Abstract
The physical properties of the organic–inorganic hybrid crystals having the formula [NH3(CH2)3NH3]ZnX4 (X = Cl, Br) were investigated. The phase transition temperatures (TC; 268K for Cl and 272K for Br) of the two crystals bearing different halogen atoms in their skeletons were determined through differential scanning calorimetry. The thermodynamic properties of the two crystals were investigated through thermogravimetric analysis. The structural dynamics, particularly the role of the [NH3(CH2)3NH3] cation, were probed through 1H and 13C magic-angle spinning nuclear magnetic resonance spectroscopy as a function of temperature. The 1H and 13C NMR chemical shifts did not show any changes near TC. In addition, the 1H spin–lattice relaxation time (T1ρ) varied with temperature, whereas the 13C T1ρ values remained nearly constant at different temperatures. The T1ρ values of the atoms in [NH3(CH2)3NH3]ZnCl4 were higher than those in [NH3(CH2)3NH3]ZnBr4. The observed differences in the structural dynamics obtained from the chemical shifts and T1ρ values of the two compounds can be attributed to the differences in the bond lengths and halogen atoms. These findings can provide important insights or potential applications of these crystals.
Similar content being viewed by others
Introduction
Organic–inorganic compounds based on hybrid perovskites, particularly [(CnH2n+1NH3)]2BX4 (n = 1, 2, 3,···; B = Mn, Co, Cu, Zn, Cd; X = Cl, Br) and [NH3(CH2)nNH3]BX4 (n = 2, 3,…) have attracted considerable attention in recent years. Monoammonium series [(CnH2n+1NH3)]2BX41,2,3,4,5 and diammonium series [NH3(CH2)nNH3]BX4 have been extensively studied because of their relative stability and potential applications6,7,8,9,10,11. The physical and chemical properties of the organic–inorganic hybrid perovskites depend on the characteristics of the organic cations, geometry of the inorganic anions (metal halide ions; (BX6)2− or (BX4)2−), and reaction stoichiometry1,2,3,12,13,14,15,16,17,18. For B = Mn, Cu, or Cd, the structure is consists of the corner shared octahedral (BX6)2− alternated with organic layers. While for B = Co or Zn, the structures are tetrahedral (BX4)2− sandwiched between layers of organic cations. These compounds have gained research attention because of the multiplicity of their crystal structures, which is correlated to the structural dynamics of the cations and anions. Organic–inorganic hybrid materials based on perovskite structures are of interest due to their potential applications19,20,21. Recently, solid-state NMR on hybrid perovskite materials has also garnered considerable attention22,23,24,25.
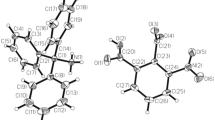
The [NH3(CH2)3NH3]ZnCl4 (1,3-propanediammonium tetrachlorozincate (II)) complex (n = 3; B = Zn; X = Cl), a member of the diammonium [NH3(CH2)nNH3]BX4 series, belongs to the monoclinic crystal system (space group: P21/n) at room temperature. Its lattice constants have been reported as a = 10.692 Å, b = 10.611 Å, c = 10.786 Å, β = 118.47°, and Z = 426. Figure 1 shows the structure of the [NH3(CH2)3NH3]ZnCl4 crystal at 300 K (CCDC number : 1227730). The crystal structure of the [NH3(CH2)3NH3]ZnBr4 (1,3-propanediammonium tetrabromozincate (II)) complex (n = 3, B = Zn; X = Br) is identical to that of the [NH3(CH2)3NH3]ZnCl4 complex; both belong to the same space group. Its unit cell dimensions were determined as a = 11.084 Å, b = 10.968 Å, c = 11.185 Å, β = 117.07°, and Z = 427. The [ZnBr4]2− anion forms a tetrahedron interconnected via the N‒H···Br hydrogen bonds to the [NH3(CH2)3NH3] cation. There is no center of symmetry within the [NH3(CH2)3NH3] cation in [NH3(CH2)3NH3]ZnBr4.
To date, the structures of [NH3(CH2)3NH3]ZnX4 (X = Cl and Br) have been reported by Kallel et al.22 and Ishihara et al.23, who studied the single crystals of the complexes through X-ray diffraction analysis. Although [NH3(CH2)3NH3]ZnX4 (X = Cl and Br) has many applications, the physical properties and structural dynamics of its crystals have not been elucidated.
Herein, the phase transition temperatures and thermodynamic properties of the crystals of [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 complexes are determined by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The structural dynamics of the crystals of [NH3(CH2)3NH3]ZnX4 (particularly the role of the [NH3(CH2)3NH3] cation) were probed by 1H magic-angle spinning nuclear magnetic resonance (MAS NMR) and 13C MAS NMR spectroscopy as a function of temperature. The 1H and 13C NMR spectral profiles were recorded to determine the changes in the chemical shifts. A change in the chemical shift values reflects a change in the structural environment. In addition, the spin–lattice relaxation times (T1ρ) in the rotating frame were discussed according to the change of temperature. Based on the MAS NMR results, the effects of different halogen atoms on the hydrogen bond and carbon atoms in the [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 crystals were investgated. Moreover, comparison of the physical properties of the two crystals revealed important information regarding the basic mechanisms.
Experimental
An aqueous solution containing NH2(CH2)3NH2·2HCl and ZnCl2 was slowly evaporated at 300 K to produce the single crystals of [NH3(CH2)3NH3]ZnCl4. Similarly, the slow evaporation at 300 K of an aqueous solution containing NH2(CH2)3NH2·2HBr and ZnBr2, produced the single crystals of [NH3(CH2)3NH3]ZnBr4. Transparent hexagonal crystals of [NH3(CH2)3NH3]ZnCl4 and thin-plate-shaped crystals of [NH3(CH2)3NH3]ZnBr4 were produced.
The structures of the [NH3(CH2)3NH3]ZnX4 (X = Cl and Br) crystals at 298 K were analysed using an X-ray diffraction system equipped with a Cu-Kα radiation source. And, the lattice parameters were determined by single-crystal X-ray diffraction methods at the Western Seoul Center of Korea Basic Science Institute. The crystals were mounted on a Bruker D8 Venture equipped with IμS micro-focus sealed tube Mo-Kα and a PHOTON III M14 detector.
DSC (TA, DSC 25) experiments were carried out at a scanning speed of 10 K/min in the temperature range of 190–620 K under an atmosphere of nitrogen. TGA experiments were performed on a thermogravimetric analyzer (TA Instrument) in the temperature range of 300–870 K. The same heating rate was maintained. The amounts of [NH3(CH2)3NH3]ZnCl4 used for the DSC and TGA experiments were 6.04 and 6.72 mg, respectively and the amounts of [NH3(CH2)3NH3]ZnBr4 used were 6.40 and 8.96 mg, respectively.
The NMR spectra of the crystals of [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 were recorded on a 400 MHz Avance II + Bruker solid-state NMR spectrometer equipped with 4 mm MAS probes (at KBSI, Western Seoul Center). The MAS 1H and 13C NMR experiments were conducted at the Larmor frequency of 400.13 and 100.61 MHz, respectively. It was observed that an MAS rate of 10 kHz can minimize the spinning sidebands. Tetramethylsilane (TMS) was used as the standard to record the NMR spectra. As preparation for the MAS NMR experiments, the single crystal was ground into powder. The T1ρ values were obtained using a variable length spin lock pulse. For the two compounds, the width of the π/2 pulse for 1H was 3.5 μs and the width of the π/2 pulse for 13C was 3.96–4.3 μs. The radiofrequency power of the spin-lock pulses was 71.42 kHz for 1H and 62.50 kHz for 13C. An almost constant temperature (error ± 0.5 K) was maintained even when the rate of flow of nitrogen gas and the heater current were adjusted.
Experimental results
X-ray results
The X-ray powder diffraction patterns of the [NH3(CH2)3NH3]ZnX4 (X = Cl and Br) crystals at 298 K are displayed in Fig. 2. And, the lattice constants for [NH3(CH2)3NH3]ZnCl4 were determined to be a = 10.670 Å, b = 10.576 Å, c = 10.755 Å, and β = 118.477°, and those for [NH3(CH2)3NH3]ZnBr4 were determined to be a = 11.146 Å, b = 10.995 Å, c = 11.188 Å, and β = 117.215°. These results are consistent with those reported previously26,27.
Thermal properties
Two endothermic peaks at 268 and 597 K were observed in the DSC curves of [NH3(CH2)3NH3]ZnCl4, as shown in Fig. 3. The DSC curves of [NH3(CH2)3NH3]ZnBr4 revealed the presence of two endothermic peaks at 272 and 589 K, as shown in Fig. 3. The results obtained from the DSC experiments revealed that the peaks that appeared at ~ 590 K were significantly larger than the other peaks. An additional TGA and differential thermal analysis (DTA) experiments were performed to determine whether these endothermic peaks are related to the structural phase transitions or melting. The results of the TGA and DTA experiments conducted with the two crystals are presented in Fig. 4a,b. The onset of thermal decomposition temperature (= Td) was observed at temperatures > 550 K. This was characterized by a loss in the weight of the compounds. It was observed that [NH3(CH2)3NH3]ZnCl4 (Mw = 283.34 mg) and [NH3(CH2)3NH3]ZnBr4 (Mw = 461.15 mg) started losing weight at higher temperatures. The amounts of solid residues obtained were calculated from the molecular weights of the compounds and balanced chemical reactions. The amounts were estimated from Eqs. (1), (2), (3), and (4)28.
[NH 3 (CH 2 ) 3 NH 3 ]ZnCl 4
The amount of residue obtained is determined as follows:
The amount of residue obtained is determined as follows:
[NH 3 (CH 2 ) 3 NH 3 ]ZnBr 4
The amount of residue obtained is determined as follows:
The amount of residue obtained is determined as follows:
[NH3(CH2)3NH3]ZnCl4 was found to lose approximately 13% and 26% of its weight when the temperature was approximately 606 and 621 K, respectively. [NH3(CH2)3NH3]ZnBr4 was found to lose approximately 17% and 35% of its weight when the temperature was approximately 622 and 649 K, respectively. The weight loss can be potentially attributed to the decomposition of HX and 2HX (X = Cl and Br) moieties, respectively, as shown in Fig. 4a,b. Based on the DSC and TGA results, the phase transition temperatures for [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 were 268 K and 272 K, respectively. In addition, it was found that the endotherm peaks of 597 K for [NH3(CH2)3NH3]ZnCl4 and 589 K for [NH3(CH2)3NH3]ZnBr4 were related to Td.
MAS 1H NMR results
The temperature-induced 1H NMR chemical shifts for [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 crystals were recorded through MAS NMR spectroscopy. A single resonance signal was observed in each of the NMR spectra of both the compounds. The 1H NMR spectrum of [NH3(CH2)3NH3]ZnBr4 recorded at 300 K is shown in Fig. 5. The observed resonance signal was asymmetric. The line width represented as symbol 1 and that represented as symbol 2 at the full-width at half-maximum (FWHM) value are not the same as those at 3.54 and 6.11 ppm for 1 and 2, respectively. This asymmetric signal is attributed to the overlapping lines of the two 1H for CH2 and NH3 present in the [NH3(CH2)3NH3] cations. The spinning sidebands were marked with open circles. At 300 K, the 1H line width of the spectrum recorded for [NH3(CH2)3NH3]ZnCl4 was 10.57 ppm, whereas that of the spectrum recorded for [NH3(CH2)3NH3]ZnBr4 was 9.65 ppm. The 1H NMR chemical shifts for two 1H in the [NH3(CH2)3NH3] cations are temperature independent. The chemical shift values remained practically constant over a wide range of temperature, indicating that the structural environment of the protons present in the [NH3(CH2)3NH3] cation does not change when the temperature is increased.
The 1H T1ρ was measured at each temperature by applying a spin lock of variable duration, t. The relationship between the intensities of the NMR signals and the duration of the spin lock is as follows29,30,31:
where I(t) and I(0) are the signal intensities at times t and t = 0, respectively. The 1H NMR signals were plotted as a function of the delay times at each temperature. The 1H NMR signals for [NH3(CH2)3NH3]ZnCl4 at 300 K were recorded by varying the delay times (in the range of 1–150 ms, Fig. 6). The decay curves were fit to an exponential function, as shown in Eq. (5). The T1ρ values of the protons in [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 were obtained as a function of inverse temperature, as shown in Fig. 6. The T1ρ values of the atoms in the two compounds did not change near TC (268 K for [NH3(CH2)3NH3]ZnCl4 and 272 K for [NH3(CH2)3NH3]ZnBr4). The T1ρ values initially increased and then decreased when the temperature was increased. In addition, the trend of changes in the 1H T1ρ values of [NH3(CH2)3NH3]ZnBr4 and [NH3(CH2)3NH3]ZnCl4 with temperature was similar. The T1ρ values of the protons in [NH3(CH2)3NH3] in both the crystals were 10–1000 ms. The 1H T1ρ values of [NH3(CH2)3NH3]ZnCl4 decreased abruptly at 360 K and that of [NH3(CH2)3NH3]ZnBr4 decreased abruptly at 300 K (as represented by lines). The T1ρ value of the protons in [NH3(CH2)3NH3]ZnCl4 was higher than that in [NH3(CH2)3NH3]ZnBr4 at all studied temperatures. For both the compounds, lower T1ρ values were recorded at higher temperatures. On the slow side of T1ρ, a decrease in T1ρ results in smaller valued of the correlation time τC. The T1ρ values for Arrhenius-type random motions with τC are described in terms of slow motions. When τC < < ω1, T1ρ ∝ τC = τ0 exp(-Ea/kBT), where ω1 denotes the spin-lock frequency and Ea represents the activation energy. The decrease in T1ρ values with temperature indicates an increase in proton mobility at higher temperatures.
MAS 13C NMR results
The MAS 13C NMR chemical shifts in [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 were measured according to the change in temperature. Two resonance signals were observed in the MAS 13C NMR spectra of the compounds. The chemical shift values of the other carbon atoms were determined relative to the TMS signal. Here, the CH2 group sandwiched between two other CH2 groups is labeled as CH2-1. The CH2 group close to NH3 is labeled CH2-2. At 300 K, the signals corresponding to the CH2-1 and CH2-2 carbon atoms in [NH3(CH2)3NH3]ZnCl4 appear at δ = 26.41 and δ = 38.53 ppm, respectively. The chemical shift values of the carbon atoms in [NH3(CH2)3NH3]ZnBr4 are similar to the chemical shift values of the carbon atoms in [NH3(CH2)3NH3]ZnCl4 (Fig. 7, inset). It was observed that for both the crystals, the 13C chemical shift values remained almost constant with changes in temperature (Supplementary Fig. 1).
The change in FWHM for 13C NMR spectra with temperature for both crystals is shown in Fig. 7. In the case of [NH3(CH2)3NH3]ZnCl4, the 13C line widths (CH2-1 and CH2-2) decreased monotonically with an increase in temperature. Significant anomaly due to phase transition was not observed. The 13C line widths in [NH3(CH2)3NH3]ZnBr4 changed with the increase in temperature. A transformation from a Gaussian to Lorentzian shape was observed. The line width reduced because of internal molecular motion. The line width of CH2-1 was lower than that of CH2-2. The decrease in line width with an increase in temperature can be attributed to the internal molecular motion.
The intensities of the 13C NMR signals were determined by varying the delay times at each temperature. The 13C NMR signals (CH2-1 and CH2-2) recorded for [NH3(CH2)3NH3]ZnCl4 and [NH3(CH2)3NH3]ZnBr4 were plotted as a function of delay time. The decay curves for CH2-1 and CH2-2 were fitted to an exponential equation of Eq. (5). From the slopes of the recovery traces, the 13C T1ρ values were obtained (CH2-1 and CH2-2) as a function of inverse temperature, as shown in Fig. 8. The 13C T1ρ values of [NH3(CH2)3NH3]ZnCl4 were higher than those of [NH3(CH2)3NH3]ZnBr4. The 13C T1ρ value of CH2-1 was slightly higher than the 13C T1ρ value of CH2-2. The T1ρ values of the atoms in both the compounds were temperature independent at > 200 K.
Conclusion
The physical properties of organic–inorganic hybrid crystals [NH3(CH2)3NH3]ZnX4 (X = Cl, Br) were investigated through DSC, TGA, and NMR spectroscopy. The TGA results revealed that the [NH3(CH2)3NH3]ZnX4 (X = Cl, Br) complex exhibited good thermal stability. When the temperature was increased, the 1H and 13C chemical shifts remained almost constant. Although the TC was obtained from the DSC experiments, in particular, changes in the chemical shift values were not observed at temperatures near TC. This indicated that the chemical environment surrounding the protons and the carbon atoms in the [NH3(CH2)3NH3] cation remained unaltered.
The structural dynamics were discussed in terms of the 1H T1ρ and 13C T1ρ values. The 1H T1ρ values changed with the temperature, whereas the T1ρ value of the carbon atom located in the middle of the N–C–C–C–N chain did not change significantly with the temperature. The 1H T1ρ and 13C T1ρ values of the atoms in [NH3(CH2)3NH3]ZnCl4 were higher than those in [NH3(CH2)3NH3]ZnBr4 (Table 1). The reason why 13C T1ρ values in [NH3(CH2)3NH3]ZnCl4 are longer than 13C T1ρ values in [NH3(CH2)3NH3]ZnBr4 are as follows. The 13C T1ρ is most likely driven by 1H-13C interactions, and hence, the H-C bond lengths are important.
However, the H-C bond lengths of the two materials are unlikely to be different, and also cannot be accurately measured from X-ray results. We assume that the differences in the dynamics between the materials contribute more to the difference in T1ρ. Although the structures and lattice constants of two crystals are similar, the differences between the local environments and structural dynamics obtained from the chemical shifts and T1ρ values of the two compounds can be attributed to the different bond lengths and halogen atoms. Thus, the results of this study elucidate, the physical properties of [NH3(CH2)3NH3]ZnX4, which will expand the application scope of this organic–inorganic hybrid crystals.
References
Narita, N. & Yamada, I. Nonlinear magnetic-susceptibility of two-dimensional magnets (CnH2 n+1NH3)2CuCl4 with n = 1, 2 and 3. J. Phys. Soc. Jpn. 65, 4054 (1996).
Mitzi, D. B., Chondroudis, K. & Kagan, C. R. Organic-inorganic electronics. IBM J. Res. Dev. 45, 29 (2001).
Manaka, H., Yamada, I. & Goto, T. Disappearance of the weak ferromagnetic moment under high pressure observed in the two-dimensional antiferromagnet (C3H7NH3)2CuCl4 through magnetic susceptibility measurements. J. Phys. Soc. Jpn. 71, 2822 (2002).
Cheng, Z. & Lin, J. Layered organic-inorganic hybrid perovskites: structure, optical properties, film preparation, patterning and templating engineering. Cryst. Eng. Com. 12, 2646 (2010).
Gonzalez-carrero, S., Galian, R. E. & Perez-Prieto, J. Organometa halide perovskites: bulk low-dimension materials and nanoparticles. Part. Syst. Charact. 32, 709 (2015).
Kind, R., Plesko, S., Gunter, P., Ross, J. & Fousek, J. Structural phase transitions in the perovskite-type layer compounds NH3(CH2)3NH3CdCl4, NH3(CH2)4NH3MnCl4, and NH3(CH2)5NH3CdCl4. Phys. Rev. B 23, 5301 (1981).
Mostafa, M. F., Emrick, R. & Atallah, J. A. S. Mossbauer investigation of some layered Fe(II)Cl compounds. Appl. Phys. 81, 4134 (1997).
Staskiewicz, B., Czupinski, O. & Czapla, Z. On some spectroscopic properties of a layered 1,3-diammoniumpropylene tetrabromocadmate hybrid crystal. J. Mol. Struct. 1074, 723–731 (2014).
Staskieqicz, B., Turowska-Tyrk, I., Baran, J., Gorecki, C. Z. & Czapla, Z. Structural characterization, thermal, vibrational properties and molecular motions in perovskite-type diamonopropanetetrachlorocadmate NH3(CH2)3NH3CdCl4 crystal. J. Phys. Chem. Solids 75, 1305–1317 (2014).
Czapla, Z. et al. Structural phase trasition in a perovskite-type NH3(CH2)3NH3CuCl4 crystal X-ray and optical studies. Phase Transition 90, 637–643 (2017).
Abdel-Adal, S. K., Kocher-Oberlehner, G., Ionov, A. & Mozhchil, R. N. Effect of organic chain length on structure, electronic composition, lattice potential energy, and optical properties of 2D hybrid perovskites [(NH3)(CH2)n(NH3)]CuCl4, n = 2–9. Appl. Phys. A 123, 531–541 (2017).
Wei, Y., Audebert, P., Galmiche, L., Lauret, J. S. & Deleporte, E. Photostability of 2D organic-inorganic hybrid perovskites. Materials 7, 4789 (2014).
Mitzi, D. B. Templating and structural engineering in organic-inorganic perovskites. J. Chem. Soc. Dalton Trans. 1, 1 (2001).
Ahmad, S., Hanmandlu, C., Kanaujia, P. K. & Vijaya Prakash, G. Direct deposition strategy for highly ordered inorganic organic perovskite thin films and their optoelectric applications. Opt. Mater. Express 4, 1313 (2014).
Saikumar, I., Ahmad, S., Baumberg, J. J. & Vijaya Prakash, G. fabrication of excitonic luminescent inorganic-organic hybrid nano- and microcrystals. Scr. Mater. 67, 834 (2012).
Pradeesh, K., Yadav, G. S., Singh, M. & Vijaya Prakash, G. Synthesis, structure and optical studies of inorganic-organic hybrid semiconductor, NH3(CH2)12NH3PbI4. Mater. Chem. Phys. 124, 44 (2010).
Pradeesh, K., Baumberg, J. & Vijaya Prakash, G. Exciton switching and peierls transitions in hybrid inorganic-organic self-assembled quantum wells. Appl. Phys. Lett. 95, 173305 (2009).
Abdel-Aal, S. K. & Abdel-Rahman, A. S. J. Cryst. Growth 457, 282 (2017).
Rao, C. N. R., Cheetham, A. K. & Thirumurugan, A. Hybrid inorganic-organic materials: a new family incondensed matter physics. J. Phys. Condens. Matter 20, 83202–83223 (2008).
Elseman, M. et al. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Interfaces 10, 11699–11707 (2018).
Aramburu, J. A., Garcia-Fernandez, P., Mathiesen, N. R., Garcia-Lastra, J. M. & Moreno, M. Changing the usual interpretation of the structure and ground state of Cu2+-layered perovskites. J. Phys. Chem. C 122, 5071–5082 (2018).
Franssen, W. M. J. & Kentgens, A. P. M. Solid-state NMR of hybrid perovskites. Solid State Nucl. Mag. Reson. 100, 36–44 (2019).
Milic, J. V. et al. Supramolecular engineering for formamidinium-based layered 2D perovskite solar cells: structural complexity and dynamics revealed by solid-state NMR spectroscopy. Advan. Energy Mater. 9, 1900284–1900296 (2019).
Lee, J., Lee, W., Kang, K., Lee, T. & Lee, S. K. Layer-by layer structural identification of 2D ruddlesden-popper hybrid lead iodide perovskites by solid-state NMR spectroscopy. Chem. Mater. 33, 370–377 (2021).
Dahlman, C. J. et al. Dynamic motion of organic spacer cations in ruddlesden-popper lead perovskites probed by solid-state NMR spectroscopy. Chem. Mater. 33, 642–656 (2021).
Kallel, A., Fail, J., Fuess, H. & Daoud, A. 1,3-propanediammonium tetrachlorozincate (II). Acta Cryst. B36, 2788 (1980).
Ishihara, H. et al. Isolated versus condensed anion structure III: the influence of the cations 1,3-propanediammonium, 1,4-butanediammonium, n-propylammonium, n-butylammonium and n-pentylammonium on structures and phase transitions of ZnBr42- salts. A 81Br NQR and X-ray structure analysis. Z. Naturforsch. 52, 550 (1997).
Lim, A. R. & Kim, S. H. Study on paramagnetic interactions of (CH3NH3)2CoBr 4 hybrid perovskites based on nuclear magnetic resonance (NMR) relaxation time. Molecules 24, 2895 (2019).
Abragam, A. The Principles of Nuclear Magnetism (Oxford University Press, 1961).
Koenig, J. L. Spectroscopy of Polymers (Elsevier, 1999).
Harris, R. K. Nuclear Magnetic Resonance Spectroscopy (Pitman Pub., 1983).
Acknowledgements
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2018R1D1A1B07041593 and 2016R1A6A1A03012069).
Author information
Authors and Affiliations
Contributions
A.R. L. designed the project and wrote the manuscript. S.H. K. performed NMR experiments. Y.L. J performed TGA experiments and comment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, A.R., Kim, S.H. & Joo, Y.L. Physicochemical properties and structural dynamics of organic–inorganic hybrid [NH3(CH2)3NH3]ZnX4 (X = Cl and Br) crystals. Sci Rep 11, 8408 (2021). https://doi.org/10.1038/s41598-021-87940-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87940-2
- Springer Nature Limited
This article is cited by
-
Exploring the potential applications of lead-free organic–inorganic perovskite type [NH3(CH2)nNH3]MCl4 (n = 2, 3, 4, 5, and 6; M = Mn, Co, Cu, Zn, and Cd) crystals
Scientific Reports (2024)
-
Investigating the physicochemical properties, structural attributes, and molecular dynamics of organic–inorganic hybrid [NH3(CH2)2NH3]2CdBr4·2Br crystals
Scientific Reports (2023)
-
Advances in physicochemical characterization of lead-free hybrid perovskite [NH3(CH2)3NH3]CuBr4 crystals
Scientific Reports (2022)
-
Growth, structure, phase transition, thermal properties, and structural dynamics of organic–inorganic hybrid [NH3(CH2)5NH3]ZnCl4 crystal
Scientific Reports (2022)