial patterns of genetic diversity, community composition and occurrence of native and non-native amphipods in naturally replicated tributary streams. BMC Ecol. 16, 23. https://doi.org/10.1186/s12898-016-0079-7 (2016).
Paz-Vinas, I. et al. Systematic conservation planning for intraspecific genetic diversity. Proc. R. Soc. Lond. B: Biol. Sci. 285, 20172746. https://doi.org/10.1098/rspb.2017.2746 (2018).
Sitzia, T., Kudrnovsky, H., Müller, N. & Michielon, B. Biological flora of Central Europe Myricaria germanica (L.) Desv. Perspect. Plant Ecol. Evol. Syst. 52, 125629. https://doi.org/10.1016/j.ppees.2021.125629 (2021).
Egger, G., Steineder, R. & Angermann, K. Verbreitung und Erhaltungszustand des FFH-Lebensraumtyps 3230 “Alpine Flüsse mit Ufergehölzen von Myricaria germanica” an der Isel und deren Zubringern (Osttirol, Österreich). Carinthia II 204, 391–432 (2014).
Schletterer, M., Gewolf, S., Egger, G. & Fink, S. Forschungsprojekt Tamariske: Genetische Untersuchung von Populationen an der Isel – Dokumentation der Beprobungen 2018. 32 (Innbruck, 2019).
Scheidegger, C. & Wiedmer, A. Genetische Untersuchung zur Deutschen Tamariske in Tirol. (Eidg. Forschungsanstalt WSL, Birmensdorf, 2014).
Hedrick, P., Lacy, R., Allendorf, F. & Soule, M. Directions in conservation biology: Comments on caughley. Conserv. Biol. 10, 1312–1320 (1996).
Sampson, J., Byrne, M., Gibson, N. & Yates, C. Limiting inbreeding in disjunct and isolated populations of a woody shrub. Ecol. Evol. 6, 5867–5880 (2016).
Kudrnovsky, H. & Stöhr, O. Myricaria germanica (L.) Desv. historisch und aktuell in Österreich: Ein dramatischer Rückgang einer Indikatorart von europäischem Interesse. STAPFIA Rep. 99, 13–34 (2013).
Hoban, S. et al. Genetic diversity targets and indicators in the CBD post-2020 global biodiversity framework must be improved. Biol. Conserv. 248, 108654. https://doi.org/10.1016/j.biocon.2020.108654 (2020).
Auffret, A. G., Plue, J. & Cousins, S. A. O. The spatial and temporal components of functional connectivity in fragmented landscapes. Ambio 44, 51–59. https://doi.org/10.1007/s13280-014-0588-6 (2015).
Herrmann, J. et al. Connectivity from a different perspective: Comparing seed dispersal kernels in connected vs. unfragmented landscapes. Ecology 97, 1274–1282 (2016).
Mortelliti, A., Amori, G. & Boitani, L. The role of habitat quality in fragmented landscapes: A conceptual overview and prospectus for future research. Oecologia 163, 535–547 (2010).
Mosner, E., Liepelt, S., Ziegenhagen, B. & Leyer, I. Floodplain willows in fragmented river landscapes: Understanding spatio-temporal genetic patterns as a basis for restoration plantings. Biol. Conserv. 153, 211–218 (2012).
Chambers, J., MacMahon, J. & Brown, R. Alpine seedling establishment: The influence of disturbance type. Ecology 71, 1323–1341 (1990).
Bill, H.-C. Besiedlungsdynamik und Populationsbiologie charakteristischer Pionierpflanzenarten nordalpiner Wildflüsse PhD thesis, Philipps-Universität Marburg, (2000).
Lite, S. J., Bagstad, K. J. & Stromberg, J. C. Riparian plant species richness along lateral and longitudinal gradients of water stress and flood disturbance, San Pedro River, Arizona, USA. J. Arid Environ. 63, 785–813. https://doi.org/10.1016/j.jaridenv.2005.03.026 (2005).
Andersson, E., Nilsson, C. & Johansson, M. E. Plant dispersal in boreal rivers and its relation to the diversity of riparian flora. J. Biogeogr. 27, 1095–1106 (2000).
Aguiar, F. et al. The abundance and distribution of guilds of riparian woody plants change in response to land use and flow regulation. J. Appl. Ecol. 55, 2227–2240 (2018).
Leyer, I. Dispersal, diversity and distribution patterns in pioneer vegetation: The role of river-floodplain connectivity. J. Veg. Sci. 17, 407–416 (2006).
Crookes, S. & Shaw, P. W. Isolation by distance and non-identical patterns of gene flow within two river populations of the freshwater fish Rutilus rutilus (L. 1758). Conserv. Genet. 17, 861–874. https://doi.org/10.1007/s10592-016-0828-3 (2016).
Werth, S. & Scheidegger, C. Gene flow within and between catchments in the threatened riparian plant Myricaria germanica. PLoS ONE 9, e99400 (2014).
Jacquemyn, H., Honnay, O., Van Looy, K. & Breyne, P. Spatiotemporal structure of genetic variation of a spreading plant metapopulation on dynamic riverbanks along the Meuse River. Heredity 96, 471–478. https://doi.org/10.1038/sj.hdy.6800825 (2006).
Mayer, C., Schiegg, K. & Pasinelli, G. Patchy population structure in a short-distance migrant: evidence from genetic and demographic data. Mol. Ecol. 18, 2353–2364 (2009).
Benda, L. E. E. et al. The network dynamics hypothesis: How Channel networks structure riverine habitats. Bioscience 54, 413–427 (2004).
Miettinen, A. et al. A large wild salmon stock shows genetic and life history differentiation within, but not between, rivers. Conserv. Genet. 22, 35–51. https://doi.org/10.1007/s10592-020-01317-y (2021).
Fink, S., Lanz, T., Stecher, R. & Scheidegger, C. Colonization potential of an endangered riparian shrub species. Biodivers. Conserv. 26, 2099–2114. https://doi.org/10.1007/s10531-017-1347-3 (2017).
Merritt, D. & Wohl, E. Plant dispersal along rivers fragmented by dams. River Res. Appl. 22, 1–26 (2006).
Sitzia, T., Michielon, B., Iacopino, S. & Kotze, D. J. Population dynamics of the endangered shrub Myricaria germanica in a regulated Alpine river is influenced by active channel width and distance to check dams. Ecol. Eng. 95, 828–838 (2016).
Wöllner, R., Scheidegger, C. & Fink, S. Gene flow in a highly dynamic habitat and a single founder event: Proof from a plant population on a relocated river site. Glob. Ecol. Conserv. 28, e01686. https://doi.org/10.1016/j.gecco.2021.e01686 (2021).
McLaughlin, B. et al. Hydrologic refugia, plants, and climate change. Glob. Change Biol. 23, 2941–2961 (2017).
Chiu, M. C. et al. Branching networks can have opposing influences on genetic variation in riverine metapopulations. bioRxiv https://doi.org/10.1101/550194 (2020).
Catford, J. & Jansson, R. Drowned, buried and carried away: Effects of plant traits on the distribution of native and alien species in riparian ecosystems. New Phytol. 204, 19–36 (2014).
Schletterer, M. & Scheiber, T. Wiederansiedlung der deutschen tamariske (Myricaria germanica (L.) DESV.) an der Leutascher Ache (Nordtirol, Österreich). B. Naturwiss. Med. Ver. Innsbr. 95, 53–65 (2008).
Riehl, S. & Zehm, A. in ANLiegen Natur Vol. 40, 17–20 (ANL Bayern, Laufen, 2017).
Egger, G., Angermann, K. & Gruber, A. Wiederansiedlung der Deutschen Tamariske (Myricaria germanica (L.) Desv.) in Kärnten. Carinthia II 393–418 (2010).
Kudrnovsky, H. Alpine rivers and their ligneous vegetation with Myricaria germanica and riverine landscape diversity in the Eastern Alps: Proposing the Isel river system for the Natura 2000 network. Eco. Mont 5, 5–18 (2013).
Lener, F. P. Etablierung und Entwicklung der Deutschen Tamariske (Myricaria germanica) an der oberen Drau in Kärnten Master thesis (University of Vienna, Vienna, 2011).
Schiechtl, H. M. in Alpenländ. Bienenzeitung Vol. 4 125–131 (1957).
Bill, H.-C., Poschlod, P., Reich, M. & Plachter, H. Experiments and observations on seed dispersal by running water in an Alpine floodplain. Bull. Geobot. Inst. ETH 65, 13–28 (1999).
Nilsson, C., Brown, R., Jansson, R. & Merritt, D. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 85, 837–858 (2010).
Lener, F. P., Egger, G. & Karrer, G. Sprossaufbau und entwicklung der deutschen tamariske (Myricaria germanica) an der Oberen Drau (Kärnten, Österreich). Carinthia II(203), 515–552 (2013).
Werth, S. & Scheidegger, C. Isolation and characterization of 22 nuclear and 5 chloroplast microsatellite loci in the threatened riparian plant Myricaria germanica (Tamaricaceae, Caryophyllales). Conserv. Genet. Resour. 3, 445–448 (2011).
R Core Team. R: A language and environment for statistical computing. R Found. Stat. Comp., <http://www.R-project.org/> (2016).
Excoffier, L., Laval, G. & Schneider, S. Arlequin ver 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50 (2005).
Cornuet, J. M. & Luikart, G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144, 2001–2014 (1996).
Luikart, G., Allendorf, F. W., Cornuet, J. M. & Sherwin, W. B. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 89, 238–247. https://doi.org/10.1093/jhered/89.3.238 (1998).
Falush, D., Stephens, M. & Pritchard, J. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 7, 574–578 (2007).
Earl, D. A. & von Holdt, B. M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Res. 4, 359–361 (2012).
Smouse, P. E., Peakall, R., GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28, 2537–2539. https://doi.org/10.1093/bioinformatics/bts460 (2012).
Piry, S. et al. GENECLASS2: A software for genetic assignment and first-generation migrant detection. J. Hered. 95, 536–539. https://doi.org/10.1093/jhered/esh074 (2004).
Paetkau, D., Slade, R., Burden, M. & Estoup, A. Genetic assignment methods for the direct, real-time estimation of migration rate: A simulation-based exploration of accuracy and power. Mol. Ecol. 13, 55–65. https://doi.org/10.1046/j.1365-294X.2004.02008.x (2004).
Wilson, G. A. & Rannala, B. Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163, 1177–1191 (2003).
Rannala, B. (ed University of California Davis) 1–12 (2007).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904. https://doi.org/10.1093/sysbio/syy032 (2018).
Meirmans, P. G. Nonconvergence in Bayesian estimation of migration rates. Mol. Ecol. Resour. 14, 726–733. https://doi.org/10.1111/1755-0998.12216 (2014).
Greenland, S. et al. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 31, 337–350. https://doi.org/10.1007/s10654-016-0149-3 (2016).
Acknowledgements
We thank Christine Schwarzmann and Christian Plössnig from the Office of the Tyrolean Regional Government and the advisory group on the research project “Genetic diversity at Isel” (Johannes Kostenzer, Norbert Müller, Christian Ragger, Michael Reischer, Peter Schönswetter, Kordula Schwarzwälder) for advice on the study design. We are grateful to Luca Hoppler and Susanne Gewolf for their participation in collecting plant material, and to the Genetic Diversity Center of ETH, especially Aria Minder and Silvia Kobel, for assistance with fragment analysis. Funding was provided for field work and genetic analysis in 2014 by the Office of the Tyrolean Regional Government, Austria, for data collection, lab work and analysis in 2018 and 2019 by TIWAG, Austria, and for data collection in 2016 and genetic analysis in 2020 by FOEN Switzerland, project “riverscape: sediment dynamics and connectivity”. Sampling in 2014 and 2016 was carried out on behalf of the Office of the Tyrolean Regional Government. Sampling in 2018 was carried out based on the permit (for sampling parts of protected plants) NSCH/N-269/6-2017 from 06.09.2017 (district authority Lienz) and the decision of the State Administrative Court of Tyrol LVwG-2017/41/2267-19 from 23.05.2018.
Funding
Funding was provided by the Swiss Federal Offices for the Environment for the project “Riverscape—sediment dynamics and connectivity” granted to CS. Field work was funded by the Office of the Tyrolean Regional Government, Austria (sampling periods 2014–2016) and by TIWAG—Tiroler Wasserkraft AG, Austria (sampling period 2018).
Author information
Authors and Affiliations
Contributions
S.F., C.S., G.E. and M.S. contributed to the study conception and design. Material preparation and data collection were performed by all authors. Laboratory analysis were performed by A.H.-W., V.Z. and S.F., and subsequent data analysis were done by S.F. The first draft of the manuscript was written by S.F. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors have no conflicts of interest to declare.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fink, S., Hoppler-Wiedmer, A., Zengerer, V. et al. Gene flow in a pioneer plant metapopulation (Myricaria germanica) at the catchment scale in a fragmented alpine river system. Sci Rep 12, 8570 (2022). https://doi.org/10.1038/s41598-022-12172-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12172-x
- Springer Nature Limited