Abstract
Applying a pneumatic tourniquet provides surgeons with a bloodless surgical field. However, application of the tourniquet induces various physiological changes. We evaluated the effect of tourniquet deflation on the intracranial pressure by using ultrasonography to measure the optic nerve sheath diameter (ONSD) in patients undergoing lower limb surgery. The ONSD was measured in 20 patients at five time points: after anesthetic induction (T0) and immediately before (T1), immediately after (T2), 5 min after (T3), and 10 min after tourniquet deflation (T4). Hemodynamic and respiratory variables were recorded. The ONSD showed significant differences at each point (P < 0.001). The ONSDs at T2 and T3 were significantly greater than that at T1 (P = 0.0007 and < 0.0001, respectively). The change in the end-tidal carbon dioxide partial pressure (EtCO2) was similar to the change in the ONSD. The change in the ONSD was significantly correlated with the change in the EtCO2 after tourniquet deflation (r = 0.484, P = 0.030). In conclusion, the ONSD, as an indicator of intracranial pressure, increased after tourniquet deflation in patients undergoing lower limb surgery. This was correlated with an increased EtCO2 and arterial carbon dioxide partial pressure.
Trial registration: ClinicalTrials.gov (NCT03782077).
Similar content being viewed by others
Introduction
Although there has been a debate on the use of pneumatic tourniquet in orthopedic surgery, they do provide surgeons with a bloodless surgical field, which improves the surgical environment. In addition, pneumatic tourniquets reduce the surgical time and intraoperative blood loss1,2; therefore, they are still widely used during lower limb surgery. However, inflation and deflation of the tourniquet induces physiological changes, including alterations in the pH, arterial oxygen partial pressure, arterial carbon dioxide partial pressure (PaCO2), potassium ion levels, and lactate levels3. In particular, tourniquet deflation causes carbon dioxide (CO2) that has accumulated during the ischemic period to be released into the systemic circulation. The resultant elevation in PaCO2 is followed by an increase in the intracranial pressure (ICP)4. Although this increase in cerebral blood flow (CBF) has not much clinical significance and it is compensated and tolerated in most healthy patients, the increase in cerebral blood volume may cause an increase in ICP in patients with decreased intracranial compliance5,6,7. Increased intracranial pressure can induce deleterious outcomes, including cerebral ischemia7,8. Therefore, cautious monitoring and thorough management of the ICP is required in patients undergoing lower limb surgery, who have or are at high risk of cerebrovascular disease, using a pneumatic tourniquet.
Many studies have shown that ultrasonography of the optic nerve sheath diameter (ONSD) has good diagnostic accuracy to detect intracranial hypertension9. Increased ICP is transmitted to the subarachnoid space surrounding the optic nerve, causing optic nerve sheath expansion10. Therefore, increased ICP could be estimated based on the ONSD, which can be measured in a non-invasive manner that could be applied easily in orthopedic patients.
In the present study, we aimed to investigate alterations in the ICP using ultrasonography to measure the ONSD after deflation of the pneumatic tourniquet in patients undergoing lower limb surgery. We also evaluated the factors related to changes in the ONSD.
Results
In November and December 2018, 21 consecutive patients scheduled for undergoing knee surgery, using a pneumatic tourniquet were screened for eligibility. Among them, one refused to participate in the study, and 20 were enrolled, all of whom finished the study without drop-out. Ultimately, 20 patients were included in the final analysis. The patients’ baseline characteristics and intraoperative data are shown in Table 1.
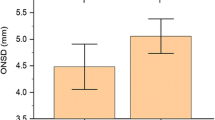
The ONSD showed significant differences at each time point (P < 0.001; Table 2 and Fig. 1). A post hoc analysis demonstrated that ONSDs at T2 and T3 were significantly greater than that at T1 (P = 0.0007 and < 0.0001, respectively). However, the ONSD at T4 was no different from that at T1. The change in the end-tidal carbon dioxide partial pressure (EtCO2) was similar to the change in the ONSD. The EtCO2 levels at T2, T3, and T4 were significantly higher than that at T1 (P < 0.0001, P < 0.0001, and P = 0.001, respectively). The PaCO2, obtained through arterial blood gas analysis, was also significantly higher at T3 than at T1 (P < 0.0001; Fig. 2). Other intraoperative variables at each time point, including hemodynamic and respiratory variables, are shown in Table 3. Mean arterial pressure (MAP) significantly decreased over time, and the MAPs at T2, T3, and T4 were lower than that at T1. Plateau airway pressure (PAP) and peak inspiratory airway pressure (PIAP) were significantly lower after tourniquet deflation compared with the PAP and PIAP at T1 except for PAP at T3. The body temperature was lower than before the tourniquet deflation only at T3 and T4.
Comparison of optic nerve sheath diameters (ONSDs) measured at after anesthetic induction (T0) and immediately before (T1), immediately after (T2), 5 min after (T3), and 10 min after deflation (T4). The ONSDs were significantly increased at T2 and T3 as compared with T1. The dot and error bars indicate the mean and standard deviation, respectively.
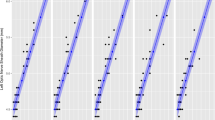
The blue lines indicate that compared of EtCO2 measured at after anesthetic induction (T0) and immediately before (T1), immediately after (T2), 5 min after (T3), and 10 min after deflation (T4). EtCO2 were moderate correlated with ONSD. The red line indicates that compared of PaCO2 measured at immediately before (T1) and at 5 min after deflation (T3).
The change in the ONSD was significantly correlated with the change in the EtCO2 from immediately before tourniquet deflation to 5 min after tourniquet deflation (Pearson’s correlation coefficient [r] = 0.484; P = 0.030; Table 4). However, there was no significant correlation between the change in the ONSD and the change in the MAP.
Discussion
In this prospective observational study, we found that the ONSD, as an indicator of the ICP, increased after tourniquet deflation in patients undergoing lower limb surgery. This was correlated with an increased carbon dioxide after tourniquet deflation.
A few methods can be used to monitor the ICP in real-time: the intraventricular catheter and the intraparenchymal catheter11. The intraventricular and intraparenchymal catheters are considered the gold standard techniques based on their accuracy12. However, these invasive techniques carry the highest risk of complications and are not always feasible12. Therefore, noninvasive methods for indirectly estimating ICP are attracting the attention of researchers: transcranial Doppler and the ONSD. Transcranial Doppler and the ONSD are both non-invasive, low-cost, and accessible at the bedside. However, transcranial Doppler has several disadvantages. For example, it is difficult to find an adequate acoustic window in some populations and the technique only measures arterial blood flow and volume, without any information on other factors that contribute to the ICP13,14. Conversely, the ONSD is easily visualized in most cases. Moreover, a pressure rise in the intracranial compartment leads to a shift of CSF from the intracranial subarachnoid space into the optic subarachnoid space15, and an increase in ONSD is an early manifestation of increased ICP. Thus, the ONSD is useful for monitoring the ICP during surgery.
During tourniquet inflation, the oxygen supply decreases because there is no arterial or venous blood flow in the limb; anaerobic processes then occur in the ischemic area3. Deflation of the tourniquet allows reperfusion of the ischemic limb, which can cause increases in oxygen consumption and CO2 production 2 min after tourniquet release16,17. The current study corroborated previous investigations, in which the EtCO2 significantly increased immediately after tourniquet deflation and the increased levels were maintained for up to 10 min. In addition, the PaCO2 level was significantly higher after tourniquet deflation. This rapid increase in the PaCO2 after tourniquet deflation is associated with transient increases in the CBF18,19. Ellingsen et al. showed that the CBF response started less than 30 s after the PaCO2 began to increase, and that it required up to 2 min to reach a peak value20. Changes in the ICP are rapidly transmitted to the optic nerve sheath, with no apparent temporal lag21. In the present study, the EtCO2 showed a peak value immediately after deflation, but the ONSD, which is an indicator of the ICP, showed the highest value after 5 min of deflation. However, this inconsistency with other studies may have arisen because the two variables were measured in different ways in previous studies—in the present study, all variables were measured intermittently, including the EtCO2 and ONSD; therefore, we could not identify the exact peak time of each variable.
The PaCO2 was not the only factor affecting the ICP and ONSD. Blood pressure may also influence the ICP by altering the CBF. After tourniquet deflation, blood pressure decreases because vasodilatory substances are released into the circulation22. In the present study, blood pressure decreased immediately after tourniquet deflation and was maintained for 10 min. Previous studies have demonstrated that a blood pressure drop is associated with decreased CBF23,24. However, we found that the EtCO2 showed moderate correlation, while the MAP was not apparently correlated with the CBF. Based on this result, we speculated that the PaCO2 was a more important determinant of the ICP than blood pressure. In addition, the MAP after tourniquet deflation stayed between 60 and 150 mmHg in the present study. CBF autoregulation typically occurs between these values25.
In healthy individuals, the respiratory drive is stimulated towards compensation and increases breathing when hypercapnia occurs26. If hyperventilation is not adequately achieved after tourniquet deflation, the CBF velocity increases27. The transient increase in CBF may have a detrimental effect in patients at risk of cerebrovascular accident, including brain injury7. When patients are self-breathing, changes to the ICP caused by hypercapnia after tourniquet deflation can be compensated by increased breathing. In previous studies on the tourniquet time and ICP28, recovery of spontaneous breathing after tourniquet deflation may have influenced results, because the researchers did not continuously infuse any neuromuscular blocking agents. In the present study, we aimed to assess the effect of tourniquet deflation itself; therefore, we continuously infused a neuromuscular blocking agent under a train of four monitoring. The present study demonstrated that tourniquet deflation was followed by related ONSD dilation and increases in the PaCO2 and EtCO2 levels without any compensation by self-breathing. To assess whether compensation via spontaneous breathing affected changes in the ICP and ONSD, future studies should compare the ONSD after tourniquet deflation between controlled ventilation and spontaneous breathing groups.
The present study had the following limitations. First, it was conducted under general anesthesia using sevoflurane. Inhalational anesthetics, such as sevoflurane have a cerebral vasodilatory effect and can increase the CBF and ICP in a dose-dependent manner29. However, sevoflurane was maintained at approximately 1 minimal alveolar concentration; therefore, its effect was likely minimal30. In addition, sevoflurane has not been shown to affect the autoregulation of cerebral vasculature, unlike other inhalational anesthetics31. Second, the ONSD and PaCO2 were not measured continuously, thus we could not identify the exact peak time of increase in the ONSD and PaCO2 after tourniquet deflation. The ONSD and PaCO2 cannot be monitored continuously. However, we did show that ONSD increases within a few minutes of tourniquet deflation and then decreases towards the baseline level within 10 min. Third, it is difficult to draw a definite conclusion due to our study was an observational study and the number of enrolled patients was small. Nevertheless, we cautiously believe that ONSD measurements could improve outcomes by detecting ICP increases after tourniquet deflation in patients at higher risk of cerebrovascular accident. In conclusion, tourniquet deflation caused an increase in the ONSD in patients undergoing lower limb surgery, and this increase was associated with an increase in the PaCO2. Furthermore, sonographic ONSD monitoring might provide useful information regarding changes in the ICP that occur during lower limb surgery using a tourniquet.
Methods
Study design
This prospective observational study was performed at Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. The study protocol was approved by the Institutional Review Board of Asan Medical Center (protocol number: 2018-1172, approval date: 01/SEP/18), and written informed consent was obtained from all patients. The study was conducted according to the 1964 Helsinki declaration and its later amendments. We registered the study on ClinicalTrials.gov (NCT03782077).
Patients
The study included patients who met the following criteria: (1) the American Society of Anesthesiologists physical status I–IV32, (2) age between 18 and 80 years, and (3) knee surgery with pneumatic tourniquet scheduled at our center. We excluded patients with cerebrovascular, neurological, or ophthalmic diseases, as well as those who refused to participate or had any contraindication to general anesthesia or tourniquet use.
Anesthetic management
The recruited patients entered the operating room without any premedication. Standard monitoring was performed, including peripheral oxygen saturation, electrocardiogram, non-invasive blood pressure, EtCO2, and body temperature. General anesthesia was induced using 2 mg/kg of propofol and 50 µg of fentanyl. When the eyelash reflex was lost, 0.6–0.8 mg/kg of rocuronium was intravenously injected, and the lung was mask-ventilated using 5–6 vol% sevoflurane in 80% oxygen for 3 min. When the patients reached an adequate anesthetic depth, an appropriately sized supraglottic airway device was inserted. The esophageal temperature probe was also inserted via a supraglottic airway device to measure body temperature. Anesthesia was maintained using 1.5–2 vol% of sevoflurane in a 50% oxygen/nitrous oxide mixture at a rate of 2L/min. The concentration of sevoflurane was controlled according to the bispectral index level and minimal alveolar concentration of inhalation agent to avoid overdose sevoflurane. Mechanical ventilation was adjusted to maintain the EtCO2 level at 35 mmHg with a tidal volume of 6–8 mL/kg and a respiratory rate of 10–12/min. To eliminate the effect of self-respiration compensating hypercapnia, rocuronium was continuously infused to maintain a train of four count of 1–2 throughout the surgery. After anesthetic induction, the radial artery was cannulated for invasive arterial blood pressure monitoring and blood gas analysis. Crystalloid solution including plasmalyte was administered at a maintenance dose to maintain normovolemia. To control pain, we administered an ultrasound-guided femoral nerve and sciatic nerve block by injecting 40 cm3 of 0.3% ropivacaine. A pneumatic thigh tourniquet was applied as close as possible to the limb root, and it was inflated to a pressure of 300 mmHg. All surgical procedures were performed with the tourniquet inflated; after the surgery, the tourniquet was deflated. When the ONSD measurements were completed, the inhalation agent and rocuronium were discontinued, and the neuromuscular blockade was reversed using sugammadex. After recovery from general anesthesia, patients were transferred to the postanesthetic care unit.
Measurements
The primary outcome of this study was a change in the ONSD, measured by ultrasonography after tourniquet deflation. Ultrasound imaging was performed by two investigators trained in ocular sonography using the 6–13 Hz linear probe of a single ultrasound machine (S-Nerve; SonoSite, Bothell, WA, USA). Patients were placed in the supine position with their heads in the neutral position. The probe was located on the closed upper eyelid in the transverse plane and moved slightly to identify the optimal image between the vertical hypoechoic band and the retrobulbar echogenic fat tissue. The outer diameter of the optic nerve sheath was measured 3 mm behind the optic disc (Fig. 3). We measured the ONSD twice in each eyeball, and used the average values of the four measurements in the analysis33. The measurements were performed at the following five points: after anesthetic induction (T0), immediately before the tourniquet deflation (T1), immediately after the tourniquet deflation (T2), 5 min after tourniquet deflation (T3), and 10 min after tourniquet deflation (T4).
MAP, respiratory variables, and body temperature were measured at the same time points. Respiratory variables included the PAP, PIAP, and EtCO2 and they were measured by the ventilator machine (Drager Primus, Lubeck, Germany). Arterial blood gas analysis was also performed to ensure accurate PaCO2 measurement at T1 and T3. In addition, the patients’ baseline characteristics and tourniquet time were recorded.
Statistical analysis
Based on our clinical experiences, the mean ONSD after anesthesia induction was 5.0 ± 0.5 mm. When an ONSD change of 10% was considered significant, the calculated sample size with a power of 0.9 and an alpha-error of 0.1 was 18 patients. Considering dropout, we decided to enroll 20 patients.
The data we obtained were recorded in a standardized case report form. In the analysis of repeated measured data, including ONSD, a linear mixed model was used. Furthermore, we performed multiple comparison using Scheffe’s method to compare variables between two time points. Pearson’s correlation coefficient analysis was used to analyze how the ONSD change was correlated with the EtCO2 change and MAP change. P-values of < 0.05 were considered statistically significant.
References
Yi, S., Tan, J., Chen, C., Chen, H. & Huang, W. The use of pneumatic tourniquet in total knee arthroplasty: A meta-analysis. Arch. Orthop. Trauma Surg. 134, 1469–1476 (2014).
Alcelik, I. et al. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: A systematic review and meta-analysis of randomized controlled trials. J. Arthroplasty 27, 331–340 (2012).
Girardis, M. et al. The hemodynamic and metabolic effects of tourniquet application during knee surgery. Anesth. Analg. 91, 727–731 (2000).
Estebe, J.-P., Davies, J. M. & Richebe, P. The pneumatic tourniquet: Mechanical, ischaemia–reperfusion and systemic effects. Eur. J. Anaesthesiol. 28, 404–411 (2011).
Conary, K. & Klemm, M. Severe increase of intracranial pressure after deflation of a pneumatic tourniquet. Anesthesiology 71, 294–295 (1989).
Sparling, R., Murray, A. & Choksey, M. Raised intracranial pressure associated with hypercarbia after tourniquet release. Br. J. Neurosurg. 7, 75–77 (1993).
Eldridge, P. & Williams, S. Effect of limb tourniquet on cerebral perfusion pressure in a head-injured patient. Anaesthesia 44, 973–974 (1989).
McGillicuddy, J. E. Cerebral protection: Pathophysiology and treatment of increased intracranial pressure. Chest 87, 85–93 (1985).
Dubourg, J., Javouhey, E., Geeraerts, T., Messerer, M. & Kassai, B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 37, 1059–1068 (2011).
Hansen, H. & Helmke, K. The subarachnoid space surrounding the optic nerves: An ultrasound study of the optic nerve sheath. Surg. Radiol. Anat. 18, 323–328 (1996).
Hawthorne, C. & Piper, I. Monitoring of intracranial pressure in patients with traumatic brain injury. Front. Neurol. 5, 121 (2014).
Zhang, X. et al. Invasive and noninvasive means of measuring intracranial pressure: A review. Physiol. Meas. 38, R143 (2017).
Maeda, H. et al. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: Evaluation by the transcranial Doppler method. Stroke 24, 670–675 (1993).
Cardim, D. et al. Non-invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: Is it possible?. Neurocrit. Care 25, 473–491 (2016).
Chacko, J. Optic nerve sheath diameter: An ultrasonographic window to view raised intracranial pressure?. Indian J. Crit. Care Med. 18, 707 (2014).
Kam, P., Kavanaugh, R. & Yoong, F. The arterial tourniquet: pathophysiological consequences and anaesthetic implications. Anaesthesia 56, 534–545 (2001).
Hoka, S. et al. VO2 and VCO2 following tourniquet deflation. Anaesthesia 47, 65–68 (1992).
Deloughry, J. & Griffiths, R. Arterial tourniquets: Continuing education in anaesthesia. Crit. Care Pain 2, 64–68 (2009).
Kumar, K., Railton, C. & Tawfic, Q. Tourniquet application during anesthesia: “What we need to know?”. J. Anaesthesiol. Clin. Pharmacol. 32, 424 (2016).
Ellingsen, I., Hauge, A., Nicolaysen, G., Thoresen, M. & Walløe, L. Changes in human cerebral blood flow due to step changes in PAO2 and PACO2. Acta Physiol. Scand. 129, 157–163 (1987).
Lesky, D. & Schott, C. Does optic nerve sheath diameter ultrasonography permit accurate detection of real-time changes in icp?. Emerg. Med. 48, 317–320 (2016).
Townsend, H., Goodman, S., Schurman, D., Hackel, A. & Brock-Utne, J. Tourniquet release: Systemic and metabolic effects. Acta Anaesthesiol. Scand. 40, 1234–1237 (1996).
Tzeng, Y.-C. & Ainslie, P. N. Blood pressure regulation IX: Cerebral autoregulation under blood pressure challenges. Eur. J. Appl. Physiol. 114, 545–559 (2014).
Numan, T. et al. Static autoregulation in humans: A review and reanalysis. Med. Eng. Phys. 36, 1487–1495 (2014).
Paulson, O., Strandgaard, S. & Edvinsson, L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 2, 161–192 (1990).
Brinkman, J. E., Toro, F. & Sharma, S. Physiology, Respiratory Drive (Springer, 2018).
Kadoi, Y. et al. Hyperventilation after tourniquet deflation prevents an increase in cerebral blood flow velocity. Can. J. Anesth. 46, 259–264 (1999).
Besir, A. & Tugcugil, E. Does tourniquet time or pressure contribute to intracranial pressure increase following tourniquet application?. Med. Princ. Pract. 28, 16–22 (2019).
Matta, B. F., Heath, K. J., Tipping, K. & Summors, A. C. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology 91, 677 (1999).
Juhász, M. et al. Effect of sevoflurane on systemic and cerebral circulation, cerebral autoregulation and CO2 reactivity. BMC Anesthesiol. 19, 1–8 (2019).
Dagal, A. & Lam, A. M. Cerebral autoregulation and anesthesia. Curr. Opin. Anesthesiol. 22, 547–552 (2009).
Knuf, K. M., Maani, C. V. & Cummings, A. K. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioperat. Med. 7, 1–6 (2018).
Kim, Y., Choi, S., Kang, S. & Park, B. Propofol affects optic nerve sheath diameter less than sevoflurane during robotic surgery in the steep trendelenburg position. BioMed Res. Int. 2019, 1–5 (2019).
Author information
Authors and Affiliations
Contributions
W.U.K., and Y.J.R. conceived and designed the study. H.J.K. and Y.J.K. collected clinical samples and data. Y.J.K., J.K., H.K., and W.U.K. analysed the data and contributed to data interpretation. H.J.K. and Y.J.K. wrote the first draft of the manuscript. H.J.K., Y.J.K., and W.U.K. reviewed the manuscript. All authors approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, HJ., Kim, Y.J., Kim, J. et al. Change in the optic nerve sheath diameter after deflation of a pneumatic tourniquet: a prospective observational study. Sci Rep 12, 521 (2022). https://doi.org/10.1038/s41598-021-04457-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04457-4
- Springer Nature Limited