Abstract
Although favourable surgical outcomes for myelopathy caused by cervical ossification of the posterior longitudinal ligament (OPLL) have been reported, factors significantly associated with post-operative neck pain attenuation still remain unclear. The primary aim of the present study was to determine factors significantly associated with post-operative neck pain attenuation in patients with cervical OPLL using a prospective multi-centre registry of surgically treated cervical OPLL. Significant postoperative neck pain reduction (50% reduction of neck pain) was achieved in 31.3% of patients. There was no significant difference in neck pain attenuation between surgical procedures. Statistical analyses with univariate analyses followed by stepwise logistic regression revealed neurological recovery as a factor having a significant positive association with post-operative neck pain attenuation (p = 0.04, odds ratio 5.68 (95% confidence interval: 1.27–22.2)). In conclusion, neurological recovery was an independent factor having a significant positive association with post-operative neck pain attenuation in patients with cervical myelopathy caused by OPLL who underwent cervical spine surgery.
Similar content being viewed by others
Introduction
Ossification of the posterior longitudinal ligament (OPLL) is a disease with heterotopic ossification in the spinal posterior longitudinal ligament1. Computed tomography screening has revealed an unexpectedly high prevalence of OPLL in the cervical spine, which affects about 7–10% of the general population in Japan2,3. Increase of thickness of ossification foci can cause compression of nerve roots and the spinal cord, possibly resulting in neurological deficits4. Favourable surgical outcomes for cervical OPLL have been reported. Nerve root and spinal cord symptoms including numbness, palsy, and vesico-rectal disturbance can be attenuated after surgery for cervical OPLL5.
In addition to neurological symptoms including radiculopathy and myelopathy, cervical OPLL can cause local symptoms such as neck pain and stiffness, which can be main complaints for patients6. Previous reports revealed neck pain attenuation by cervical spine decompression/fusion surgeries for cervical OPLL7,8,9,10. However, the precise aetiology of neck pain, postoperative change of neck pain, and factors significantly associated with post-operative neck pain attenuation in patients with cervical OPLL still remain unclear.
The primary aim of the present study was to elucidate the post-operative change of neck pain and to determine factors significantly associated with post-operative neck pain attenuation in patients with cervical OPLL using a prospective multi-centre registry of surgically treated cervical OPLL.
Results
Patient demographics are shown in Table 1. Pre-operative visual analogue scale (VAS 1–100 mm) neck pain score was 62.0 ± 21.4 mm on average (± SD), VAS neck pain score was reduced to 46.2 ± 27.2 mm at 1 year after surgery, therefore, post-operative 1 year VAS neck pain score reduction after surgery was 15.9 ± 26.1 mm and VAS neck pain score reduction was 23.1 ± 46.6%, and VAS neck pain score 2 years after surgery was 48.2 ± 28.8 mm, post-operative 2 years VAS neck pain score reduction was 13.8 ± 28.4 mm and VAS neck pain score reduction was 18.6 ± 53.4%. Fifty-six patients (21.1%) showed post-operative neck pain deterioration, whereas the remaining 209 (78.9%) showed no deterioration. Significant attenuation of neck pain, which was set to 50% reduction of VAS neck pain, was achieved in 77 out of 265 patients 1 year after surgery (29.1%) and 83 out of 265 patients 2 year after surgery (31.3%, Table 2).
There was no significant difference in pre-operative VAS neck pain score between surgical procedures. Post-operative VAS neck pain scores, neck pain reduction score, and proportion of neck pain reduction showed no significant difference between surgical procedures (Table 3).
Age, diabetes mellitus, disease duration and Japanese Orthopedic Association score for evaluating cervical myelopathy (JOA score) recovery rate were identified as possible candidates for factors having significant association with postoperative neck pain attenuation by initial uni-variate analyses (Table 4). Logistic regression analysis revealed the JOA score recovery rate as an independent factor having a significant positive association with post-operative neck pain attenuation (p = 0.04, odds ratio 5.68 (95% confidence interval: 1.27–22.2), Table 4). Receiver-operator characteristic (ROC) analysis revealed a JOA score recovery rate of 52.6% as a cut-off value to achieve at least a 50% reduction of post-operative neck pain score (area under curve (AUC) = 0.6).
Discussion
The present results demonstrated that neurological recovery was an independent factor having a significant positive association with post-operative neck pain attenuation.
There are many previous reports showing the possible aetiologies of neck pain.
Axial pain, as first reported by Hosono, which is defined as post-operative neck pain related to posterior approach-induced muscle damage, is regarded as a major cause of post-operative neck pain11. Various kinds of muscle preserving posterior approaches have been reported to attenuate post-operative axial neck pain12,13,14. The anterior approach does not invade the posterior musculo-ligamentous complex; therefore, post-operative muscle-related neck pain can be decreased compared with that in the posterior approach15. However, the present results showed that there is no significant difference in post-operative neck pain attenuation between surgical approaches (anterior and posterior) or surgical procedures (laminoplasty, PDF and ADF), suggesting that surgical damage of the cervical musculature has no significant association with post-operative neck pain in the present patient series. Possible explanations for this discrepancy in muscle damage-related neck pain between previous reports and the present data might be as follows: the posterior approach-related muscle damage decreased according to the recent popularization of muscle-preserving posterior approaches and the impact of posterior approach-related muscle damage might be limited to the early post-operative phase and not the chronic phase.
Discogenic and/or facet genic neck pain, which is caused by degenerated intervertebral disks and facet joints accompanied with segmental instability, can be another possible source of neck pain16,17,18. Fusion surgery can be indicated for discogenic/facet genic neck pain because this category of pain can theoretically be attenuated by fusion of the pain-generating segment19. However, the present results unexpectedly showed that there was no significant difference in post-operative neck pain attenuation between segmental motion-preserving laminoplasty and fusion surgeries (anterior and posterior). Therefore, discogenic/facet genic neck pain was not likely to be a major aetiology of neck pain in the present series.
The present results revealed post-operative neurological recovery as an independent factor having a significant association with post-operative neck pain attenuation. These lines of evidence suggest that neurogenic pain is one of the major causes of neck pain in patients with cervical OPLL. There might be several possible origins of myelopathy-related neck pain. Spinal cord compression can stimulate the posterior ramus of the spinal nerve, possibly resulting in neck pain19. Segmental spinal cord sign caused by compressive myelopathy may, like girdle pain, be another origin of neck pain20. Segmental spinal cord compression can cause local impairment of the spinothalamic tract at its chiasma at the central grey matter of the spinal cord21. Irrespective of the precise cause, a large-scale cohort study revealed that cervical myelopathy can cause neck pain22.
In addition, there is a possibility that the natural course of the disease is attributed to postoperative neck pain attenuation. Theoretically, progression of ossification foci can lead to spontaneous fusion of intervertebral segments, potentially resulting in neck pain attenuation. However, we could not find articles describing the natural history of neck pain in OPLL patients. Therefore, we had no proper answer for this question at present. Our multicenter study group is now constructing another prospective registry for OPLL patients receiving non-operative treatment with long term follow-up. Precise natural course of OPLL might be elucidated in future.
The present study includes several major limitations. The present registry lacks data regarding cervical sagittal alignment. Recently, the concept of sagittal alignment has been introduced to the cervical spine, similar to the thoracolumbar spine. Cervical sagittal alignment is important to evaluate neck pain because it has been reported to correlate with neck pain23. Therefore, the outcome might be changed significantly if cervical sagittal alignment data were added. To solve this problem, future collection of data regarding cervical sagittal alignment is needed. Another major limitation of the present study is that the present registry lacks information about the precise location and characteristics of neck pain and evaluation of neuropathic pain. Those data are important to elucidate the origin of neck pain. As a result, we can only speculate on the origin of neck pain using indirect evidence including post-operative change of neck pain, pre-operative patient factors, surgical factors, radiological changes, and neurological status. Future data collection of the precise characteristics of neck pain and neuropathic pain evaluation are warranted.
In conclusion, neurological recovery was an independent factor having a significant positive association with post-operative neck pain attenuation in a prospective study of a cohort of patients with cervical myelopathy caused by OPLL who underwent cervical spine surgery.
Methods
We used a prospective cohort design for the present study.
We assembled as investigator’s meeting before the initiation of the present study and twice a year during the study period for training to standardize the data collection and imaging analyses. All the clinical and imaging data were collected by physicians except for surgeons who performed surgery in each institute. Cleaning of collected data was performed by the committee member of the present study group. Missing data was mainly caused by patients’ drop out from follow-up.
The registry included data from 478 patients who underwent cervical spine surgery for myelopathy caused by cervical OPLL. Amongst these patients, we excluded data from those who lacked pre-operative neck pain evaluation (40 cases), who showed a pre-operative neck pain score < 30 mm on a visual analogue scale (VAS, 0–100 mm, 166 cases) to avoid possible ceiling effects in evaluation for pain attenuation and several previous pain trials set similar exclusion criteria24. We also excluded patients received anterior–posterior combined surgery because the number of those patients was very small (n = 7) to obtain statistical significance.
Therefore, we included data from 265 patients with cervical OPLL and a pre-operative neck pain severity score ≥ 30 mm on a VAS. Patient demographics are shown in Table 1.
Neck pain was evaluated using the VAS score pre- and post-operatively. The proportion of VAS score reduction was calculated as (pre-operative VAS neck pain—post-operative VAS neck pain) / pre-operative VAS neck pain × 100 (%). Post-operative neck pain deterioration was expressed as the negative proportion of VAS score reduction. We employed “50% pain reduction” as classification for sufficient postoperative neck pain attenuation because we think it is comprehensive at a glance and it is one of the popular outcomes in pain research field24. In addition, another reason why we adopted 50% pain reduction as the outcome measure is that the impact of absolute value of pain evaluation could differ between patients having different preoperative neck pain.
Possible explanatory factors having significant association with postoperative neck pain attenuation were as followings.
Patient factors Patients factors included age at surgery, sex, body mass index, disease duration, and diabetes mellitus.
Neurological status Pre- and post-operative neurological status were analysed using the Japanese Orthopedic Association score for evaluating cervical myelopathy (JOA score; 0–17 points25). The recovery rate of JOA score was calculated using the following method: (post-operative JOA score—pre-operative JOA score) / (17 (full mark)—pre-operative JOA score) × 100 (%)26. Post-operative neurological deterioration was expressed as the negative value JOA score recovery rate.
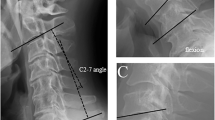
Imaging factors Imaging factors including types of OPLL (continuous, segmental, mixed and localized types Fig. 1A)27, canal narrowing rate (thickness of OPLL at its peak level / antero-posterior diameter of corresponding spinal level (%) in lateral radiogram of cervical spine (Fig. 1B), post-operative change of C2-7 angle (C2-7 angle was measured as angle between inferior endplates of C2 and C7 vertebral bodies (Fig. 1C), and post-operative change of C2-7 angle was calculated as subtraction of postoperative C2-7 angle from preoperative C2-7 angle), change of C2-7 range of motion (ROM was calculated as subtraction of C2-7 angle from extension position to flexion position (Fig. 1D), and change of C2-7 range of motion was calculated as (preoperative C2-7 ROM)—(postoperative C2-7 ROM)). Spinal cord signal intensity change in magnetic resonance imaging (MRI) T2 weighted image were assessed because the intramedullary signal change had been reported as one of the imaging factors for prediction of surgical outcome (Fig. 1E)28.
Methods for imaging analyses. (A) OPLL was classified into 4 types (continuous, segmental, mixed and localized types). (B) Canal narrowing rate was calculated as followings, thickness of OPLL at peak (b)/antero-posterior diameter (a), in lateral radiogram of cervical spine in neutral position. (C) C2-7 angle was measured as angle between inferior endplates of C2 and C7 vertebral bodies (C). Lordosis curve was expressed as positive value and kyphosis curve was expressed as negative value. Post-operative change of C2-7 angle was calculated as subtraction of postoperative C2-7 angle from preoperative C2-7 angle. (D) C2-7 range of motion (ROM) was calculated as subtraction of C2-7 angle from extension position to flexion position (D), and change of C2-7 range of motion was calculated as (preoperative C2-7 ROM)—(postoperative C2-7 ROM)). (D) Spinal cord signal intensity change in magnetic resonance imaging (MRI) T2 weighted image were assessed because the intramedullary signal change had been reported as one of the imaging factors for prediction of surgical outcome (E, arrow).
Surgical factors: Surgical factors including surgical procedures (laminoplasty, Fig. 2A), posterior decompression with instrumented fusion (PDF, Fig. 2B) and anterior decompression and fusion (ADF, Fig. 2C) and surgical approach (anterior and posterior) were evaluated.
Representative images of each surgical procedures. Laminoplasty included open-door method and double-door method. There is no significant difference in any postoperative assessment between both procedures. Preoperative (A) and postoperative (D) radiograms of the OPLL patient received laminoplasty. Posterior recompression with instrumented fusion (PDF) was performed as combinatory procedure of laminoplasty or laminectomy followed by instrumented posterior fusion. Preoperative (B) and postoperative (E) radiograms of the OPLL patient received PDF. Anterior decompression with fusion (ADF) was performed as subtotal corpectomy with extirpation or floating of ossification foci followed by autologous bone graft with anterior plating. Preoperative (C) and postoperative (F) radiograms of the OPLL patient received ADF.
Missing data were supplemented by the last observation carried forward method because the VAS score of neck pain was 46.2 ± 27.2 mm in 1 year after surgery and 48.2 ± 28.8 mm in 2 years after surgery, showing no significant difference between 2 time points.
First, we analysed the association of surgical procedures with post-operative neck pain. Pre- and post-operative VAS neck pain scores and the proportion of post-operative pain reduction were compared between surgical procedures including laminoplasty, PDF and ADF surgeries with Steel–Dwass analyses. Next, we performed uni-variate analyses followed by multi-variate analysis using stepwise logistic regression to elucidate the independent factors having a significant positive association with post-operative neck pain attenuation. Achievement of 50% or more post-operative neck pain reduction ratio was set as a response variable. The background factors for the patients as mentioned above, surgical factors, neurological factors, and imaging factors were set as explanatory variables. All the factors were checked the multicollinearity each other before univariate analyses.
Factors showing a p-value < 0.1 with initial uni-variate analyses were then analysed by stepwise logistic regression. Factors showing a p-value < 0.05 were determined as independent factors having a significant positive association with post-operative neck pain attenuation. Odds ration and 95% confidence interval was calculated for screened factors. Screened factors were then analysed using receiver-operator characteristic (ROC) curves to determine their cut-off values. All the statistical analyses were conducted with statistical analytics software JMP (version 12.0; SAS Institute, Cary, NC, USA) under the supervision by the biostatistician in our department (one of the co-author KF). Those statistical analyses were performed on data obtained 1 and 2 years after surgery.
References
Wu, J. C., Chen, Y. C. & Huang, W. C. Ossification of the posterior longitudinal ligament in cervical spine: Prevalence, management, and prognosis. Neurospine 15, 33–41 (2018).
Hirai, T. et al. Prevalence and distribution of ossified lesions in whole spine of patients with cervical ossification of the posterior longitudinal ligament: A multicenter study (JOSL CT study). PLoS ONE 1, e016117 (2016).
Fujimori, T. et al. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: Results of whole spine CT scans in 1500 Japanese patients. Spine (Phila Pa 1976) 41, 1668–1676 (2016).
Wilson, J. R. et al. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patients with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 38, S37-54 (2013).
Singhatanadgige, W., Limthongkul, W., Valone, F. 3rd., Yingsakmongkol, W. & Riew, K. D. Outcomes following laminoplasty or laminectomy and fusion in patients with myelopathy caused by ossification of the posterior longitudinal ligament: A systematic review. Glob. Spine J. 6, 702–709 (2016).
Hirai, T. et al. Clinical characteristics in patients with ossification of the posterior longitudinal ligament: A prospective multi-institutional cross-sectional study. Sci. Rep. 26, 5532 (2020).
Fujimori, T. et al. Patient satisfaction with surgery for cervical myelopathy due to ossification of the posterior longitudinal ligament. J. Neurosurg. Spine 14, 726–733 (2011).
Fujimori, T., Le, H., Ziewacz, J. E., Chou, D. & Mummaneni, P. V. Is there a difference in range of motion, neck pain, and outcomes in patients with ossification of posterior longitudinal ligament versus those with cervical spondylosis, treated with plated laminoplasty?. Neurosurg. Focus 35, E9 (2013).
Nakashima, H. et al. Comparison of outcomes of surgical treatment for ossification of the posterior longitudinal ligament versus other forms of degenerative cervical myelopathy: Results from the prospective, multicenter AO Spine CSM-international study of 479 patients. J. Bone Joint Surg. Am. 98, 370–378 (2016).
Youseff, J. A. et al. Outcomes of posterior cervical fusion and decompression: A systematic review and meta-analysis. Spine J. 19, 1714–1729 (2019).
Hosono, N., Yonenobu, K. & Ono, K. Neck and shoulder pain after laminoplasty. A noticeable complication. Spine (Phila Pa 1976) 21, 1969–1973 (1996).
Hosono, N., Sakaura, H., Mukai, Y., Fujii, R. & Yoshikawa, H. C3–6 laminoplasty takes over C3–7 laminoplasty with significantly lower incidence of axial neck pain. Eur. Spine J. 15, 1375–1379 (2006).
Kudo, H. et al. Ten-year long term results of modified cervical double-door laminoplasty with C3 laminectomy preserving the semispinalis cervicis inserted into the axis compared with those of conventional cervical laminoplasty. Clin. Spine Surg. https://doi.org/10.1097/BSD.0000000000001068 (2020).
Shiraishi, T. Skip laminectomy- a new treatment for cervical spondylotic myelopathy, preserving bilateral muscular attachments to the spinous processes: a preliminary report. Spine J. 2, 108–115 (2002).
Kato, S., Ganau, M. & Fehlings, M. G. Surgical decision-making in degenerative cervical myelopathy- anterior versus posterior approach. J. Clin. Neurosci. 58, 7–12 (2018).
DeFrancesch, F., Sperry, B. P., Aprillm, C. N., Choe, D. & McCormick, Z. L. Prevalence and discordance of the “startle response” with true discogenic pain according to Spine Intervention Society Guidelines for provocation discography: A cohort study. Pain Med. 28, 099 (2020).
Peng, B. & DePalma, M. J. Cervical disc degeneration and neck pain. J. Pain Res. 11, 2853–2857 (2018).
Lawson, G. E. et al. Medial branch blocks for diagnosis of facet joint pain etiology and use in chronic pain litigation. Int. J. Environ. Res. Public Health 17, 7932 (2020).
Heller, J. G. The syndromes of degenerative cervical disease. Orthop. Clin. North Am. 23, 381–394 (1992).
Diaz, E. & Morales, H. Spinal cord anatomy and clinical syndromes. Semin. Ultrasound CT MR 37, 360–371 (2016).
Shenoy, V. S. & Sampath, R. Syringomyelia in StatPearls (StatPearls Publishing, 2020).
Niu, S., Anastasio, A. T., Maidman, S. D., Faraj, R. R. & Rhee, J. M. The frequency of various “myelopathic symptoms” in cervical myelopathy; evaluation in a large surgical cohort. Clin. Spine Surg. 33, E448-453 (2020).
Tang, J. A. et al. ISSG. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery 76, S14–S21 (2015).
Cardenas, D. D. et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology 80, 533–539 (2013).
Japanese Orthopaedic Association. Scoring system for cervical myelopathy. Nippon Seikeigeka Gakkai Zasshi 68, 490–503 (1994) (in Japanese).
Hirabayashi, K. & Toyama, Y. Choice of surgical procedure for cervical ossification of the posterior longitudinal ligaments. In Ossification of the posterior longitudinal ligament (eds Yonenobu, K. et al.) 135–142 (Springer-Verlag, Tokyo,1997).
Tsuyama, N. Ossification of the posterior longitudinal ligament of the spine. Clin. Orthop. Relat. Res. 184, 71–84 (1984).
Nouri, A., Martin, A. R., Mikulis, D. & Fehlings, M. G. Magnetic resonance imaging assessment of degenerative cervical myelopathy: A review of structural changes and measurement techniques. Neurosurg. Focus 40, E5 (2016).
Funding
This study was supported by Japan Agency for Medical Research and Development and by Japanese Health Labor Sciences Research Grant. This study was approved by each institutional review board.
Author information
Authors and Affiliations
Contributions
M.K., T.Y., K.K., K.A. and Y.K. contributed to planning and conduct of the present study and to reporting the present manuscript. S.E., K.S., K.K., T.F., S.M., H.T., M.M., S.I., K.T., M.N., M.M., A.O. and M.Y. contributed to conception and design of the present study and to reporting the present study. Y.N., T.H., K.W., N.N., K.W., T.K., Y.N., Y.O., K.A., M.T., K.M., H.N., K.M., S.M., T.K., K.Y., S.K., S.K., T.O., S.I., S.F., H.K., H.K., G.I., M.T., contributed to conducting the present study and to edit the present manuscript. All authors reviewed the manuscript. All the authors read and approved the final manuscript. K.F. supervised all the statistical analyses in the present study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koda, M., Yoshii, T., Egawa, S. et al. Neurological improvement is associated with neck pain attenuation after surgery for cervical ossification of the posterior longitudinal ligament. Sci Rep 11, 11910 (2021). https://doi.org/10.1038/s41598-021-91268-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91268-2
- Springer Nature Limited