Abstract
The aim of this study was to assess pre- and postoperative cognitive functions in patients who underwent surgery for benign intracranial lesions. In total, 58 patients (21 men, 37 women, mean age 51.6 years [range 24–76 years]) with benign intracranial lesions (including benign tumors and vascular lesions) and neuralgia of the trigeminal nerve were included in this prospective study. Extensive cognitive testing was used to categorize attention, memory, and executive functions. Mood and pain were assessed preoperatively (t0, mean 3.7 days before surgery), immediately after surgery/during inpatient stay (t1, mean 7.6 days after surgery), and at first outpatient check-up (t2, mean 99.5 days after surgery). All 58 patients were tested at t0 and t1, but at t2 only 24 patients were available at t2. The data were categorized as improvement/stable condition or deterioration and shown as percentages. The pre- and postoperative values of BDI-II and mood were compared by the Wilcoxon test for paired samples. Binary logistic regression analyses were performed to identify parameters influencing cognition in the subgroup of meningioma patients. Immediately after surgery (t1), the percentage of patients with improvement/stable condition was > 50% in all categories in the majority of subtests (attention: 12/14 subtests, memory: 11/13 subtests, executive functions: 6/9 subtests). Similar results were shown at t2. Mood and pain did not change significantly after surgery. Factors like age, Karnofsky performance status, and tumor volume were not shown as significant influencing factors for cognitive functions in meningioma patients. The results of this study suggest that—in contrast to neuroepithelial tumors—cognitive functions do not deteriorate after surgery of benign intracranial lesions. Further studies are necessary to evaluate the results of this study.
Similar content being viewed by others
Introduction
Extensive cognitive testing is not used routinely before or after surgery of intracranial lesions. The main factors assessed in clinical routines and in neurooncological studies are age, functional independence (measured by the Karnofsky Performance Status Scale [KPS]1), neurological status, and extent of resection. However, evidence is increasing that cognition is important for not only quality of life but also overall survival2,3,4,5. Many studies have assessed pre- and postoperative cognitive functions in patients with gliomas and brain metastases6,7,8,9,10,11. Several studies have also evaluated the effect of meningioma surgery on cognitive functions12,13,14,15,16,17,18,19. Most of these studies have shown preoperative impairment and postoperative improvement of cognition in meningioma patients, sometimes with conflicting results16,17,18,20. Zweckberger et al.19 reported delayed improvement of cognitive functions, whereas another recent study showed ongoing impairment21. Little is known about pre- and postoperative cognitive functions in patients with rare intracranial tumors or vascular lesions like arterious venous malformations22,23. For unruptured aneurysms, the ISAT trial showed improved cognitive outcomes after endovascular treatment, as compared to a neurosurgical approach24.
Mood and pain are known to influence cognition25,26, so these measurements were also included in the study.
The aim of this study was to assess whether cognitive functions, mood, and pain improve or deteriorate after surgery in patients with benign intracranial lesions.
Methods
Study design
The local ethics committee approved this prospective single-center study (clinical trial registration number 3094/11). All participants signed an informed consent form. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments27.
Patient population
Patients who underwent surgery for a benign intracranial process (including benign tumors, vascular lesions, and neuralgia of the trigeminal nerve) between September 2012 and December 2014 were prospectively included in this study.
The data of the preoperative study population and the risk factors for preoperative cognitive impairment have been reported elsewhere28.
The following inclusion criteria were defined: age ≥ 18 years, informed consent, extensive neuropsychological testing of pre- and postoperative performance, surgery for a benign intracranial process, preoperative magnetic resonance imaging (MRI), knowledge of the German language, no pregnancy, and preoperative Mini-Mental Status Examination (MMSE) ≥ 18.
The exclusion criteria included age < 18 years, missing informed consent form, missing postoperative neuropsychological testing, other histopathological diagnosis (e.g., malignant intracranial process), and preoperative MMSE < 18.
Study design
Patients were included in this prospective study after detailed information and informed consent were obtained. Neuropsychological testing (including the basic test battery [MMSE] and the extended test battery [as explained below] was performed at 3 time points by medical students supervised by a certified neuropsychologist: preoperatively (t0), immediately postoperatively during inpatient stay (t1), and during follow-up at the first outpatient checkup (t2).
Basic test battery
The basic test battery was the MMSE29. This well-known test was used to examine the patients’ basic cognitive functions.
Extended test battery
The extended test battery comprised 3 categories of cognitive functions: attention, memory, and executive functions. The tests used to evaluate these 3 categories are described below.
Attention
The computer-based Test of Attentional Performance (TAP) was used to evaluate cognitive functions in the attention category (in addition to the TMT-A)30.
Alertness
Reaction times (shown in ms) for visual stimuli were recorded in this subtest, either with acoustic notification (alertness W_sound) or without acoustic notification (W_O_sound).
Divided attention
Simultaneous reaction times (ms) to visual (divided attention visual) and to auditory (divided attention auditory) stimuli were measured. Furthermore, the numbers of mistakes (divided attention failure) and of omissions (divided attention selected) were recorded.
Visual field
Visual stimuli were provided while the patients fixated at a central point. The data (reaction time [ms] and omissions [skip]) are shown separately for the right/left and central visual fields.
Trail-Making-Test A
The Trail-Making-Test A (TMT-A) task was to connect numbers (1–25) in the right order as fast as possible. Time is measured and shown in seconds31.
Memory
Wechsler Memory Scale (WMS)
The Wechsler Memory Scale Revised32 is a test battery for assessing different aspects of memory and includes 13 subtests. In this study, the block- and digit-span subtests were used to evaluate verbal and nonverbal short-term memory. A row of digits was read out, or a group of wooden blocks was arranged in a specific order. Patients were asked to repeat the tasks immediately (memory span verbal/nonverbal [ms v/ms nv]) and after a delay (working memory verbal/nonverbal [wm v/wm nv]).
Verbal learning and memory test
The Verbal Learning and Memory Test (VLMT) was used to analyze episodic memory. A learning list of 15 words was read out 5 times, and patients were asked to repeat the words after the words were read out once (Dg1) and after being read out 5 times (Dg5). The gained knowledge was measured (Dg1-5). An interference list with different words was read out, and the patients again were asked to repeat the words from the learning list immediately (Dg6) and after a delay of 30 min (Dg7). The loss of knowledge was measured after the interference list was read (Dg5-6) and after a delay (Dg5-7).
Rey Osterrieth complex figure test (ROCF)
The well-known neuropsychological Rey Osterrieth Complex Figure Test (ROCF) was used to analyze the patients’ visual memory and visual constructive capacity33 and included 3 subtests. Initially, the subjects were asked to copy a geometrical figure that was shown to them. This first subtest was not assessed in the present study. In the next 2, subtests, which were analyzed in this study, the subjects were asked to draw a geometrical figure shown to them before, both immediately (ROCF copy) and after a 30-min delay (ROCF delay).
Executive functions
Trail-Making-Test B
The Trail-Making-Test B (TMT-B) was similar to the TMT-A; however, the patients were asked to connect letters and numbers in alternation (eg, 1-A-2-B)31. The time needed for correct connection was measured in seconds.
Regensburg word fluency test
Formal lexical and semantic fluency was measured by the Regensburg Word Fluency Test (RWT)34. Words with a specific initial letter (eg, words beginning with the letter A or L) were requested for formal lexical fluency, and words in a specific category (eg, in the category animals) were requested for semantic fluency. Another subtest was used to analyze the capability to change between both categories.
Stroop word color test
Color-word interference was analyzed with the Stroop Word Color Test35. The first subtest was word reading, in which the patients were asked to read words (green, blue, etc.) written in black. The second subtest was line naming, in which the colors of different lines were recorded. In the third and last subtest (interference), patients were asked to read out the color of words that were written in different colors (eg, the word “green” was written in blue).
Assessment of mood and pain
Patients’ mood was assessed by the well-known Beck Depression Inventory-II (BDI-II) in 52/58 patients at t0, in 37/58 patients at t1, and in 19/24 at t236. The BDI-II is scored from 0 to 63, with higher scores for higher extent of depression. The median score of the normative population was 7.4 (population of n = 582 depressive patients and n = 260 healthy controls, according to the manual of Hautziger et al.37 The participants were divided into 5 groups according to scores from the BDI-II: no depression (0–8), minimal depression (9–13), slight depression (14–9), moderate depression (20–28), and severe depression (> 28).
Pain, especially headache, in this cohort was assessed using the IBK, the German version of the Headache Disability Inventory (HDI)38. This test was available for 43/58 patients at t0, 33/58 patients at t1, and 18/24 patients at t2. Pain was divided into 4 scales: no headache, slight headache, moderate headache, and severe headache.
Volumetric measurement
A neuroradiologist performed manual segmentation of the contrast-enhancing part of the intracranial lesion pre- and postoperatively (iPlan Net Cranial 3.0, Brainlab AG, Munich, Germany). No volumetric measurement was performed of vascular lesions or trigeminal neuralgia.
Surgery
Surgery was performed at the Department of Neurosurgery with the aim of maximum tumor resection in patients with benign tumors. Pituitary adenomas were resected using a transnasal-transsphenoidal approach, and other benign tumors were resected using trepanation. Aneurysms were treated by clipping and pterional trepanation, whereas trigeminal nerve neuralgia was treated by microvascular decompression.
Statistical analysis
IBM SPSS Statistics versions 24.0, 25.0, and 26.0 (SPSS Inc., IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Normally distributed data were shown as means/standard deviations, and non-normally distributed data were shown as medians/interquartile range (IQR). The delta between the pre- and postoperative percentile ranks was recorded, and the patients were divided into improvement/stable condition or deterioration groups. The Wilcoxon test for paired samples was used for pre- and postoperative comparisons of mood and pain and of the basis test battery (MMSE). Binary logistic regression analyses were performed to identify risk factors for postoperative changes of cognitive functions. P < 0.05 was defined as significant.
Ethical approval and informed consent
The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments and approved by the local ethics committee (Ethics committee technical university munich). Informed consent was signed by all study participants.
Results
Patient population
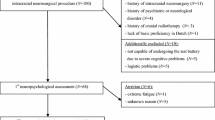
Initially, 81 patients were included in the study. Of them, 23 patients were excluded: 22 patients did not perform neuropsychological testing after surgery due to reduced general condition or lack of retrieved informed consent (for further neuropsychological testing after surgery), and 1 patient was excluded due to missing surgery (neuroradiological intervention).
Therefore, 58 patients (21 men, 37 women, mean age 51.6 years [range 24–76]) were included in this study, with meningioma (n = 23, 19/23 WHO grade I, 3/23 WHO grade II, 1/23 WHO grade III), pituitary adenoma (n = 8), vestibular schwannoma (n = 7), neuralgia of the trigeminal nerve (n = 3), cavernoma (n = 3), intracranial aneurysm (n = 4), pineocytoma (n = 2), arterial-venous malformation (n = 2), hemangiopericytoma (n = 1, WHO grade II), clivus chordoma (n = 1), colloidal cyst (n = 1), subependymoma (n = 1), and others (n = 2). Of the 58 lesions, 20 were located in the right hemisphere, 23 were located in the left hemisphere, and 15 were located in the midline (pituitary adenomas, pineocytoma, aneurysm of the basilar artery, chordoma, subependymoma, and 2 meningiomas).
The main tumor locations were the frontal lobe (23/58) and infratentorial region (16/58). The baseline patient characteristics also included information about initial symptoms and adjuvant treatment, as shown in Table 1. During follow-up (at t2), 24/58 patients (10 men, 14 women, mean age 46.8 years [range 24–73], with meningioma [n = 8], vestibular schwannoma [n = 3], pituitary adenoma [n = 5], pineocytoma [n = 2], clivus chordoma [n = 1], cavernoma [n = 2], aneurysm [n = 1], neuralgia of the trigeminal nerve [n = 1], and others [n = 1]) underwent neuropsychological testing. The others either were lost to follow-up or withdrew informed consent. The mean time from preoperative testing (t0) to surgery was 3.7 days (range 1–23 days), the mean time from surgery to postoperative testing during inpatient stay (t1) was 7.6 days (range 2–55 days), and the mean time from surgery to follow-up at the first outpatient control (t2) testing was 99.5 days (range 61–197 days).
MMSE
No significant differences were observed between preoperative MMSE (29.0 [IQR 28.0–30.0] and postoperative MMSE (29.0 [27.0–30.0]; P = 0.278) as well as between preoperative MMSE and follow-up MMSE (28.0 [27.3–29.8]); P = 0.522).
Pre- and postoperative comparisons
Analyses were performed for all patients and the patients in the meningioma and pituitary adenoma subgroups (Table 2). Improvement of cognition was defined as stable/improving cognitive functions in more than 50% of the patients. In the attention category, 12/14 subtests showed early postoperative improvement/stable condition at t1, as compared to 11/13 subtests in the memory category and 6/9 subtests in the executive functions category. Similar results were observed in the subgroup of meningioma patients (Table 2). The percentage of patients with improvement during follow-up (t2) was similar, comprising 11/14 subtests in the attention category and, even higher, 13/13 subtests in the memory category and 9/9 subtests in the executive functions category. Among the meningioma patients, this rate at t2 was lower in the memory category (8/13 subtests). In the subgroup of patients with pituitary adenomas, 12/14 subtests improvement/stable condition immediately postoperatively (t1) in the attention category, as compared to 7/13 subtests in the memory category and 6/9 subtests in the executive functions category. At t2, 12/14 subtests in the attention category showed improvement/stable condition, compared to 12/13 subtests in the memory category and 7/9 subtests in the executive functions category (Table 2).
Figure 1 shows the distributions of improvement and deterioration in the subtests of the attention, memory, and executive functions categories at t1 and t2.
Figure 2 presents the results of the 8 meningioma patients at different time points in each category (attention, memory, and executive functions).
Mood and pain
Mood did not change significantly after surgery at t1 (P = 0.484) and at t2 (P = 0.306). In addition, pain did not change significantly at t1 (P = 0.060) or at t2 (P = 0.564). The distributions of depression and pain at the different time points are shown in Fig. 3.
Multivariate analysis
For the homogenous subgroup of meningioma patients, binary logistic regression analyses were performed with change of cognition after surgery as the dependent variable and age, KPS, and tumor volume as the independent variables. Odds ratios (95% CI) are presented in Table 3. No significant influencing factors could be identified in this analysis.
Discussion
Cognitive functions in patients with benign intracranial lesions improved or remained stable immediately postoperatively and during follow-up among the majority of patients in all categories of cognitive functions for the cohort of 58 patients. No significant influencing factors, like age, KPS, or tumor volume, were identified for changes in cognitive functions among meningioma patients.
The preoperative neurocognitive functions of patients with benign intracranial lesions were analyzed in a previous study, which showed that age and KPS were the main risk factors for impaired neurocognitive functions before operation28. In this study, we analyzed a subgroup of patients with available, extensive postoperative neurocognitive testing.
This study showed improvement or stable condition of cognitive functions in the attention, memory, and executive functions categories. These results agree with those of Tucha et al., who studied (elderly) meningioma patients, showing postoperative improvement mainly in the attention and memory domains and no deterioration of preoperative cognitive functions15,17,39. Another recent study be Meskal et al. showed postoperative improvement in almost all cognitive domains, except for psychomotor speed and reaction time16.
The mentioned studies by Tucha et al. reported no improvement in executive functions after meningioma surgery17,39. A recent previous study on meningioma patients also reported postoperative improvement of cognitive functions but with lower ongoing cognitive scores as compared to healthy controls40.
The rate of improvement/stable condition was even higher during follow-up (t2), as compared to immediate postoperative testing (t1), in this cohort. These results might be explainable by postoperative edema or reduced postoperative functional independence (KPS), a known risk factor for cognitive impairment7. Previous studies also showed a transient decline of cognition with recovery at follow-up after surgery and after irradiation15,19,41. The time point when postoperative evaluation occurs might be important for these findings. In this study, the first postoperative testing was performed quite early (mean time from surgery to t1 of 7.6 days). Thus, the aftereffects of surgery might be more prominent at this date. In the other mentioned studies on meningioma patients, postoperative testing was performed later (about 3 months after surgery), which agrees with our follow-up examination (t2)15,17,39.
However, the results of the follow-up analyses should be taken with caution. According to the study design (t1 = postoperative testing during inpatient stay; t2 = first follow-up testing on outpatient controls), there were major differences in the time periods, with some overlapping results. Furthermore, only patients with a MMSE > 18 underwent extensive neuropsychological testing, thus only selecting patients without major deterioration after surgery.
The highest rate of improvement/stable condition was observed in the ROCF delay subtest, at almost 100%. This improvement might have been because the patients remembered that they had to perform the same task in 30 min. Therefore, these results should be considered with caution.
In contrast to other studies focusing mainly on 1 entity (eg, meningioma or pituitary adenoma), this study included different entities and also vascular lesions. This might introduce a bias due to the high majority of the diseases. However, considering a more heterogeneous group could also provide additional information, as compared to focusing on only 1 entity.
Among the meningioma patient subgroup, tumor size was not observed as a significant influencing factor, contrasting a previous study by Liouta et al.14 These differences might be explained by the different study designs. The present study focused on patients with benign intracranial lesions and with therefore had a lower number of meningioma patients, whereas Liouta et al. focused only on meningioma patients and therefore had a larger number of patients.
The surgical approach and tumor location might affect cognitive functions. Due to the low number of patients in the present study cohort, no further analyses were conducted regarding these possibilities. Surgery was performed according to the neurosurgical standards at our department and did not significantly differ between the patient subgroups.
This study has several limitations. The high dropout rate during follow-up (at t2) was a main limitation and might have introduced unavoidable bias. Patients with less cognitive deficits might be more likely to perform cognitive testing during follow-up than patients with considerable restrictions would. This might have biased the results and resulted in overestimation of the rate of patients without deterioration after surgery. Further studies with a lower dropout rate are necessary to address this.
Additional limitations include the low numbers of patients with some diseases and the variety of diseases included in this cohort. However, subependymoma, clivus chordoma, arterial-venous malformation, and cavernoma are rare intracranial lesions, and to our knowledge, cognitive functions have not been assessed before in patients with these lesions. To address this, a subgroup analysis was performed with meningioma patients only.
In particular, the number of unruptured aneurysms was very low in this cohort (n = 4), and the study does not add any new findings to the already known results of the ISAT trial, which showed cognitive improvement after endovascular treatment of aneurysms24.
The study population remains a very heterogeneous population with small numbers of each individual pathology. The significance of this study might be limited due to this heterogeneous cohort and many other possible confounding variables that would affect cognitive outcomes. However, the aim of this prospective study was to include all types of benign intracranial lesions and not to select special subgroups (eg, meningioma patients) as previous studies on such cohorts already exist. This exploratory study might draw attention to this heterogeneous patient cohort and might be of interest for further (prospective) studies.
Conclusions
Cognitive functions improved or remained stable in the attention, memory, and executive functions categories after surgery of benign intracranial lesions in the majority of our cohort of 58 patients. Due to the high dropout rate and the various intracranial lesions included in this study, the results of this study should be taken with caution, and further studies are necessary to confirm the results.
References
Sacko, A. et al. Evolution of the Karnosky Performance Status throughout life in glioblastoma patients. J. Neurooncol. 122, 567–573. https://doi.org/10.1007/s11060-015-1749-6 (2015).
Krupp, W. et al. Assessment of neuropsychological parameters and quality of life to evaluate outcome in patients with surgically treated supratentorial meningiomas. Neurosurgery 64, 40–47. https://doi.org/10.1227/01.NEU.0000336330.75381.39 (2009) (discussion 47).
Meyers, C. A., Hess, K. R., Yung, W. K. & Levin, V. A. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J. Clin. Oncol. 18, 646–650. https://doi.org/10.1200/JCO.2000.18.3.646 (2000).
Taphoorn, M. J. & Klein, M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 3, 159–168. https://doi.org/10.1016/S1474-4422(04)00680-5 (2004).
Waagemans, M. L. et al. Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery 69, 72–78. https://doi.org/10.1227/NEU.0b013e318212badb (2011) (discussion 78-79).
Chang, E. L. et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 10, 1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3 (2009).
Gempt, J. et al. Factors influencing neurocognitive function in patients with neuroepithelial tumors. Sci. Rep. 7, 17764. https://doi.org/10.1038/s41598-017-17833-w (2017).
Habets, E. J. et al. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol. 18, 435–444. https://doi.org/10.1093/neuonc/nov186 (2016).
Noll, K. R., Sullaway, C., Ziu, M., Weinberg, J. S. & Wefel, J. S. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol. 17, 580–587. https://doi.org/10.1093/neuonc/nou233 (2015).
van Kessel, E., Baumfalk, A. E., van Zandvoort, M. J. E., Robe, P. A. & Snijders, T. J. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: A systematic review of neurocognitive functioning prior to anti-tumor treatment. J. Neurooncol. 134, 9–18. https://doi.org/10.1007/s11060-017-2503-z (2017).
Wu, A. S. et al. Neurocognitive function before and after surgery for insular gliomas. J. Neurosurg. 115, 1115–1125. https://doi.org/10.3171/2011.8.JNS11488 (2011).
Dijkstra, M. et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. J. Neurol. Neurosurg. Psychiatry 80, 910–915. https://doi.org/10.1136/jnnp.2007.138925 (2009).
Hendrix, P. et al. Neurocognitive function surrounding the resection of frontal WHO grade I meningiomas: A prospective matched-control study. World Neurosurg. 98, 203–210. https://doi.org/10.1016/j.wneu.2016.10.095 (2017).
Liouta, E., Koutsarnakis, C., Liakos, F. & Stranjalis, G. Effects of intracranial meningioma location, size, and surgery on neurocognitive functions: A 3-year prospective study. J. Neurosurg. 124, 1578–1584. https://doi.org/10.3171/2015.6.JNS1549 (2016).
Meskal, I., Gehring, K., Rutten, G. J. & Sitskoorn, M. M. Cognitive functioning in meningioma patients: A systematic review. J. Neurooncol. 128, 195–205. https://doi.org/10.1007/s11060-016-2115-z (2016).
Meskal, I., Gehring, K., van der Linden, S. D., Rutten, G. J. & Sitskoorn, M. M. Cognitive improvement in meningioma patients after surgery: Clinical relevance of computerized testing. J. Neurooncol. 121, 617–625. https://doi.org/10.1007/s11060-014-1679-8 (2015).
Tucha, O. et al. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J. Neurosurg. 98, 21–31. https://doi.org/10.3171/jns.2003.98.1.0021 (2003).
Yoshii, Y. et al. Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg. Neurol. 69, 51–61. https://doi.org/10.1016/j.surneu.2007.07.064 (2008) (discussion 61).
Zweckberger, K. et al. Prospective analysis of neuropsychological deficits following resection of benign skull base meningiomas. J. Neurosurg. 127, 1242–1248. https://doi.org/10.3171/2016.10.JNS161936 (2017).
Koizumi, H. et al. Cognitive dysfunction might be improved in association with recovered neuronal viability after intracranial meningioma resection. Brain Res. 1574, 50–59. https://doi.org/10.1016/j.brainres.2014.05.047 (2014).
van der Vossen, S., Schepers, V. P., Berkelbach van der Sprenkel, J. W., Visser-Meily, J. M. & Post, M. W. Cognitive and emotional problems in patients after cerebral meningioma surgery. J. Rehabil. Med. 46, 430–437. https://doi.org/10.2340/16501977-1795 (2014).
Brundl, E. et al. Treatment of unruptured intracranial aneurysms and cognitive performance: Preliminary results of a prospective clinical trial. World Neurosurg. 94, 145–156. https://doi.org/10.1016/j.wneu.2016.06.112 (2016).
Hendrix, P. et al. Cognitive function surrounding resection of nonfunctioning pituitary adenomas with suprasellar extension: A prospective matched-control study. J. Clin. Neurosci. 40, 109–114. https://doi.org/10.1016/j.jocn.2017.02.028 (2017).
Scott, R. B. et al. Improved cognitive outcomes with endovascular coiling of ruptured intracranial aneurysms: neuropsychological outcomes from the International Subarachnoid Aneurysm Trial (ISAT). Stroke 41, 1743–1747. https://doi.org/10.1161/STROKEAHA.110.585240 (2010).
Listunova, L. et al. Cognitive impairment along the course of depression: non-pharmacological treatment options. Psychopathology https://doi.org/10.1159/000492620 (2018).
Moriarty, O., McGuire, B. E. & Finn, D. P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 93, 385–404. https://doi.org/10.1016/j.pneurobio.2011.01.002 (2011).
World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. https://doi.org/10.1001/jama.2013.281053 (2013).
Bette, S. et al. Risk factors for neurocognitive impairment in patients with benign intracranial lesions. Sci. Rep. 9, 8400. https://doi.org/10.1038/s41598-019-44466-y (2019).
Cockrell, J. R. & Folstein, M. F. Mini-Mental State Examination (MMSE). Psychopharmacol. Bull. 24, 689–692 (1988).
Zimmermann, P. F. Testbatterie zur Aufmerksamkeitsprüfung (TAP). Psychologische Testsysteme (2009).
Tombaugh, T. N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. https://doi.org/10.1016/S0887-6177(03)00039-8 (2004).
Russell, E. W. Factor analysis of the Revised Wechsler Memory Scale tests in a neuropsychological battery. Percept. Mot Skills 54, 971–974. https://doi.org/10.2466/pms.1982.54.3.971 (1982).
Shin, M. S., Park, S. Y., Park, S. R., Seol, S. H. & Kwon, J. S. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat. Protoc. 1, 892–899. https://doi.org/10.1038/nprot.2006.115 (2006).
Lange, K. W., Aschenbrenner, S. & Tucha, O. Regensburg Word Fluency Task: A new test for the assessment of verbal fluency. Eur. Arch. Psychiatry Clin. Neurosci. 25 (2000).
Stroop, J. R. Studies of intereference in serial verbal reactions. J. Exp. Psychol. 18, 643–662 (1935).
Kuhner, C., Burger, C., Keller, F. & Hautzinger, M. Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples. Nervenarzt 78, 651–656. https://doi.org/10.1007/s00115-006-2098-7 (2007).
Hautzinger, M., Keller, F. & Kühner, C. BDI-II Beck Depressions-Inventar Revision Manual 2nd edn. (Pearson Assessment and Information GmbH, 2009).
Bauer, B., Evers, S., Gralow, I. & Husstedt, I.-W. Psychosoziale Beeinträchtigung durch chronische Kopfschmerzen Evaluation des Inventars zur Beeinträchtigung durch Kopfschmerzen (IBK). Nervenarzt 70, 522–529 (1999).
Tucha, O., Smely, C. & Lange, K. W. Effects of surgery on cognitive functioning of elderly patients with intracranial meningioma. Br. J. Neurosurg. 15, 184–188 (2001).
Rijnen, S. J. M. et al. Cognitive outcomes in meningioma patients undergoing surgery: Individual changes over time and predictors of late cognitive functioning. Neuro Oncol. 21, 911–922. https://doi.org/10.1093/neuonc/noz039 (2019).
Steinvorth, S. et al. Neuropsychological outcome after fractionated stereotactic radiotherapy (FSRT) for base of skull meningiomas: A prospective 1-year follow-up. Radiother. Oncol. 69, 177–182 (2003).
Acknowledgements
CZ has served on scientific advisory boards for Philips and Bayer Schering; serves as co-editor on the Advisory Board of Clinical Neuroradiology; has received speaker honoraria from Bayer-Schering and Philips and has received research support and investigator fees for clinical studies from Biogen Idec, Quintiles, MSD Sharp & Dome, Boehringer Ingelheim, Inventive Health Clinical UK Ltd., Advance Cor, Brainsgate, Pfizer, Bayer-Schering, Novartis, Roche, Servier, Penumbra, WCT GmbH, Syngis, SSS Internartional Clinical Research, PPD Germany GmbH, Worldwide Clinical Trials Ltd., Phenox, Covidien, Actelion, Medivation, Medtronic, Harrison Clinical Research, Concentric, Penumbra, Pharmtrace, Reverse Medical Corp., Premier Research Germany Ltd., Surpass Medical Ltd. and GlaxoSmithKline. SB, JG and BM work as consultants for Brainlab (Brainlab AG, Munich). All named potential conflicts of interest are unrelated to this study. BW received funding from KKF TU Munich. This funding is unrelated to this study. This study received no financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.B.: data analysis, statistical analysis, interpretation of the data, drafting the manuscript. J.R.: data collection, analysis and interpretation, reviewed the manuscript for intellectual content. B.W.: data interpretation, reviewed the manuscript for intellectual content. M.B.: data collection, reviewed the manuscript for intellectual content. C.Z.: interpretation of the data, reviewed the manuscript for intellectual content. B.M.: interpretation of the data, reviewed the manuscript for intellectual content. Y.R.: interpretation of the data, reviewed the manuscript for intellectual content. F.R.: study design and supervision, interpretation of the data, reviewed the manuscript for intellectual content. J.G.: study design, study supervision, data collection and analysis, interpretation of the data, reviewed the manuscript for intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bette, S., Ruhland, J.M., Wiestler, B. et al. Postoperative cognitive functions in patients with benign intracranial lesions. Sci Rep 11, 8757 (2021). https://doi.org/10.1038/s41598-021-88061-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88061-6
- Springer Nature Limited