Abstract
Effects of a novel dietary mixture of selenium nanoparticles (Se-NPs) and omega-3-fatty acids i.e., Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on mitigating arsenic pollution, high-temperature stress and bacterial infection were investigated in Pangasianodon hypophthalmus. To aim this, four isocaloric and iso-nitrogenous diets were prepared: control feed (no supplementation), Se-NPs at 0.2 mg kg−1 diet with EPA + DHA at 0.2, 0.4 and 0.6% as supplemented diets. Fish were reared under normal condition or concurrent exposure to arsenic (2.65 mg L−1), and temperature (34 °C) (As + T) stress for 105 days. The experiment was conducted with eight treatments in triplicates. Response to various stresses i.e., primary (cortisol), secondary (oxidative stress, immunity, and stress biomarkers) and tertiary stress response (growth performance, bioaccumulation and mortality due to bacterial infection) were determined. Supplementation of dietary Se-NPs at 0.2 mg kg−1 diet and EPA + DHA at 0.2 and 0.4% reduced the primary stress level. Exposure to arsenic and temperature (As + T) and fed with control diet and EPA + DHA at 0.6% aggravated the cortisol level. Anti-oxidative enzymes (Catalase, superoxide dismutase, glutathione peroxidase and glutathione-s-transferase) and immunity (Nitroblue tetrazolium, total protein, albumin, globulin, A:G ratio, total immunoglobulin and myeloperoxidase) of the fish were augmented by supplementation of Se-NPs and EPA + DHA at 0.2 and 0.4%. Neurotransmitter enzyme, HSP 70, Vitamin C were significantly enhanced (p < 0.01) with supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.2 and 0.4%. Whereas total lipid, cholesterol, phospholipid, triglyceride and very low-density lipoprotein (VLDL) were reduced (p < 0.01) with the supplementation of Se-NPs at 0.2 mg kg−1 diet and EPA + DHA at 0.2 and 0.4%. Tertiary stress response viz. growth performance was also significantly enhanced with supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.2 and 0.4% reared under As + T. Whereas arsenic bioaccumulation in fish tissues was significantly reduced with dietary supplementation of Se-NPs and EPA + DHA. Cumulative mortality and relative percentage survival were reduced with Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.2 and 0.4%. The investigation revealed that a novel combination of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.4% followed by 0.2% has the potential to alleviate temperature stress, bacterial infection and arsenic pollution. Whereas diet containing Se-NPs at 0.2 mg kg−1 diet and EPA + DHA at 0.6% was noticeably enhanced the stress in P. hypophthalmus.
Similar content being viewed by others
Introduction
Climate change and pollution are the major challenges affecting the functioning of ecosystems worldwide for last two decades1. Global warming has been one of the contributing factors for arsenic contamination of food and water, especially in Asian countries dependent on groundwater withdrawals2. It is now established that chronic exposure to water and foodborne arsenic can cause adverse health effects, including cancer, in humans. Since the North-Eastern states of India have high arsenite content in groundwater, crops and fish, which may accumulate arsenic from water and cause arsenicosis (toxicity from chronic exposure to arsenic) in humans3,4,5. Arsenic can contaminate ecosystems through natural as well as anthropogenic activity6. In addition to the toxic effects of arsenic on human a higher concentration of arsenic in aquatic systems can also deteriorate the condition of aquatic animals including fishes7,8. Arsenite binds with sulfhydryl groups of biomolecules viz. glutathione (GSH), lipoic acid and the cysteinyl residues of many enzymes, causing toxicity9.
Eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) are strong antioxidants and essential fatty acid (EFAs) which belong to omega-3-fatty acid. Linolenic (18:3n-3) and linoleic acid (18:2n-6) are the precursor for synthesis of EPA and DHA which has been absent in the fish10. The omega-3 fatty acids play essential roles in a wide variety of physiological functions, such as neural and visual system11,12, pigmentation13,14, bone development15,16, reproduction17, stress resistance18,19 and immunity20,21 as well as growth and development of the fish22,23, and anxiety-like behaviour and emotions24,25 in animals. Despite the essential roles of EPA and DHA in physiology, fish have a negligible capacity to synthesize them, thus necessitating dietary intake10,26. Selenium (Se) is an essential trace element for growth, immunity and maintains redox homeostasis through selenoproteins such as the glutathione peroxidase, which reduces hydrogen peroxide through the glutathione-mediated pathway in fish27,28. Selenium dependent glutathione peroxidase is very useful to control the redox state in the cell. Other selenoenzymes, viz. methionine sulfoxide reductase and thioredoxin reductase also maintain redox homeostasis and prevent oxidative damage of lipids and other biomolecules by terminating redox-reactive radicals29,30. Furthermore, selenoproteins have crucial roles in cell proliferation activation, cell differentiation, innate and adaptive immunity31,32. Selenium is also known to antagonize toxicity of elements such as cadmium and arsenite in fishes, primarily by attenuation of oxidative stress33,34. Our previous works have demonstrated that Se-NPs supplementation in fish diet could stimulate growth performance, immunity, anti-oxidative status and thermal tolerance35,36,37,38,39 in fish. Moreover, Se-NPs are more efficient and less toxic than the usual form of elemental selenium40.
Oxidative stress is the biological process in which an unpaired electron, present on reactive radical, damages the tissues in living organisms including animal and fish. These oxidizing radicals are formed as by-products of aerobic respiration41. Oxidizing radicals could exist as free radicals such as (OH·), superoxide (O2·−), nitric oxide (NO·), nitrogen dioxide (NO2·), peroxyl (ROO·) and lipid peroxyl (LOO·) and non-free radicals hydrogen peroxide (H2O2), ozone (O3), singlet oxygen (1O2), hypochlorous acid (HOCl), nitrous acid (HNO2), peroxynitrite (ONOO−), dinitrogen trioxide (N2O3), lipid peroxide (LOOH)42. Such free radicals and non-free radicals are neutralized by enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione-s-transferase (GST) and glutathione peroxidase (GPx). Therefore, a combination of Se-NPs and EPA + DHA can protect the cell against oxidative stress through enhancing anti-oxidative enzymatic systems. The mixture may also have role in improving the immunity of the fish. The present study was carried out to evaluate the protective role of dietary mixture of Se-NPs and EPA + DHA against arsenic pollution, high temperature stress and bacterial infection in Pangasianodon hypophthalmus.
Material and methods
Ethics statement
The study protocol and the end-points of the experiments were approved by the Research Advisory Committee of ICAR-NIASM. All methods were carried out in accordance with relevant national and international guidelines and regulations. The study is in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Diet preparation
Four iso-nitrogenous (35% crude protein) and iso-caloric (393 kcal/100 g) experimental diets were prepared. The basal diet containing soybean meal, fish meal, groundnut meal, wheat flour, cod liver oil, carboxymethyl cellulose (CMC), lecithin, and vitamin C. Selenium-free vitamin-mineral mixture was prepared manually for inclusion in the experimental diet. The heat-labile components were incorporated after cooking the feed ingredients (Table 1). The control diet received no Se-NP or EPA + DHA supplementation. The rest of the three experimental diets contained 0.2 mg Se-NP kg−1 diet, in mixture with EPA + DHA at 0.2, 0.4 and 0.6%. Proximate composition of four experimental diets were analyzed using AOAC method43 (Table 1). Crude protein (CP) was estimated using nitrogen content and ether extract (EE) by solvent extraction method. Ash (mineral content) was estimated by completely burning the feed overnight in a muffle furnace at 550 °C. Total carbohydrate % was calculated using the following equation:
The gross energy of the diets were calculated by the use of the method described by Halver44.
Experimental procedure
Pangasianodon hypophthalmus (weight, 4.73 ± 0.52 g, length, 4.11 cm) were procured from West Bengal, India. Fish was transported in healthy condition to Central Wet Laboratory facility of ICAR-NIASM, Baramati, Pune. Before stocking in fibre-reinforced plastic tanks, fishes were treated with salt (1%) and KMnO4 solution (2 ppm) and then acclimatized for 40 days before the commencement of the experiment. The fishes were fed with basal diet until the commencement of the experiment. Water quality were also recorded periodically using the methods suggested by the American Public Health Association, APHA45. The experiment was conducted in triplicates in twenty-four (24) tanks (capacity 150 L) with eighteen (18) fish (mean weight of 5.6 ± 1.68) in each replicate for 105 days. Eight (8) treatments were designed as follows and shown in Table 2.
Two-thirds of the tank’s water was manually exchanged on every alternate day, the arsenic concentration was maintained, and continuous aeration was provided with a compressed air pump. Feed was presented to fish in each tank until they reached apparent satiation, when feeding activity ceased. The amount of feed consumed by the fish and uneaten feed as well as faecal matters in each tank was removed daily by siphoning. In the stressors group arsenic was added as 1/10th of LC50 2.68 mg L−1 of arsenic3 and temperature (34 °C) was maintained with thermostatic heater3.
Green synthesis and characterization of Se-NPs
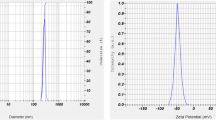
Fish gill tissue was used for the synthesis of Se-NPs. The tissues were neatly dissected out and cleaned carefully under running water, cut into several small pieces and homogenized. Tissues homogenates were centrifuged at 5000–6000 rpm for 15 min at 4 °C. The supernatant was obtained and filtered. After that, supernatant was mixed with 2 M sodium selenite (200 ml) on a shaker for 96 h. The final solution was centrifuged at 6000 rpm at 15 min at 4 °C and then pellet was obtained. The pellet was isolated and dried for 5 h. Before diet preparation, Se-NPs were crushed in to a fine powder35,36. Se-NPs were characterized through a spectrophotometer in an absorption spectrum at 360–380 nm. Particles size of 203.5 nm and zeta potential − 41.8 mV (Fig. 1A,B) was confirmed through Nanoparticles Analyzer (Horiba Scientific Nanoparticles Analyzer, nano-partica SZ-100 series Kyoto, Japan) at 25 °C.
Tissue homogenate preparation and blood collection
Fishes were anesthetized with clove oil (100 µl L−1) before collecting the gill, kidney, liver and brain tissues. The collected tissues were homogenized in chilled sucrose (5% w/v, 0.25 M) and EDTA solution (1 mM) using tissue homogenizer (Omni Tissue Master Homogenize, Kennesaw, GA). The sample containing tube was kept on ice during homogenization to avoid denaturation of the enzyme activities. The sample homogenates were centrifuged for supernatant collection at 5000×g for 15 min at 4 °C in a cooling centrifuge (Eppendorf AG, 5430R, Hamburg, Germany). After that, the supernatant were stored at − 20 °C until further analysis. Blood was also collected from four (4) fish and used for serum preparation (heparin free syringe) and blood from three (3) fish was collected with heparinized syringe to avoid blood clotting. The tissue protein was also determined using Lowry method46.
Sample preparation for analysis of arsenic and selenium
Muscle, liver, gill, kidney and brain were collected for determination of arsenic concentration. Elements such as selenium (Se), lithium (Li), sodium (Na), magnesium (Mg), potassium (K), calcium (Ca), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), gallium (Ga), arsenic (As), rubidium and molybdenum (Mo) were analyzed in experimental diet. The tissues and diets were processed in microwave digestion system (Microwave Reaction System, Multiwave PRO, Anton Paar GmbH, Austria, Europe) using HNO3 and H2O2 in 5:1 ratio. After acidic digestion the digested sample were filtered using 0.45 µm pore size filter paper and made up to volume of 50 mL for final process. Water used for experiments was acidified with HNO3 (100 µL HNO3 in 10 mL water samples) and used for arsenic determination. Then samples were analyzed in an Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Agilent 7700 series, Agilent Technologies, USA). The multi-element standard was used for calibration curve at R2 > 0.99947,48.
Growth performance
Weight gain (%), feed conversion ratio (FCR), specific growth rate, protein efficiency ratio (PER), daily growth index (DGI), thermal growth coefficient (TGC) and relative feed intake (RFI) were determined in the study. The weight of the fish was observed on every 15 days intervals up to 105 days.
where \(\Sigma {\text{D}}0\,{\text{is}}\,{\text{the}}\,{\text{thermal}}\,{\text{sum}}\,({\text{feeding}}\,{\text{days}} \times {\text{average}}\,{\text{temperature}},^\circ {\text{C}})\)
Antioxidant enzyme activities
Activity of superoxide dismutase (EC 1.15.1.1) was estimated using method described by Misra and Fridovich49. Activity of catalase (EC 1.11.1.6) was determined using method described by Takahara et al.50. Glutathione-s-transferase, GST (EC 2.5.1.18) and glutathione peroxidase, GPx (EC 1.11.1.9) were determined using method described by Habing et al.51, and Paglia and Valentine52, respectively.
Lipid peroxidation (LPO)
LPO was determined using the method of Uchiyama and Mihara53. Briefly, 0.25 mL of liver and kidney tissues homogenates were mixed with 25 μL of 10 mM butylated hydroxytoluene (BHT) to which 3 mL phosphoric acid (1%) with 1 mL of 0.67% thiobarbituric acid (TBA) were added. The homogenate was then incubated at 90 °C for 45 min and absorption was read at 535 nm in spectrophotometer.
Neurotransmitter enzyme activity
Acetylcholine esterase (AChE) (EC. 3.1.1.7) activity was measured using the Hestrin et al.54 and modified by Augustinsson55.
Cortisol and HSP-70
Serum cortisol and HSP 70 were determined using ELISA kit (Cortisol EIA kit, catalogue no. 500360, Cayman Chemicals, USA; HSP 70 kit EKS-700B, Bioguenix/Enzo Life Science, Mumbai, India). The assay was performed as per instruction provided with the kit.
Ascorbic acid (vitamin C)
Ascorbic acid was estimated from brain and muscle tissue, followed by the method of Roe and Keuther56.
Nitroblue tetrazolium (NBT), serum protein and A:G ratio
NBT activities were determined as followed as Secombes57 and modified by Stasiack and Baumann58. The serum protein was estimated by using a protein estimation kit. Albumin was estimated by method of Doumas et al.59 and globulin was quantified by subtracting albumin values from total plasma protein.
Myeloperoxidase content (MPO) and total immunoglobulin level
The myeloperoxidase was quantified as method of Quade and Roth60 with some modifications61 and total immunoglobulin level was determined using method of Anderson and Siwicki62.
Blood glucose
The determination of blood glucose was determined as per the method of Nelson63 and Somoyogi64. The final reading was obtained at 540 nm against the blank.
Lipid profiling
Total lipid was determination as per method of Bligh and Dyer65. Similarly, phospholipid was determined as per method of Bartlett66 and modified by Marinetti67. Total cholesterol was determined as per the procedure of Henly68. Further, triglyceride was calculated based on total lipid, phospholipid and cholesterol. The very low density lipoprotein (VLDL) was calculated by dividing triglyceride by 5.
Challenge study with Aeromonas hydrophila
P. hypophthalmus was infected with Aeromonas hydrophilla (Lot no. 637-51-5 and Ref 0637P, HiMedia, Mumbai) after 105 days experimental trial. A. hydrophila was culture in nutrient broth for 24 h at 37 °C in an incubator and the culture was harvested and after centrifuging the culture broth at 6000 rpm for 15 min at 4 °C. The harvested cells were washed thrice in PBS (pH 7.2) and finally diluted to obtain 108 CFU mL−1. Thereafter, 0.15 mL of bacterial suspension was injected to fishes and observed morality up to 7 days. The tissues were collected from morbid fish for confirmation of A. hydrophilla. Cumulative mortality and relative survival were obtained as follows:
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences program version 16.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean ± standard error of mean and tested for normality and homogeneity of variance using the Shapiro–Wilk’s and Levene’s test, respectively. When both tests were satisfied, one-way ANOVA (Analysis of variance) with Duncan’s multiple range tests (DMRT) was employed to test the statistically significant difference at p < 0.05.
Results
Concurrent exposure to arsenic and temperature elicits primary stress response (cortisol) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
The results of cortisol are shown in the Fig. 2A. Primary stress response is the resultant of first indicator of stress induced by arsenic and high temperature in fish. In the present investigation the cortisol was noticeably elevated (p = 0.0032) with concurrent exposure to arsenic (As) and high temperature (34 °C) (As + T) when not fed with supplemented diet, in comparison to the control group. Cortisol was significantly reduced (p < 0.01) with supplementation of Se-NPs (0.2 mg kg−1 diet) and EPA + DHA at 0.2 and 0.4% with or without exposure to As + T in comparison to control group. However, group supplemented with Se-NPs at 0.2 mg kg−1 diet and EPA + DHA at 0.6% with or without stressors (As + T) was similar to control group.
(A, B) Mitigation of primary and secodary stress response (Cortisol and HSP 70) through dietary supplementation of selenium nanoparticles, eicosapentanoic acid and dicosahexanoic acid fed to P. hypophthalmus reared in control or under arsenic and high temperature (As + T) for 105 days. Within endpoints and groups, bars with different superscripts differ significantly (a–d) cortisol (p = 0.032), HSP-L (p = 0.026), HSP-G (p = 0.014). Data expressed as Mean ± SE (n = 3).
Concurrent exposure to arsenic and temperature elicits secondary stress response (HSP 70; Oxidative stress; LPO) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
Chaperon protein are highly conservative in nature. In the present investigation, we have studied heat shock protein 70 (HSP 70) in liver and gill tissues (Fig. 2B). The HSP 70 in liver (p = 0.026) and gill (p = 0.014) were noticeably elevated in the group concurrently exposed to As + T and fed with control diet in comparison to unexposed group (control group). Se-NP at 0.2 mg kg−1 and EPA + DHA at 0.4% diet significantly reduced the HSP 70 in liver and gill tissues with or without exposure to As + T in comparison to unexposed group (control group) and group exposed to As + T and fed with control diet. Gills and livers of the fish exposed to As and thermal stress and fed with diet containing 0.6% EPA + DHA showed highest HSP-70, which was also statistically similar to the fish exposed to As and thermal stress alone.
CAT activity in liver (Table 3) was significantly higher (p = 0.0023) in fish concurrently exposed to As + T and fed with control diet in comparison to control and other supplemented group except Se-NPs at 2 mg kg−1 and EPA + DHA at 0.6% diet and exposed to As + T. Whereas, CAT activity was noticeably lower (p < 0.01) in supplemented group fed with Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.4% in comparison to all other groups. Whereas, in kidney tissue, the remarkably highest (p = 0.002) activities was observed in control fed and concurrently exposed to As + T followed by group fed with Se-NPs at 0.2 mg kg−1 with EPA + DHA at 0.6% diet with or without exposure to As + T. The CAT activities were significantly (p < 0.01) reduced with supplementation of Se-NPs and EPA + DHA at 0.4% diet in comparison to all other groups. Similarly, in case of gill catalase, highest activity was observed (p = 0.014) in group exposed to As + T and fed with control diet in comparison to unexposed group. The supplementation of Se-NPs at 0.2 mg kg−1 with EPA + DHA at 0.4% significantly reduced the catalase activity in gill tissues with or without As + T exposure, followed by 0.2% fed group in comparison to control group, stressors group and Se-NPs and EPA + DHA at 0.6%. Similarly, superoxide dismutase (SOD) activities in liver (p = 0.034) and gill (p = 0.027) were significantly elevated in group concurrently exposed to As + T and fed with control diet group. Further, the activity of SOD in liver was noticeably reduced (p < 0.01) with supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% diet group in comparison to all other treatments. In case of kidney, the highest activities were observed in the group concurrently exposed to As + T and fed with control diet as well as Se-NPs and EPA + DHA at 0.6% diet group. Whereas, the activities were significantly reduced (p = 0.043) with application of Se-NPs and EPA + DHA at 0.4% and 0.2% diet in comparison to stressors group. The activity of SOD in kidney was similar in control group and supplemented group (Se-NPs and EPA + DH at 0.4 and 0.2%). Whereas, in case of gill, the supplementation with Se-NPs and EPA + DHA at 0.4, 0.2 and 0.6% diet group was did not affect SOD activity in comparison to control group (Table 3). GST activities in gill (p = 0.041) and kidney (p = 0.0013) were significantly higher in group concurrently exposed to As + T and fed with control diet as well as Se-NPs and EPA + DHA at 0.6% in comparison to control group. Whereas, in case of liver (p = 0.011) the highest activities were observed in the group concurrently exposed to As + T and fed with control diet and Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.6% diet group then unexposed group. Dietary supplementation with Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.4 and 0.2% with or without stressors (As + T) significantly reduced (p < 0.01) the GST activities in liver and kidney, in comparison to all other treatments group. Similarly, gill GST activities were remarkably reduced (p < 0.01) with supplemented group of Se-NPs and EPA + DHA at 0.4% diet group in comparison to other treatments (Table 4). Glutathione peroxidase (GPx) activities in liver, gill and kidney were significantly enhanced (p < 0.01) with exposure to As + T and fed with control diet and Se-NPs and EPA + DHA at 0.6% diet group in comparison to unexposed group (control group). GPx in liver (p = 0.0035), gill (p = 0.041) and kidney (p = 0.0023) were noticeably reduced (p < 0.01) with supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.4% and 0.2% diet group with or without stressors. The supplemented group with Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.6% diet, without exposure to stressors, showed GPx activity similar to control diet group in liver, gill and kidney tissues, whereas, with exposure to stressors (As + T) the group (Se-NPs at 0.2 mg kg−1 diet and EPA + DHA at 0.6%) showed a significantly higher GPx activity in comparison to control, but similar to that of the fish exposed to As + T and fed with control diet (Table 4).
Lipid peroxidation (LPO) level in gill (p = 0.023), liver (0.028) and kidney (p = 0.034) tissues were noticeably higher (p < 0.01) in group exposed to As + T and fed with control diet as well as Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.6% diet in comparison to control group. The LPO in liver and kidney were significant reduced (p < 0.01) with dietary supplementation of Se-NPs and EPA + DHA at 0.4% diet group in comparison to all other treatment groups. Whereas, LPO in gill was significantly lowered (p = 0.023) with dietary supplementation of Se-NPs and EPA + DHA at 0.2 or 0.4%, with or without stressors, (Table 4) in comparison to all other treatment groups.
Concurrent exposure to arsenic and temperature elicits secondary stress response (Acetylcholine esterase, Vitamin C) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
Figure 3A presents the AChE activity in brain and muscle tissues of P. hypophthalmus fed with EPA + DHA and Se-NPs reared in control or under As + T. AChE activities in brain (p = 0.0028) and muscle (p = 0.029) tissues were noticeably inhibited (p < 0.01) with concurrent exposure to As + T and fed with control diet or Se-NPs with EPA + DHA at 0.6%. Whereas AChE activity in muscle and brain was noticeably enhanced (p < 0.01) with supplementation of Se-NPs and EPA + DHA at 0.4% diet group with or without exposure to As + T in comparison to other treatments. Similarly, Vitamin C in muscle (p = 0.038) and brain (p = 0.0056) was significantly reduced with exposure to As + T and fed with control diet as well as Se-NPs and EPA + DHA at 0.6% diet group in comparison to unexposed group (control group). Further, Vitamin C in brain and muscle was remarkably enhanced (p < 0.01) with dietary supplementation of Se-NPs and EPA + DHA at 0.4% diet group with or without exposure to stressors (As + T), in comparison to other treatments. Results also revealed that supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.6% diet group noticeably reduced the Vit C in brain and muscle tissues with or without exposure to As + T in comparison to other supplemented and control diet group (Fig. 3B).
(A, B) Mitigation of secondary stress response (acetylcholine esterase and vitamin C) through dietary selenium nanoparticles, eicosapentanoic acid and dicosahexanoic acid fed to P. hypophthalmus reared in control or under arsenic and high temperature (As + T) for 105 days. Within endpoints and groups, bars with different superscripts differ significantly (a–f) AChE-B (p = 0.0028), AChE-M (p = 0.029), VitC-B (p = 0.0056), VitC-M-G (p = 0.038). Data expressed as Mean ± SE (n = 6).
Concurrent exposure to arsenic and temperature elicits secondary stress response (total protein, albumin, globulin, A:G ratio, NBT, blood glucose, total immunoglobulin and myeloperoxidase) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
Table 5 summarizes the results of immunological attributes of P. hypophthalmus exposed to As + T and fed with normal and experimental diets. Total protein was significantly enhanced (p = 0.001) with supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.4% diet group with or without exposure to stressors (As + T), in comparison to other treatments including EPA + DHA at 0.2 and 0.6% diet group. Supplemented group with Se-NPs and EPA + DHA at 0.2% with or without exposure to As + T showed similar level of TP to control group. However, globulin (p = 0.0041) and NBT (p = 0.002) were significantly reduced with exposure to arsenic and high temperature (As + T) and fed with control diet or mixture of Se-NPs and EPA + DHA at 0.6%, in comparison to the other treatment groups. Moreover, the globulin and NBT were significantly higher in fish with supplementation of Se-NPs and EPA + DHA at 0.4% or 0.2%, with or without stressors (As + T), in comparison to control group, stressor group (As + T) and EPA + DHA at 0.6% diet group. Similarly, albumin and A: G ratio were significantly enhanced (p < 0.01) with exposure to As + T fed with control diet. Further, the albumin was significantly reduced (p = 0.038) with dietary supplementation of Se-NPs and EPA + DHA at 0.2, 0.4 and 0.6% diet group with or without exposure to As + T in comparison to unexposed group and stressor group and fed with control diet. Similarly, A:G ratio was significantly reduced with (p = 0.0051) supplementation of Se-NPs and EPA + DHA at 0.2 and 0.4% diet with or without exposure to As + T in comparison to control group and stressor group (As + T) (Table 4). Blood glucose of P. hypophthalmus exposed to As + T and fed with control diet was significantly elevated (p = 0.0063) in comparison to all other groups. Results showed that dietary supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% with or without exposure to stressors (As + T) were remarkably reduced the blood glucose level in comparison to control group and other supplemented group. The blood glucose in group fed with mixture of Se-NPs and EPA + DHA (without stressors) at 0.6% was similar to control diet group, whereas blood glucose was significantly higher in Se-NPs and EPA + DHA (with stressors) at 0.6% than control group (Table 5).
Other immunological attributes viz. total immunoglobulin and MPO are reported in the Fig. 4A, B. Total immunoglobulin (p = 0.0042) and MPO (p = 0.017) were significantly reduced with concurrent exposure to As + T and fed with control diet in comparison to control group and supplemented groups except EPA + DHA at 0.6 diet group in P. hypophthalmus. Whereas, total immunoglobulin was significantly enhanced (p = 0.0042) with supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% diet groups with or without exposure to stressors (As + T) in comparison to other treatments. Similarly, MPO was significantly enhanced (p = 0.017) with combinatorial mixture of Se-NPs and EPA + DHA at 0.4 and 0.2% with or without exposure to As + T in comparison to other treatments. Results revealed that supplemented group of Se-NPs and EPA + DHA at 0.6% has not much potential to improve the total immunoglobulin and MPO in P. hypophthalmus.
(A,B) Mitigation of secondary stress response (total immunoglobulin and myeloperoxiadase) through dietary supplementation of selenium nanoparticles, eicosapentanoic acid and dicosahexanoic acid fed to P. hypophthalmus reared in control or under arsenic and high temperature (As + T) for 105 days. Within endpoints and groups, bars with different superscripts differ significantly (a–f) Total immunoglobulin (p = 0.0042), Myeloperoxidase (p = 0.017). Data expressed as Mean ± SE (n = 6).
Concurrent exposure to arsenic and temperature elicits secondary stress response (lipid profiling) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
Data pertaining to lipid profiling (total lipid, cholesterol, phospholipid, triglyceride and very low density lipoprotein, VLDL) of P. hypophthalmus reared in control or under arsenic and high temperature (As + T) are recorded in Table 6. Total lipid, cholesterol, phospholipid and VLDL were noticeably higher (p < 0.01) with from exposeure to As + T and when fed with control diet in comparison to control diet group. However, results of total lipid, cholesterol, triglyceride and VLDL in group fed to Se-NPs and EPA + DHA at 0.6% diet group without and not exposed to stressors (As + T) were similar with control group. Total lipid, cholesterol (p = 0.0073) and phospholipid (p = 0.039) were significantly reduced by supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% diet groups with or without exposure to As + T in comparison to other treatment groups. Whereas, VLDL (p = 0.024) and triglyceride (p = 0.018) were significantly reduced (p = 0.018) with combinatorial mixture of Se-NPs and EPA + DHA at 0.4% in comparison to fed group of 0.2 and 0.4% EPA + DHA with or without stressors (As + T).
Concurrent exposure to arsenic and temperature elicits tertiary stress response (growth performance) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
Data pertaining to growth performance (final weight gain %, FCR, PER, SGR, DGI, TGC and RFI) of P. hypophthalmus fed with control and supplemented diets (Se-NPs and EPA + DHA) reared in control or under As + T are recorded in Table 7. Final body weight gain (%), specific growth rate (SGR), protein efficiency ratio (PER), daily growth index (DGI), thermal growth coefficient (TGC) and relative feed intake (RFI) of P. hypophthalmus were noticeably inhibited (p < 0.01) by concurrent exposure to As + T and fed with control diet in comparison to control group. Whereas FCR (p = 0.016) was significantly enhanced with concurrent exposure to As + T and fed with control diet in comparison to unexposed group (control group). SGR (p = 0.0078), DGI (p = 0.0043), TGC (p = 0.012) and RFI (p = 0.0038) were noticeably increased in group fed with combinatorial mixture of Se-NPs and EPA + DHA at 0.4 and 0.2% diet group with or without exposure to As + T in comparison to unexposed group (control), stressors group (As + T) and Se-NPs and EPA + DHA at 0.6% diet group. Similarly, final body weight gain (%) (p = 0.029) and PER (p = 0.031) were remarkably increased with Se-NPs and EPA + DHA at 0.4% supplemented diet, in comparison to other treatments, regardless of exposure to stressors. The FCR (p = 0.016) was significantly lower with combinatorial mixture of Se-NPs and EPA + DHA at 0.4% diet group in comparison to other treatments. The group fed with combinatorial mixture of Se-NPs and EPA + DHA at 0.6% showed similar final body weight gain (%), PER, DGI, TGC and RFI with control diet group (unexposed to As + T). Overall results revealed that growth performance of the P. hypophthalmus was drastically reduced by exposure to As + T and fed with control diet.
Concurrent exposure to arsenic and temperature elicits tertiary stress response (Bacterial challenges, relative survival (%) and cumulative mortality) but alleviate by dietary EPA + DHA and Se-NPs in P. hypophthalmus
The results of relative survival (%) (RPS) and cumulative mortality (%) of P. hypophthalmus reared in control or under As + T and injected with A. hydrophila after experimental period of 105 days are presented in Fig. 5A, B. A 70 and 60% higher RPS was observed in groups fed with Se-NPs and 0.4% EPA + DHA, either in combination with As + T stress or absence of it, respectively. Whereas, RPS of 40 and 50% was observed in groups fed with Se-NPs and EPA + DHA at 0.2% with or without exposure to stressors (As + T), respectively. RPA in the groups exposed to As + T stress and fed with control diet or combination of Se-NPs and 0.6% EPA + DHA was observed lowest; 10 and 20%, respectively. Correspondingly, the cumulative mortality was also highest (66.66 and 62.5%, respectively) in the treatment groups exposed to As + T and fed with control diet or 0.6% EPA + DHA-based diet. The lowest cumulative mortality (%) was observed in the group treated with Se-NPs and EPA + DHA at 0.2 or 0.4% as 41.67 and 33.33% respectively, whereas in the same diet group (0.2 or 0.4% EPA + DHA), with exposure to As + T, the cumulative mortality was observed as 37.20 and 29.17%, respectively.
(A,B) Mitigation of tertiary stress response (relative survival (%) and cumulative mortality) through dietary supplementation of selenium nanoparticles, eicosapentanoic acid and dicosahexanoic acid fed to P. hypophthalmus reared in control or under arsenic and high temperature (As + T) for 105 days. Data expressed as Mean ± SE (n = 30).
Bioaccumulation of arsenic and selenium in fish tissues and water
The concentration of arsenic in water was significantly higher in group treated with As + T and fed with control diet (868.47 µg L−1) and EPA + DHA at 0.6% group (774.05 µg L−1), in comparison to control and other groups. The lowest arsenic concentration was determined in the group fed with EPA + DHA at 0.4, 0.6 and 0.2% and control group (unexposed group) (Table 8). The bioaccumulation of the arsenic was higher in kidney > liver > gill > muscle > brain. The arsenic bioaccumulation was noticeably reduced with group fed with combinatorial mixture of Se-NPs and EPA + DHA at 0.4 and 0.2% in comparison to other groups. The selenium bioaccumulation in muscle was significantly higher (p < 0.01) in group fed with Se-NPs and EPA + DHA at 0.4% without As + T in comparison to all other experimental groups (Table 7). Similarly, significantly lower (p < 0.01) Se bioaccumulation was found in the group fed with Se-NPs and EPA + DHA at 0.6% regardless of exposure to stressors, in EPA + DHA at 0.4% diet group exposed to stressors and in group fed EPA + DHA at 0.2% regardless to exposure to stressors (Table 8).
Mineral profiling of experimental diets
Data pertaining to mineral profiling of experimental diets is recorded in Table 9. Li, Na, Mg, K, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As and Mo was determined in the experimental diets. Li concentration varies from 0.47 to 0.93, Na 452.62–642.81, Mg 368.518.67, K 719.56–802, Ca 642–845, V 0.024–0.047, Cr 0.89–1.56, Mn 4.18–4.28, Mn 3.45–4.63, Fe 98.65–132, Co 0.21–0.42, Ni 0.47–0.78, Cu 1.85–2.37, Zn 5.89–7.18, Ga 0.26–0.62, As 0.12–0.24, Ru 0.75–1.01, and Mo 0.19–0.86 mg kg−1 of diet.
Discussion
Climate change and arsenic pollution are the major challenges for the aquatic ecosystem, which may interfere with the production cycle of the aquatic environment. The arsenic contaminated fish may cause diseases, including cancer in human. The present investigation deals with dietary intervention using combinatorial mixture of Se-NPs at 0.2 mg kg−1 diet and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) at 0.2, 0.4 or 0.6%, to mitigate arsenic pollution and temperature induced stress in P. hypophthalmus. The results and their discussion are categorized based on the hierarchy of animal stress response as primary, secondary and tertiary.
The primary stress response as cortisol was elevated in fish exposed to arsenic and elevated temperature (As + T). Cortisol is an endocrine hormone secreted from interrenal cells of fish kidney, which is regulated by corticotropin-releasing hormone (CRH)69. It can be used as an indicator of fish health due to its role in metabolism, immunity and osmoregulation. Cortisol mobilizes free fatty acid, glucose and amino acid to meet the immediate energy demands of the animal, however, excess mobilization of these metabolites by cortisol reduced the body and muscle mass due to increased energy expenditures70,71. Arsenic can target several sites on hypothalamus-pituitary-interrenal axis which might be the reason for increased cortisol secretion in this study. Arsenic can alter the ACTH and effect blood cortisol levels in fish3,35,72. In the current study, the cortisol was remarkably reduced with combinatorial mixture of Se-NPs and EPA + DHA at 0.4 and 0.2% diet. Se-NPs have very important role in synthesis of thioredoxin reductase, glutathione peroxidase and deiodinase73. It has also functional role in stimulation of adrenocorticotropic hormone (ACTH) which then bind with the steroidogenic membrane receptor to activate the second messenger pathway (cAMP)74. Similarly, EPA + DHA are also essential for activation and feedback mechanism of HPA-axis which affects the gluco-corticoid receptor function and transports of the cortisol across the blood brain barrier. EPA and DHA also influence on secretion of corticotrophin releasing hormone75. However, EPA + DHA along with Se-NPs are important for cortisol regulation in fish and have role in stress mitigation76 and modulatory effect on HPA activity. It is also reported that deficiency of EPA + DHA could increase corticotrophin releasing factor (CRF) and cortisol levels77. In the present investigation, the higher level of EPA + DHA at 0.6% diet along with Se-NPs enhanced the cortisol level and revealed that higher inclusion of EPA + DHA induces stress in fish.
Brain work like machineries to control oxidative challenges and if it fails the heat shock protein (HSP) is upregulated to provide the protection against the stress78. HSP are multifaceted and can be expressed during a variety of stress including metal pollution79,80 and thermal stress39,81 and therefore serves as a biomarker for cellular stress response. HSPs comprise a highly conserved, ubiquitously stress-response proteins and function like molecular chaperones which have role in synthesis, protein folding, transport and translocalization of protein and prevent protein aggregation78. In the present investigation the supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% diet significantly reduced the HSP 70 in liver and gill. Se-NPs have essential role in passing the signal from peptides to antigen cells and have their own function such as seleno-methionine. It has role in regulation of HSP during stress condition4,35. Data from present study suggested that Se-NPs and EPA + DHA at 0.4 or 0.2% might also be helping in synthesis for maintenance of the cellular homeostasis through correct folding of nascent and stress-accumulated misfolded proteins in the cell82,83.
Oxidative stress enzymes viz. CAT, SOD, GST and GPx were elevated after exposure to arsenic and high temperature. Arsenic interacts with cellular antioxidant system and induce oxidative stress resulting in higher inflammation rate and finally accumulates free radicals in the cell41. Further, arsenic can also induce generation of reactive oxygen species such as hydroxyl radical (·OH), hydrogen peroxide (H2O2), superoxide anion (O2·−), peroxyl radicals and singlet oxygen (−O2). The primary reactive oxygen species (ROS) formed by superoxide anions through metabolic process and or after oxygen activation, can further directly interact with enzymes or generate secondary ROS through metal catalyzed process84. ROS can be also formed by three other sources viz. (1) Arsenic reacts with acid and forms arsine which produce large number of free radicals85. (2) Methylated can formed redox-active iron from ferritin which has important role in generating oxygen species through conversion of O2·− and H2O2 into the highly reactive ·OH radical86. (3) Generated during oxidation of arsenite to arsenate87. The oxidative stress induced by arsenic and temperature was significantly reduced with combinatorial mixture of Se-NPs and EPA + DHA at 0.4 and 0.2%. Se-NPs and EPA + DHA acts as strong antioxidant which reduced the oxidative stress and enhanced anti-oxidative status of the fish22,88. Glutathione peroxidase is the important selenoprotein and shows anti-oxidative activity through selenocysteine which protect cell against oxidative stress and injuries89. EPA has an important role in production of eicosanoids and its metabolites improve cellular anti-oxidative mechanisms, which could have resulted in reduced ROS generation against arsenic and high temperature. They also reduce the 8-isoprostane levels and ease oxidative stress90. EPA and DHA also have major function in free radical metabolism by activating peroxisome proliferator-activated receptors (PPARs) which have role in neutralization of oxidative stress91. At higher level of EPA + DHA (0.6%), elevated oxidative stress in fish as revealed by our present investigation. The present study showed that higher dose of EPA + DHA at 0.6% enhanced the oxidative stress which might be due to production of excessive reactive oxygen species (ROS)92,93. A similar result was reported by Todorcevic et al.93, that supplementation of higher n-3 HUFAs (High unsaturated fatty acid) enhanced the oxidative stress and apoptosis in Salmo solar (Atlantic salmon). However, HUFAs also reduce the fatty acid β-oxidation capacity which facilitates oxidative stress.
In the present investigation, lipid peroxidation (LPO) in liver, gill and kidney were significantly elevated with exposure to As + T, when fed with basal diet or the diet containing higher (0.6%) dose of EPA + DHA. The elevated LPO might be due to production of free radical leading to oxidative damage of PUFAs of cell membranes94. Increased LPO was perhaps resultant of free radical mediated damage, including direct reaction with free radicals and deactivation of anti-oxidative mechanisms, as evident from the increased activities of CAT, SOD, GST and GPx in the present study95. The application of combinatorial mixture of Se-NPs and EPA + DHA at 0.4% diet reduced the level of LPO which might be due to role of Se in defence mechanism of tissues and organs through GPx and other seleno-enzymes96. EPA + DHA at 0.2% also reduced the LPO level in tissues which might be due to efficient anti-oxidative function of EPA and DHA97.
Acetylcholine esterase (AChE) is the cholinergic enzyme that breaks down acetylcholine into acetic acid and choline. The AChE activity is mostly present in the brain and to some extent in the muscle tissue. Generally, AChE hydrolyses, acetylcholine into choline and acetate at the postsynaptic membrane from acetylcholine receptors. Therefore, it is mainly found in the neuromuscular junctions and cholinergic synapses in the central nervous system. Hence, it is essential for central and peripheral nervous system for its proper functioning98. In the present investigation, AChE was inhibited by concurrent exposure to As + T in fish fed with control diet and higher inclusion levels of EPA + DHA at 0.6% diet. However, the AChE activity was augmented by combinatorial mixture of Se-NPs with EPA + DHA at 0.4 or 0.2% diet, in both brain and muscle tissues. The augmentation of AChE by Se-NPs and EPA + DHA at 0.2 and 0.4% diet might be due to role of selenium in maintenance of central nervous systems as selenoprotein which arise from cortical and hippocampal neurons of brain99. EPA has capacity to control the regulation of AChE due to change of neuron membrane fluidity and viscosity, mainly at neuronal synapses100.
Vitamin C in the present study was elevated by dietary Se-NPs and EPA + DHA at 0.4 or 0.2% diet group. However, exposure to arsenic and high temperature with basal diet or 0.6% EPA + DHA failed to maintain the vitamin C level in brain and muscle tissues. Vitamin C is potent anti-oxidant needed for collagen synthesis101 and has crucial role in metabolism of several biomolecules viz. steroids and detoxification of xenobiotics. Selenium is known to spare vitamin C due to anti-oxidative activities of selenoenzymes which can help in regeneration of ascorbic acid from dehydroascorbic acid. This could be the reason behind the maintained the high level of vitamin C in the tissues102.
Immunological attributes viz. total protein, albumin, globulin, A:G ratio, nitro blue tetrazolium (NBT), blood glucose, total immunoglobulin and myeloperoxidase are indicators of better immunity in animal including fish. In the present investigation, arsenic and high temperature had a detrimental effect on the immunological parameters but administration of dietary Se-NPs with EPA + DHA at 0.4 or 0.2% diet reasonably improved immune parameters. Reduced non-specific immunity in the fish observed in the current study could be due to the stress induce by As + T exposure and also from higher inclusion of EPA + DHA at 0.6% diet which lead to non-specific immuno-suppression in fish. Se-NPs are nutritionally important and have activities similar to selenite, methyl selenocysteine and selenomethionine. Although the bioavailability of Se-NPs is higher which cause upregulation of selenoproteins and lower toxicity in animals40. Total protein, albumin and globulin are the important parts of nonspecific immunity. Heightened immune response is often correlated with accelerated protein synthesis and was also evident in the current study where Se-NPs and EPA + DHA increased serum protein and the immunity of the fish reared under As + T. Due to nutritional enrichment of the diets, the B-lymphocytes play an important role in enhancement of immunity in fish103.
Albumin is needed for transportation of metal, hormones, vitamin, drug, bilirubin, fat metabolites and regulates the free available hormones103. Albumin is mainly secreted from liver in fish to fulfil the high energy demand during stress through protein synthesis. In the present investigation, the albumin was less than globulin, however rapid utilization of albumin to meet the immediate energy demand could also increases its rate of synthesis in the liver.
Further, it was also revealed that fish with low globulin is more susceptible to water pollution due to poor immune resistance104. Furthermore, nitro blue tetrazolium (NBT) test estimates the functioning of phagocytes, and higher NBT indicates superior non-specific immunity105. However, total immunoglobulin is the indictor for strong immunity against microbes and pathogen. It also has role in prevention of tissues damage and proliferation of infectious agent106. Cortisol also has depressive role in immunity of the fish. Study conducted by Esteban et al.107 revealed that higher cortisol level reduced the immunity of the fish. This claim was also revealed by Gamperl et al.108 that deleterious effects of stress on the immune system have been attributed mainly due to elevated levels of cortisol. The depressive effect of cortisol on immune response in fish depend upon both dosage and incubation time.
Myeloperoxidase (MPO) is also crucial for strong immunity. It has role during respiratory burst and forms hypochlorous acid from hydrogen peroxide109. The hypochlorous acid is a strong oxidant with cytotoxic effects. In the present study the combinatorial mixture of Se-NPs and EPA + DHA improved MPO level, suggesting their role in increased levels of neutrophils and the control the tissues damaged by other abiotic factors. MPO uses O2 derived species (H2O2) released by neutrophils to oxidize Cl− ions and form HOCl which is important for bactericidal activity110. The present study also demonstrated that higher intake of EPA + DHA altered the immunity of the fish. It can also enhance the lipid peroxidation and oxidative stress111.
Cortisol, as primary stress response upregulates blood glucose through gluconeogenesis and glycogenolysis pathways112. Similarly, the chromaffin cells also enhanced the release of catecholamines, as stress response to increase glycogenolysis and regulate respiratory and cardiovascular function113. This process stimulates the glucose to produce enough energy to fulfil the demand. During stress condition, chromaffin cells release adrenaline noradrenaline and catecholamine hormones toward blood circulation which mobilizes cortisol and increases glucose production in fish through glycogenolysis and glucogenesis pathways112 to cope with the energy demand produced by the stressor. Moreover, the production of glucose is mostly dependent upon the cortisol, which stimulates liver gluconeogenesis114. Finally, the blood glucose released toward the blood circulation enters into cells via insulin action114. In the present investigation concurrent exposure to As + T and fed with control diet and Se-NPs and EPA + DHA at 0.6% diet group significantly enhanced blood glucose level. Dietary supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% reduced blood glucose level could be due to regulate the gluconeogenesis of glucose from protein and amino acid (non-carbohydrate source)115. Blood glucose is also correlated with HSP 70, which increases insulin sensitivity and uptake of glucose to meet high energy demands116.
Lipid profile viz. total lipid, cholesterol, phospholipid, triglyceride and VLDL was negatively affected by concurrent exposure to As + T, whereas augmented with supplementation of Se-NPs and EPA + DHA at 0.4 and 0.2% diet. The higher total lipid, cholesterol, phospholipid, triglyceride and VLDL were significantly higher with concurrent exposure to As + T and could be due to enhanced lipogenesis by stressors in the fish which is also reflected in the present results of LPO and cortisol. Cortisol also have role in lipolysis and lipogenesis but it is still a matter of debate. The study conducted by Djurhuus et al.117 mentions that cortisol could inhibit basal and catecholamine which stimulate lipolysis in cultured human adipocytes, but similar reports in fish are still scanty. Total lipid, cholesterol, phospholipid and VLDL were reduced by selenium supplementation as it has important role in lipid metabolism of the fish10. In contrast to our results the triglyceride was enhanced with Se supplementation diet which was reported by Khalil et al.118. Selenium deficit diet increases VLDL level in rat which might be due to excessive esterification119. Supplementation of dietary PUFAs can reduced the cholesterol level due to enhancement of membrane fluidity, which was reported in gilthead sea bream by Magalhaes et al.120.
Growth performance (weight gain %, FCR, PER, TGC, and RFI) comes under tertiary stress response. Results clearly demonstrated that exposure to arsenic and temperature and fed with control diet reduced the growth performance. This is accordance with a previous study where dietary exposure to arsenite impeded growth in rainbow trout (Oncorhynchus mykiss)8. Se-NPs and EPA + DHA have growth promoter property with smaller concentration. Selenium has very diverse function in metabolism for enzymatic oxidation–reduction and nucleic acid as well as increase protein and water in the cell through oxidizing materials such as carotenoids and vitamin A121. Earlier report also suggested that application of dietary EPA + DHA improved growth performance in fish under ideal condition (non-stressors)118. In the present investigation, the supplemented groups with 0.2 and 0.4% EPA + DHA have lowered FCR and highest SGR and PER. Hence the weight gain (%) of the fish was higher in all the treatments where EPA + DHA supplemented diets were administered. Luo et al.122 had shown on Acipenser baerii that EPA and DHA are required for normal growth performance. Similarly, EPA and DHA also helped to improve growth performance in large yellow croaker exposed to biotic stress20.
Our previous study on P. hypophthalamus fed selenium nanoparticles at 1 mg kg−1 diet has also showed improved growth performance35,36,37 in fish. Results of daily growth index, thermal growth coefficient and relative feed intake support the higher body weight gain in the present study. The group supplementation with higher dose of EPA + DHA at 0.6% and Se-NPs at 0.2 mg kg−1 did not show improved growth performance in comparison to the control group. Perhaps, higher dose of EPA + DHA at 0.6%, induce oxidative stress made cells more vulnerable to oxidative stress through incorporation of more HUFAs in the cells, leading to compromised growth rate and immunity123.
The concentration and bioaccumulation of arsenic in fish tissues revealed that supplementation of Se-NPs and EPA + DHA significantly reduced arsenic bioaccumulation. Se bioaccumulation in muscle tissues was also determined and found that higher Se accumulation in the groups fed with Se-NPs and EPA + DHA at 0.4%. These findings showed that Se have capacity for absorption of arsenic. The study conducted by Moulick et al.123 demonstrated that selenium priming of rice seed reduced arsenic bioaccumulation in plants when grown in arsenic contaminated water. Similarly, in the present investigation we found less arsenic in the water in the tanks where Se-NPs supplemented diet was given. This is perhaps due to arsenic absorbing capacity of selenium. EPA + DHA might also aid detoxification of arsenic through liver and kidney as showed in the present investigation35. We have also performed the mineral profiling in 17 elements in the experimental diet. The results showed all essential (macro and micro elements) were present adequately in experimental diet.
At the end of experimental trial of 105 days the P. hypophthalmus was infected with A. hydrophila and cumulative mortality and relative survival (%) (RPS) was observed for 7 days. The present investigation clearly demonstrated that exposure to As + T with basal diet or inclusion of high concentration of EPA + DHA (0.6%) caused higher cumulative mortality and lower RPS. Nevertheless, supplementation of Se-NPs and EPA + DHA with low inclusion level (0.2 and 0.4%) reduced the cumulative mortality and enhanced the RPS. Our previous study on P. hypophthalmus reared under multiple stressors and infection with pathogenic bacteria showed that, dietary Se-NPs can reduce the mortality35,37. Perhaps, this is due to anti-oxidative and immunomodulatory role Se-NPs through multiple selenoenzymes. The similar results were also reported by Zuo et al.20 where DHA/EPA protected the large yellow croaker fish against infestation of parasites.
Conclusion
The present investigation is the first study to report the role of selenium nanoparticles (Se-NPs) (synthesized from fisheries waste) and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in mitigation of arsenic pollution, high temperature stress and pathogenic infection in P. hypophtahlmus. It also describes the mechanistic role of Se-NPs and EPA + DHA in mitigation of primary stress, secondary stress and tertiary stress response. The growth performance, immune-modulation and most importantly anti-oxidative status was improved by supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.4% followed or 0.2%. Overall results also concluded that supplementation of Se-NPs at 0.2 mg kg−1 and EPA + DHA at 0.6% is not effective against arsenic pollution and high temperature stress as well as infection against pathogenic bacteria. The combinatorial mixture of Se-NPs and EPA + DHA in the diet may be an efficient feed supplement to develop high nutritive feed against multiple stressors. Therefore, it is recommended that Se-NPs at 0.2 mg kg−1 diet and EPA + DHA at 0.4% followed by 0.2% could be included in fish diets for enhancement of growth performance, immunity, anti-oxidative status and RPS (relative percentage survival) as well as reduction of cumulative mortality due to pathogenic bacterial and bioaccumulation of arsenic in critical fish tissues.
References
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308(5730), 1912–1915 (2005).
Bondu, R., Cloutier, V., Rosa, E. & Benzaazoua, M. Mobility and speciation of geogenic arsenic in bedrock groundwater from the Canadian Shield in western Quebec, Canada. Sci Total Environ. 574, 509–519 (2017).
Kumar, N., Gupta, S. K., Bhushan, S. & Singh, N. P. Impacts of acute toxicity of arsenic(III) alone and with high temperature on stress biomarkers, immunological status and cellular metabolism in fish. Aquat. Toxicol. 4(214), 105233 (2019).
Kumar, N. Dietary riboflavin enhances immunity and anti-oxidative status against arsenic and high temperature in Pangasianodon hypophthalmus. Aquaculture. 533, 736209 (2021).
Kumar, N., Krishnani, K. K. & Singh, N. P. Oxidative and cellular metabolic stress of fish: An appealing tool for biomonitoring of metal contamination in the Kolkata, Wetland, a Ramsar Sit. Arch. Environ. Contam. Toxicol. 76(3), 469–482 (2019).
EFSA, EFSA panel on contaminants in the food chain, scientific opinion on arsenic in food. EFSA J. 7, 1351 (2009).
Jamwal, A. & Niyogi, S. Dose and chemical species-specific effects of selenium against arsenite toxicity in cultured hepatocytes of rainbow trout (Oncorhynchus mykiss). Metallomics 9(6), 744–756 (2017).
Jamwal, A., Saibu, Y., MacDonald, T., Graham, G. N. & Niyogi, S. The effects of dietary selenomethionine on tissue-specific accumulation and toxicity of dietary arsenite in rainbow trout (Oncorhynchus mykiss) during chronic exposure. Metallomics 11(3), 643–655 (2019).
Aposhian, H. V. & Aposhian, M. M. Arsenic toxicology: Five questions. Chem. Res. Toxicol. 19, 1–15 (2006).
Tocher, D. R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11(2), 107–184 (2003).
Noffs, M. D. et al. Dietary fish oil replacement with lard and soybean oil affects triacylglycerol and phospholipid muscle and liver docosahexaenoic acid content but not in the brain and eyes of surubim juveniles Pseudoplatystoma sp. Fish Physiol. Biochem. 35(3), 399–412 (2009).
Benitez-Santana, T. et al. Dietary n-3 HUFA deficiency induces a reduced visual response in gilthead seabream Sparus aurata larvae. Aquaculture 264(1–4), 408–417 (2007).
Villalta, M., Estévez, A., Bransden, M. P. & Bell, J. G. Effects of dietary eicosapentaenoic acid on gowth, survival, pigmentation and fatty acid composition in Senegal sole (Solea senegalensis) larvae during Artemia feeding period. Aqua. Nutr. 14(3), 232–241 (2008).
Vizcaino-Ocho, V., Lazo, J. P., Baron-Sevilla, B. & Drawbridge, M. A. The effect of dietary docosahexaenoic acid (DHA) on growth, survival and pigmentation of California halibut Paralichthys californicus larvae (Ayres, 1810). Aquaculture 302(3–4), 228–234 (2010).
Roo, F. J. et al. Effect of DHA content in rotifers on the occurrence of skeletal deformities in red porgy Pagrus pagrus (Linnaeus, 1758). Aquaculture 287(1–2), 84–93 (2009).
Gapasin, R. S. J. & Duray, M. N. Effects of DHA-enriched live food on growth, survival incidence of opercular deformities in milkfish (Chanos chanos). Aquaculture 193, 49–63 (2001).
Mazorra, C. et al. Dietary lipid enhancement of broodstock reproductive performance and egg and larval quality in Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 227(1–4), 21–33 (2003).
Liu, J. et al. Necessity of dietary lecithin and eicosapentaenoic acid for growth, survival, stress resistance and lipoprotein formation in gilthead sea bream Sparus aurata. Fish. Sci. 68(6), 1165–1172 (2002).
Kanazawa, A. Effects of docosahexaenoic acid and phospholipids on stress tolerance of fish. Aquaculture 155, 129–134 (1997).
Zuo, R. et al. Effects of dietary docosahexaenoic to eicosapentaenoic acid ratio (DHA/EPA) on growth, nonspecific immunity, expression of some immune related genes and disease resistanc e of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans). Aquaculture 334, 101–109 (2012).
Wu, F. C., Ting, Y. Y. & Chen, H. Y. Dietary docosahexaenoic acid is more optimal than eicosapentaenoic acid affecting the level of cellular defence responses of the juvenile grouper Epinephelus malabaricus. Fish Shellf. Immunol. 14(3), 223–238 (2003).
Hossein, Y. M. et al. Effect of oral supplementation of biogenic selenium nanoparticles on white blood cell profile of BALB/c mice and mice exposed to X-ray radiation. Avicenna J. Med. Biotechnol. 5(3), 58–167 (2013).
Glencross, B. D., Rutherford, N. R. & Jones, J. B. The docosahexaenoicacid (DHA) requirements of juvenile barramundi (Lates calcarifer). Aqua. Nutr. 17, 536–548 (2011).
Song, C., Leonard, B. E. & Horrobin, D. F. Dietary ethyl eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress 7(1), 43–54 (2004).
Chalon, S. et al. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J. Nutr. 128, 2512–2519 (1998).
Sargent, J. R., Tocher, D. R. & Bell, J. G. The lipids. In Fish Nutrition (eds Halver, J. E. & Hardy, R. W.) 181–257 (Academic Press, Elsevier, 2002).
Felton, S. P., Landolt, M. L. & Grace, R. Effects of selenium dietary enhancement on hatchery-reared coho salmon, Oncorhynchus kisutch (Walbaum), when compared with wild coho: Hepatic enzymes and seawater adaptation evaluated. Aquat. Res. 27, 135–142 (1996).
Arteel, G. E. & Sies, H. The biochemistry of selenium and the glutathione system. Environ. Toxicol. Pharm. 10, 153–158 (2001).
Driscoll, D. M. & Copeland, P. R. Mechanism and regulation of selenoprotein synthesis. Ann. Rev. Nutr. 23, 17–40 (2003).
Papp, L. V., Lu, J., Holmgren, A. & Khanna, K. K. Selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxi. Redox Signal. 9(7), 775–806 (2007).
Kudva, A. K., Shay, A. E. & Prabhu, K. S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastro. Liver Physiol. 309(2), G71–G77 (2015).
Huang, Z., Rose, A. H. & Hoffmann, P. R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 16(7), 705–743 (2012).
Jamwal, A., Naderi, M. & Niyogi, S. An in vitro examination of selenium-cadmium antagonism using primary cultures of rainbow trout (Oncorhynchus mykiss) hepatocytes. Metallomics 8(2), 2018–2227 (2016).
Jamwal, A., Lemire, D., Driessnack, M., Naderi, M. & Niyogi, S. Interactive effects of chronic dietary selenomethionine and cadmium exposure in rainbow trout (Oncorhynchus mykiss): A preliminary study. Chemosphere 197, 550–559 (2018).
Kumar, N. et al. Mitigation potential of selenium nanoparticles and riboflavin against arsenic and elevated temperature stress in Pangasianodon hypophthalmus. Sci. Rep. https://doi.org/10.1038/s41598-020-74911-2 (2020).
Kumar, N. et al. Synergistic effect of dietary selenium nanoparticles and riboflavin on the enhanced thermal efficiency of fish against multiple stress factors. J. Therm. Biol. 85, 102417 (2019).
Kumar, N. et al. Immuno-protective role of biologically synthesized dietary selenium nanoparticles against multiple stressors in Pangasianodon hypophthalmus. Fish Shellf. Immunol. 78, 289–298 (2018).
Kumar, N. & Singh, N. P. Effect of dietary selenium on immuno-biochemical plasticity and resistance against Aeromonas veronii biovar sobria in fish reared under multiple stressors. Fish Shellf. Immunol. 84, 38–47 (2019).
Kumar, N., Krishnani, K. K., Gupta, S. K. & Singh, N. P. Selenium nanoparticles enhanced thermal tolerance and maintain cellular stress protection of Pangasius hypophthalmus reared under lead and high temperature. Res. Physiol. Neurobiol. 246, 107–116 (2017).
Wang, H., Zhang, J. & Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Rad. Biol. Med. 42(10), 1524–1533 (2007).
Halliwell, B. Oxidative stress and neurodegeneration: Where are we now?. J. Neurochem. 97(6), 1634–1658 (2006).
Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal 19(9), 1807–1819 (2007).
AOAC. Official Methods of Analysis Vol. 1, 16th ed. In: Cunnif PA (ed.) AOAC International, Arlington, pp. 31–65 (1995).
Halver, J.E. The nutritional requirements of cultivated warm water and cold water fish species. In: Report of the FAO Technical Conference on Aquaculture, Kyoto, Japan, 26 May–2 June 1976. FAO Fisheries Report No. 188 FI/ R188 (En), p 9 (1976).
APHA-AWWA-WEF. In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard Methods for the Estimation of Water and Waste Water, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC (1998).
Lowry, O. H., Ronebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Kumar, N., Krishnani, K. K., Meena, K. K., Gupta, S. K. & Singh, N. P. Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 171, 265–274 (2017).
Kumar, N., Krishnani, K. K., Gupta, S. K. & Singh, N. P. Cellular stress and histopathological tools used as biomarkers in Oreochromis mossambicus for assessing metal contamination. Environ. Toxicol. Pharmacol. 49, 137–147 (2017).
Misra, H. P. & Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247(10), 3170–3175 (1972).
Takahara, S. et al. Hypocatalesemia, a new generis carrier state. J. Clin. Invest. 39(4), 610–619 (1960).
Habing, W. H., Pabst, M. N. & Jakoby, W. B. Glutathione S-transferases—The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249(22), 7130–7139 (1974).
Paglia, D. E. & Valentine, W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70(1), 158–169 (1967).
Uchiyama, M. & Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86(1), 271–278 (1978).
Hestrin, S. The reaction of acetyl choline and other carboxylic acid derivatives with hydroxylamine and its analytical application. J. Biol. Chem. 180(1), 249–261 (1949).
Augustinsson, K. B. Substrate concentration and specificity of choline ester-splitting enzymes. Arch. Biochem. 23, 111–126 (1949).
Roe, J. H. & Keuther, C. A. The determinations of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine (DNPH) derivative of dehydroascorbic acid. J. Biol. Chem. 147, 399–407 (1943).
Secombes, C. J. Isolation of Salmonid macrophage and analysis of their killing activity. In Techniques in Fish Immunology (eds Stolen, J. S. T. C. et al.) 137–152 (SOS Publication, 1990).
Stasiack, A. S. & Bauman, C. P. Neutrophil activity as a potent indicator concomitant analysis. Fish Shellf. Immunol. 37, 539–546 (1996).
Doumas, B. T., Watson, W. & Biggs, H. G. Albumin standards and measurement of serum albumin with bromocresol green. Clin. Chim. Acta. 31, 87–96 (1971).
Quade, M. J. & Roth, J. A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 58(3–4), 239–248 (1997).
Sahoo, P. K., Kumari, J. & Mishra, B. K. Non-specific immune responses in juveniles of Indian major carps. J. Appl. Ichthyol. 21(2), 151–155 (2005).
Anderson, D. P. & Siwicki, A. K. Basic haematology and serology for fish health programmes. In Diseases in Asian Aquaculture II, Fish Health Section (eds Shhariff, J. R. & Subasinghe, R. P.) 185–202 (Asian Fisheries Society, 1995).
Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 153(2), 375–380 (1944).
Somoyogi, M. A new reagent for the determination of sugars. J. Biol. Chem. 160, 61–68 (1945).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Bartlett, G. R. Phosphorus assay in column chromatography. J. Biol. Chern. 234, 466–468 (1959).
Marinetti, G. V. Chromatographic separation, identification and analysis of phosphatides. J. Lipid. Res. 3, 1–20 (1962).
Henly, A. A. Determination of serum cholesterol. Analyst 82, 286–287 (1957).
Hontela, A. Interrenal dysfunction in fish from contaminated sites: In vivo and in vitro assessment. Environ. Toxicol. Chem. 17(1), 44–48 (1998).
Rizza, R. A., Mandarino, L. J. & Gerich, J. E. Cortisol-induced insulin resistance in man: Impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J. Clin. Endocrinol. Metab. 54(1), 131–138 (1982).
Djurhuus, C. B. et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol. Endocrinol. Metab. 283, E172–E177 (2002).
Thang, N. Q., Huy, B. T., Tan, L. V. & Phuong, N. T. K. Lead and arsenic accumulation and its effects on plasma cortisol levels in oreochromis sp. Bull. Environ. Contam. Toxicol. 99(2), 187–193 (2017).
Janz, D. M. et al. Ecological Assessment of Selenium in the Aquatic Environment 141–231 (CRC Press, 2010).
Hagen, J. I., Kusakabe, M. & Young, G. Effects of ACTH and cAMP on steroidogenic acute regulatory protein and P450 11β-hydroxylase messenger RNAs in rainbow trout interrenal cells: Relationship with in vitro cortisol production. Gen. Comp. Endocrinol. 145(3), 254–262 (2006).
Mocking, R. J. T. et al. Relationship between the hypothalamic-pituitaryadrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuro 38(9), 1607–1617 (2013).
Bradbury, J., Myers, S. P. & Oliver, C. An adaptogenic role for omega-3 fatty acids in stress: A randomized placebo-controlled double blind intervention study (pilot) [ISRCTN22569553]. Nutr. J. 3, 20–23 (2004).
Delarue, J. et al. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 29, 289–295 (2003).
Richter-Landsberg, C. & Bauer, N. G. Tau-inclusion body formation in oligodendroglia: The role of stress proteins and proteasome inhibition. Int. J. Dev. Neurosci. 22(7), 443–451 (2004).
Kumar, N., Chandan, N. K., Wakchaure, G. C. & Singh, N. P. Synergistic effect of zinc nanoparticles and temperature on acute toxicity with response to biochemical markers and histopathological attributes in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 229, 108678 (2020).
Kumar, N., Krishnani, K. K. & Singh, N. P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. Int. 25(9), 8914–8927 (2018).
Kumar, et al. Dietary nano-silver: Does support or discourage thermal tolerance and biochemical status in air-breathing fish reared under multiple stressors?. J. Therm. Biol. 77, 111–121 (2018).
Sikora, A. & Grzesiuk, E. Heat shock response in gastrointestinal tract. J. Physiol. Pharmacol. 58, 43–62 (2007).
Saluja, A. & Dudeja, V. Heat shock proteins in pancreatic diseases. J. Gastroen. Hepatol. 23, S42–S45 (2009).
Valko, M., Morris, H. & Cronin, M. T. D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161–1208 (2005).
Yamanaka, A. et al. Oral administration of dimethylarsinic acid, a main metabolite of inorganic arsenic, in mice promotes skin tumorigenesis initiated by dimethylbenz(a)anthracene with or without ultraviolet B as a promoter. Biol. Pharm. Bull. 24, 510–514 (2001).
Ahmad, S., Kitchin, K. T. & Cullen, W. R. Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch. Biochem. Biophys. 382, 195–202 (2000).
Rossman, T. G. Mechanisms of arsenic carcinogenesis. An integrated approach. Mutat. Res. 533, 37–65 (2003).
Hossain, M. S., Hashimoto, M. & Masumura, S. Influence of docosahexaenoic acid on cerebral lipid peroxide level in aged rats with and without hypercholesterolemia. Neurosci. Lett. 244(3), 157–160 (1998).
Flohe, L. The selenoprotein glutathione peroxidase. In Glutathione Chemical, Biochemical, and Medical Aspects (eds Dolphin, D. et al.) 643–731 (Wiley, 1989).
Meital, L. T. et al. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 9(1), 12978 (2019).
Camps, J. et al. PPARs in regulation of paraoxonases: control of oxidative stress and inflammation pathways. PPAR Res. https://doi.org/10.1155/2012/616371 (2012).
Ji, H., Li, J. & Liu, P. Regulation of growth performance and lipid metabolism by dietary n-3 highly unsaturated fatty acids in juvenile grass carp, Ctenopharyngodon idellus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 159(1), 49–56 (2011).
Todorcevic, M. et al. N-3 HUFAs affect fat deposition, susceptibility to oxidative stress, and apoptosis in Atlantic salmon visceral adipose tissue. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 152(2), 135–143 (2009).
Levent, G. et al. Oxidative stress and antioxidant defense in patients with chronic hepatitis C patients before and after pegylated interferon alfa-2b plus ribavirin therapy. J. Transl. Med. 4(1), 1–6 (2006).
Koruk, M. et al. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann. Clin. Lab. Sci. 34(1), 57–62 (2004).
Klotz, L. O., Kroncke, K. D., Buchczyk, D. P. & Sies, H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J. Nutr. 133(5), 1448–1451 (2003).
Leonardi, F. et al. Effect of arachidonic, eicosapentaenoic and docosahexaenoic acids on the oxidative status of C6 glioma cells. Free Radic. Res. 39(8), 865–874 (2005).
Lionetto, M. G., Caricato, R., Calisi, A., Giordano, M. E. & Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. Biomed. Res. Int. 2013(1), 321213 (2013).
Roth, S., Zhang, S., Chiu, J., Wirth, E. K. & Schweizer, U. Development of a serum free supplement for primary neuron culture reveals the interplay of selenium and vitamin E in neuronal survival. J. Trace Elem. Med. Biol. 24(2), 130–137 (2010).
Puskas, L. G. & Klara Kitajka, K. Nutrigenomic approaches to study the effects of n-3 PUFA diet in the central nervous system. Nutr. Health 18(3), 227–32 (2006).
Padayatty, S. J. et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 22(1), 18–35 (2003).
May, J. M., Mendiratta, S., Hill, K. E. & Burk, R. F. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J. Biol. Chem. 272(36), 22607–22610 (1997).
Kumar, N. et al. Dietary zinc promotes immuno-biochemical plasticity and protects fish against multiple stresses. Fish Shellf. Immunol. 17(62), 184–194 (2017).
Javed, M. & Usmani, N. Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by Thermal Power Plant effluent. Saudi J. Biol. Sci. 22(2), 237–242 (2015).
Sharp, G. J. E. & Secombes, C. J. The role of reactive oxygen species in the killing of the bacterial fish pathogen Aeromonas salmonicida by rainbow trout macrophages. Fish Shellf. Immunol. 3(2), 119–129 (1993).
Uribe, C., Folch, H., Enriquez, R. & Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 56(10), 486–503 (2011).
Esteban, M. A., Rodríguez, A., Ayala, A. G. & José Meseguer, J. Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.). Gen. Comp. Endocrinol. 137(1), 89–98 (2020).
Gamperl, A. K., Vijan, M. M. & Boutilier, R. G. Experimetnal control of stress hormone levels in fishes: Techniques and applications. Rev. Fish Biol. Fish. 4, 215–255 (1994).
Beutler, B. Innate immunity: An overview. Mol. Immunol. 40, 845–859 (2004).
Caipang, C. M. A., Berg, I., Brinchmann, M. F. & Kiron, V. Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 295, 110–115 (2009).
Kris-Etherton, P. M., Harris, W. S. & Appel, L. J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106(21), 2747–2757 (2002).
Iwama, G. K., Pickering, A. D., Sumpter, J. P. & Schreck, C. B. (ed.). Fish stress and health in aquaculture. Society for Experimental Biology Seminar Series 62. Cambridge University Press (1997).
Wedemeyer, G. A., Barton, B. A. & McLeay, D. J. 1990. Stress and acclimation. In: Schreck, C. B. & Moyle, P. B. (eds). Methods for Fish Biology 491–527. American Fisheries Society.
Reid, W. H., Mason, M. & Hogan, T. Suicide prevention effects associated with clozapine therapy in schizophrenia and schizoaffective disorder. Psychiatr. Serv. 49(8), 1029–1033 (1998).
Wickson, M. E. & Morgan, A. F. The effect of riboflavin deficiency upon carbohydrate metabolism in anoxia. J. Biol. Chem. 162, 209–220 (1946).
Kumar, N., Krishnani, K. K., Gupta, S. K. & Singh, N. P. Effects of silver nanoparticles on stress biomarkers of Channa striatus: Immuno-protective or toxic?. Environ. Sci. Pollut. Res. 25, 14813–14826 (2018).
Djurhuus, C. B. et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Environ. Sci. 283(1), E172–E177 (2002).
Khalil, H. S., Mansourb, A. T., Goda, A. M. A. & Omar, E. A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture 501, 135–143 (2019).
Scott, R. L., Kheshti, A., Heimberg, M., Wilcox, H. G. & Stone, W. L. The role of selenium in the secretion of very-low-density lipoprotein in the isolated perfused rat liver. Biochem. J. 279(3), 741–745 (1991).
Magalhaes, R. et al. Effect of dietary ARA/EPA/DHA ratios on growth performance and intermediary metabolism of gilthead sea bream (Sparus aurata) juveniles. Aquaculture 516, 734644 (2020).
Luo, L. et al. Effect of dietary DHA/EPA ratio on the early development, antioxidant response and lipid metabolism in larvae of Siberia sturgeon (Acipenser baerii, Brandt). Aqua. Nutr. 25(1), 239–248 (2019).
Kjær, M. A., Todorčević, M., Torstensen, B. E., Vegusdal, A. & Ruyter, B. Dietary n-3 HUFA affects mitochondrial fatty acid beta-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon. Lipids 43(9), 813–827 (2008).
Moulick, D., Santra, S. C. & Ghosh, D. Effect of selenium induced seed priming on arsenic accumulation in rice plant and subsequent transmission in human food chain. Ecotoxicol. Environ. Saf. 152, 67–77 (2018).
Acknowledgements
The present work is part of an institutional project (#IXX15014) conducted at ICAR-National Institute of Abiotic Stress Management. Authors sincerely acknowledge to Indian Council of Agricultural Research (ICAR), New Delhi, India for providing financial support. Authors also express sincere gratitude to Director ICAR-NIASM for providing research facilities.
Author information
Authors and Affiliations
Contributions
N.K.: Conceived and designed the experiments; performed the experiments; analysed the data; contributed reagents; wrote the paper D.K.: Analysed the data S.B.: Drawn graph, analysis of the data A.J.: Manuscript edited.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, N., Singh, D.K., Bhushan, S. et al. Mitigating multiple stresses in Pangasianodon hypophthalmus with a novel dietary mixture of selenium nanoparticles and Omega-3-fatty acid. Sci Rep 11, 19429 (2021). https://doi.org/10.1038/s41598-021-98582-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98582-9
- Springer Nature Limited
This article is cited by
-
Manganese nutrient mitigates ammonia, arsenic toxicity and high temperature stress using gene regulation via NFkB mechanism in fish
Scientific Reports (2024)
-
Multifunctional role of dietary copper to regulate stress-responsive gene for mitigation of multiple stresses in Pangasianodon hypophthalmus
Scientific Reports (2024)
-
Significance of dietary quinoa husk (Chenopodium quinoa) in gene regulation for stress mitigation in fish
Scientific Reports (2024)
-
Understanding the molecular mechanism of arsenic and ammonia toxicity and high-temperature stress in Pangasianodon hypophthalmus
Environmental Science and Pollution Research (2024)
-
Manganese nanoparticles control the gene regulations against multiple stresses in Pangasianodon hypophthalmus
Scientific Reports (2023)