Abstract
Higher volumes of conventional and novel chemical insecticides are applied by farmers to control resistant strains of armyworm (Spodoperta litura) in Pakistan without knowing their risks to the environment and to public health. Ten reduced-risk insecticides were tested for their compatibility with two entomopathogenic nematodes (EPNs); Heterorhabditis indica and Steinernema carpocapsae against S. litura. The insecticide emamectin benzoate was highly toxic (LC50 = 2.97 mg/l) against 3rd instar S. litura larvae when applied alone whereas, novaluron and methoxyfenozide were the least toxic (LC50 = 29.56 mg/l and 21.06 mg/l), respectively. All the insecticides proved harmless against the two EPNs even 96 h after treatment. Indoxacarb, flubendiamide and spinetoram produced the greatest mortalities (72–76%) of S. litura larvae after 72 h when applied in mixtures with H. indica. Lowest mortalities (44.00 ± 3.74% and 48.00 ± 2.89) were observed for mixtures of H. indica with methoxyfenozide and chlorfenapyr, respectively. The positive control treatments with both EPNs (S. carpocapsae and H. indica) produced > 50% mortality 96 h after treatment. For insecticide mixtures with S. carpocapsae, only indoxacarb produced 90% mortality of larvae, whereas, indoxacarb, flubendiamide, emamectin benzoate, and spinetoram produced 90–92% mortality of larvae when applied in mixtures with H. indica. Additive interactions (Chi-square < 3.84) of EPN mixtures with reduced volumes of reduced-risk insecticides suggest opportunities to develop more environmentally favorable pest management programs for S. litura.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Spodoptera spp. (Lepidoptera: Noctuidae), commonly known as armyworms, are polyphagous insect pests, causing serious losses to many cash crops like cotton, maize, tobacco, groundnut, vegetables, legumes and fodder crops in Pakistan1,2,3. Armyworms damage plants by feeding on leaves and neonate larvae feed mainly on epidermal leaf tissue. Leaves with holes are the typical damage symptom of armyworm feeding and at high larval density complete defoliation is possible4.
Numerous insecticides belonging to conventional (organophosphates, carbamates, and pyrethroids) and novel classes (insect growth regulators, diamides, spinosyns, and avermectins) are extensively sprayed by farmers to control this pest in Pakistan5. Imprudent over-spraying of pesticides may elevate risks to the environment, impair non-target biodiversity and threaten public health6. Intensive spraying and repeated use of insecticides from the same chemical group often results in the development of resistance in this pest4,7,8. Insecticide resistance in armyworm populations to deltamethrin, cypermethrin, chlorpyrifos, profenofos, spinosad, emamectin benzoate, abamectin, indoxacarb, lufenuron, methoxyfenozide, chlorfluazuron, flufenoxuron, triflumuron, flubendiamide and spinetoram has been reported in Pakistan2,3,5.
The development of alternative strategies is badly needed to overcome resistance in this pest. Biopesticides with high selectivity including entomopathogenic nematodes (EPNs), entomopathogenic fungi (EPFs), entomopathogenic bacteria (EPBs), and nuclear polyhedrosis viruses (NPVs) are frequently reported to be an efficient tool in integrated pest management programs9. Entomopathogenic nematodes are recognized as important microbial control agents for certain pests of economic significance10,11,12,13. EPNs belonging to genera Steinernema and Heterorhabditis are the most studied for their potential to control certain pests like armyworms (Spodoptera spp.)7,11,12,14,15,16, diamondback moth (Plutella xylostella)17,18, tomato leafminer (Tuta absoluta)19,20,21, wax moth (Galleria mellonella), pink bollworm (Pectinophora gossypiella), eggplant fruit borer (Leucinodes arbonalis)13, oriental beetle (Anomala orientalis), Japanese beetle (Popillia japonica), native masked chafer (Cyclocephala borealis)22,23,24 and white grubs (Holotrichia parallela Motschulsky)24.
The compatibility of these EPNs with many insecticides has been widely studied against S. litura. Reports on toxicity of certain conventional and non-conventional insecticides with novel chemistries indicate that the EPNs (S. carpocapsae and H. indica) possess significant potential for controlling many insect pests of economic significance in agriculture12,15,18,19,20,21,25.
Considering the pest status of S. litura in Pakistan, and the challenge to overcome the resistance development and pesticide pollution, we investigated the compatibility of some selected insecticides with two EPNs viz., S. carpocapsae and H. indica as part of an effective pest management strategy. Toxicity of the selected insecticides to S. litura larvae were determined alone and in mixtures with S. carpocapsae and H. indica under laboratory conditions.
Materials and methods
All insect rearing (Galleria mellonella and Spodoptera litura), culturing of EPNs (Heterorhabditis indica and Steinernema carpocapsae) and bioassays were performed in the Insect Diversity and Biosystemics Laboratory of the Department of Entomology, University of Agriculture, Faisalabad, Pakistan.
Entomopathogenic nematodes (EPNs)
The two entomopathogenic nematode species S. carpocapsae and H. indica were reared on the late instar larvae of wax worms in the laboratory12,26. Entomopathogenic nematodes were collected from dead wax worms using the white trap method, modified for the collection of nematodes27. After 8–20 days of infection, infective juveniles emerged from the cadaver when incubated at 20–27 °C. Infective juveniles moved down to water through filter paper and these juveniles were harvested every day until no juveniles were present in the cadaver. All infective juveniles were rinsed from containers and were transferred to a beaker (100 ml). To obtain a clean suspension, the solution was diluted with distilled water by filling the beaker to the top, and nematodes were then collected and stored at 10–15 °C.
Insects
Wax worms, G. mellonella, were collected from infested beehives, and resulting adults were released in plastic jars, measuring 5 cm diameter and 30 cm depth. These jars were provided with cotton swabs soaked with a 10% honey solution for moth feeding and folded card sheets for oviposition. Newly emerged larvae were reared on a wheat-based semi-natural diet, in the laboratory following the mass-rearing technique reported by Khan et al.28 and Ashfaq et al.29.
Although extensive field surveys were performed to collect S. litura larvae, we were only successful in collecting insects from the research fields (Lat. 31.437778°; Long. 73.063611° and Lat. 31.390556°; Long. 73.018056°) of the University of Agriculture, Faisalabad. Upon reaching the laboratory, larvae were reared on washed and dried, fresh leaves of cotton, Gossypium hirsutum L., and kept in an insect rearing chamber at 26 ± 2 ºC, 75 ± 10% RH, and 12:12 LD. Field collected adults and those emerging from pupae in the laboratory were kept in well-ventilated cages (30 × 30 × 30 cm) and provided a cotton swab dipped in a sucrose-solution (100 g/l), also containing vitamin solution (20 ml/l) and methyl 4-hydroxybenzoate (2 g/l) for adult feeding 3,8,12. The homogenous lots of insects were used for bioassays with EPNs and insecticides.
Insecticide formulations
Ten insecticides with novel modes of action were selected for evaluation along with the selected EPN species against S. litura. All of the selected insecticides are registered for use against Spodoptera spp. in Pakistan30. Further details about the recommended dose rates, active ingredients, mode of action, target pests, and suppliers/distributors in Pakistan are provided in Table 1. The test concentrations of each formulated insecticide were prepared in distilled water and were used in all bioassay tests1,3,8,31.
Toxicity of insecticides to S. litura
Preliminary bioassays were performed to estimate the range of insecticides’ lethal concentrations killing 5–95% of 3rd instar larvae of S. litura. Groups of 10 larvae were used for each insecticide treatment, with insecticide solutions applied using a leaf dip method1,3,8. A single leaf disc (5 cm diameter) dipped in each insecticide concentration for 10 s and dried on a paper towel, was placed on moist filter papers to avoid desiccation in Petri plates. Each insecticide concentration was repeated five times and leaves were treated separately at different time with new insecticide solution of same concentration prepared on each occasion. The leaves dipped in distilled water alone served as the untreated check in all replicates. All treated and untreated larvae were kept in an insect growth chamber at 26 ± 2 ºC, 75 ± 10% RH, and 12:12 LD. Mortality was determined 96 h after treatment applications. Larvae were considered dead when no movement of appendages was seen upon touching with a dissecting needle.
Toxicity of insecticides to EPNs
Toxicity of the field recommended dose rates of the selected insecticides was studied against the two species of EPNs to estimate their compatibility with the test chemicals. A series of conventional mortality bioassay tests were performed to determine the toxic effects of the ten insecticides against EPNs under laboratory conditions of 26 ± 2 ºC, 75 ± 10% RH, and 12:12 LD. In a sterilized glass tube, 1 ml insecticide test solution was added along with 10 µl Ringer’s solution containing 100 entomopathogenic infective juveniles (IJs) of the selected species. The treatment solutions were thoroughly mixed by gently tapping the tubes. All treatments were repeated five times and a distilled water treatment was applied as a control. The replications were performed with new insecticide solutions of same concentrations but prepared at a different time under same set of conditions. Non-responding EPNs upon touching with a probe or dissecting needle were considered dead from the treatment7,12,21,25,26. Mortality of the IJs was determined after 48, 72, and 96 h. The insecticides resulting in less than 25% mortality were denoted as harmless or least toxic32.
Toxicity of insecticides and EPN mixtures to S. litura
The toxicity of ten selected insecticides and the EPN mixtures was evaluated in S. litura using previously reported methodology7,12,26. Plastic containers (150 ml) were provided with wet sand (10%, w/w) and five grams of insect diet. To the sand and diet in each container was further applied a solution containing the median lethal concentration (LC50) of each insecticide (Table 2) and 500 IJs (in 50 μl Ringer’s solution) of each EPN sp., separately. A treatment containing the 500 IJs of each EPN sp., alone served as a negative control for Chi-square calculations. Ten larvae were maintained in each treated container, with five replicates maintained under laboratory conditions at 26 ± 2 ºC, 75 ± 10% RH, and 12:12 LD. Mortality was assessed after 72 and 96 h of larval release into the containers.
Statistical analysis
A completely randomized design (CRD) was used for bioassays with insecticides and EPNs. In the case of bioassays with insecticides and S. litura, mortality scores were corrected using Abbott’s formula33. The corrected mortality values were further subjected to Probit analysis and the median lethal concentrations (LC50) were calculated using Polo Plus software Version 1.0 (LeOra Software LLC). The lethal concentrations of various insecticide treatments were reported with significant differences when 95% confidence intervals (CIs) were non-overlapping34.
Data for the toxicity of the test chemical insecticides against the selected EPNs were analyzed statistically through Minitab 18 Software and the means were compared for significance using Tukey's HSD test (P < 0.05).
The mean mortality scores obtained in the combined treatments of insecticides and EPNs were also subjected to ANOVA and means were compared for significance as stated above. However, comparisons of the expected versus observed mortality of S. litura was performed through a binomial test and the interaction between insecticides and EPN mixtures was described as additive, antagonistic or synergistic 7,12,20. Expected larval mortality in each treatment was estimated as:
where, PE symbolizes mortality (expected) in mixture treatments of EPNs and insecticides, PO denotes mortality of S. litura in the control treatment, P1 symbolises the mortality of S. litura due to insecticide alone and P2 represents the mortality (observed) in the treatment with EPNs alone.
A chi-square (χ2) value was calculated by using the formula
where Lo displays the observed numbers of live larvae in the treatment, Le denotes the expected number of live larvae in the treatment, Do is the number of observed dead larvae and De is the number of expected dead larvae in the treatment.
We tested the hypothesis of treatment interactions by using a 3.84 value of Chi-square (df = n − 1; and P = 0.05). Synergy was assigned to the insecticide and EPN treatments when observed mortality (Po) was higher than the expected mortality (Pe) and the χ2 was higher than 3.84. The interaction was considered additive when the χ2 value < 3.84 and antagonism was defined as treatment mixtures where observed mortality (Po) was less than expected mortality (Pe) and the χ2 was higher than 3.847,12,20.
Ethical statement
As no human or mammalian subjects were involved in this research, hence, no ethics approvals were required for this study. However, we adapted all the standard bioassay procedures while conducting the experiments.
Results
Toxicity of insecticides to S. litura
The selected insecticides were tested for their toxicity against the S. litura larvae to obtain their relevant median lethal concentrations (LC50). These median lethal values were further used in treatment mixtures with the EPN spp. The results of Probit analyses are displayed in Table 2. The insecticide emamectin benzoate was found to be highly toxic (LC50 = 2.97 mg/l) against 3rd instar S. litura larvae. The highest median lethal concentration (LC50 = 29.56 mg/l) was determined for the insecticide novaluron which was followed by methoxyfenozide (LC50 = 21.06 mg/l), whereas the toxicity scores of chlorfenapyr (LC50 = 17.16 mg/l), spinosad (LC50 = 14.78 mg/l) and indoxacarb (LC50 = 10.92 mg/l) did not differ significantly from each other because of overlapping confidence intervals. The insecticides chlorantraniliprole, flubendiamide and lufenuron also proved very toxic against 3rd instar S. litura larvae and exhibited lower LC50 values (5.50, 7.41 and 7.85 mg/l, respectively).
Toxicity of insecticides to EPNs
A series of conventional mortality bioassay tests were performed to determine the toxic effects of the field recommended dose rates of ten insecticides against EPNs and the results are displayed in Table 3. We found significant variation among all the tested insecticides against both EPN species (S. carpocapsae and H. indica) after different exposure times (P < 0.05). All the tested insecticides proved to be harmless against the two tested EPN species (S. carpocapsae and H. indica) based on less than 20% mortality, even after 96 h of treatment exposure, and less than 10% mortality in both EPN spp., after 72 h. The highest mortality (16.27 ± 0.9) of H. indica was recorded after 96 h with chlorfenapyr, followed by spinosad (14.89 ± 1.1), flubendiamide (13.37 ± 1.0), spinetoram (13.22 ± 1.0) and indoxacarb (13.21 ± 1.0). Almost the same insecticides viz., chlorfenapyr, spinosad, indoxacarb, spinetoram and flubendiamide proved to be harmless against S. carpocapsae, causing mortality ranging from 11.00 to 14.33% after 96 h of treatment.
Toxicity of insecticides and EPN mixtures to S. litura
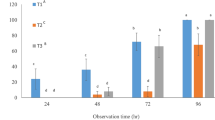
Toxicity of selected insecticides and EPN mixtures were recorded after 72 and 96 h of treatment application. In our study, insecticides proved to be an important factor in S. litura mortality, along with the EPNs. The results revealed significant variations in the toxicity levels of all the treatments and exhibited considerable differences in the mortality of S. litura larvae in all insecticide mixtures with S. carpocapsae (F = 86.15, P < 0.001, df = 11, R-sq. = 95.21%) and H. indica (F = 73.67, P < 0.001, df = 11, R-sq = 91.79%) after 72 h of treatment application (Table 4).
No synergistic, nor antagonistic interactions, were observed between the insecticides and EPNs (S. carpocapsae and H. indica). The highest mortality (74.00 ± 1.99%) was observed with an additive interaction (Chi-sq. = 2.03) when S. carpocapsae was applied along with indoxacarb. The insecticides indoxacarb, flubendiamide, and spinetoram produced the greatest mortalities (72–76%) of S. litura larvae, when applied in mixtures with H. indica after 72 h. The interaction between these insecticides and the EPN sp., (H. indica) was found to be additive (Χ2 < 3.84). Lowest mortality (44.00 ± 3.74% and 48.00 ± 2.89) was observed in mixture of H. indica with methoxyfenozide and chlorfenapyr, respectively. Similarly, the same insecticides exhibited the lowest mortalities (38.00 ± 3.99 and 44.00 ± 3.74%) when applied in mixed form with S. carpocapsae. No interaction was determined for either EPN (S. carpocapsae and H. indica) and methoxyfenozide mixtures, because mortality in the positive control (EPNs only) and the mixture were the same. However, an additive interaction was found in all other insecticide mixtures with S. carpocapsae and H. indica because of a smaller chi-square value than 3.84 (Table 4). Mixtures of emamectin benzoate, chlorantraniliprole, lufenuron and spinosad with H. indica also proved effective against S. litura larvae and exhibited an additive interaction between the EPN and insecticides (62–66% mortality and Chi-sq < 3.84).
The results obtained after 96 h also revealed significant variations in toxicity levels of the treatments and exhibited considerable differences in the mortality of S. litura larvae in all insecticide mixtures with S. carpocapsae (F = 102.76, P < 0.001, df = 11, R-sq = 95.95%) and H. indica (F = 93.43, P < 0.001, df = 11, R-sq = 93.91%) after 72 h (Table 5).
All the insecticide and EPN mixtures exhibited additive interactions indicating that both insecticides and EPNs contributed significantly to S. litura larval mortality (Table 5). However, we did not observe any synergistic or antagonistic interactions between insecticides and EPNs even after 96 h of treatment. The positive controls with both EPN spp., produced more than 50% mortality after 96 h (S. carpocapsae = 51.00 ± 1.91% and H. indica = 62.00 ± 2.58%). In the case of insecticide mixtures with S. carpocapsae, indoxacarb produced 90% mortality of S. litura larvae, whereas, indoxacarb, flubendiamide, emamectin benzoate, and spinetoram produced 90—92% mortality when applied in mixtures with H. indica. The two insecticides viz., methoxyfenozide and chlorfenapyr produced minimum mortalities (64–68%) with EPN mixtures even after 96 h.
Discussion
Insecticides are most widely used by farmers to control several key pests of agricultural significance. Many insect pests including armyworms, Spodoptera spp., have been reported to develop resistance against the insecticides used in pest management programs1,2,8. Combining certain efficient techniques like biological control agents, especially EPNs, and insecticides can help address the challenges in management of certain pest insects in agriculture7,12,13,21,25,35,36.
As mentioned earlier, S. litura has developed resistance to many of the chemical insecticides used for its management in many countries. In the present study, emamectin benzoate was found to be highly toxic to 3rd instar S. litura larvae, whereas, novaluron and methoxyfenozide were the least toxic amongst the tested insecticides. We could not find any report of resistance development in Spodoptera spp. against novaluron; however, it has been reported to methoxyfenozide, a benzylhydrazide growth regulator (IGR) through leaf-dip bioassays1. Chlorfenapyr, spinosad, and indoxacarb were moderately toxic against 3rd instar larvae compared to the other tested insecticides.
Chlorantraniliprole, flubendiamide, and lufenuron also proved very toxic against 3rd instar S. litura larvae. Low to very low resistance was reported for Pakistani strains of the armyworm against chlorantraniliprole, flubendiamide, spinetoram, and spinosad2. Since the registration of lufenuron in Pakistan in 199630, it gained popularity for successful control of the armyworm, however, moderate to high lufenuron resistance was reported in both S. litura and S. exigua in Pakistan2,5.
Before evaluating the efficacy of insecticide and EPN mixtures against S. litura, the toxic effects of the selected insecticides were first estimated against the two EPN spp., S. carpocapsae, and H. indica to appraise their compatibility with the tested insecticides. Our results indicate that all insecticides were relatively harmless or least toxic to the nematodes with mortalities less than 20% after 96 h of exposure. The insecticides caused less than 10% mortalities in both EPNs after 72 h of exposures, which encourages the integrated use of insecticides and EPNs as tank mixes are not usually prepared more than 24 h before spraying.
Studies have shown that delayed exposures of certain insecticides at higher concentrations can cause variable mortality in both EPN species (S. carpocapsae and H. indica)12, however, the insecticides remained harmless even after longer treatment exposition. Low EPN mortality was reported with certain other insecticides including chlorpyrifos37, whereas some conventional organophosphates including chlorphenvinphos and dichlorvos and their mixtures were reported to cause high mortality of Heterorhabditis amazonensis. Conversely, deltamethrin, and chlorpyrifos-ethyl did not reduce the survival of that EPN species26,38. S. carpocapsae was also reported to be more susceptible to deltamethrin compared to H. bacteriophora and H. indica25. Similarly, some commercial formulations of fipronil, malathion, cypermethrin, imidacloprid, chlorantraniliprol, and azadirachtin were also reported to cause no harm to survival or infectivity of entomopathogenic nematodes35.
In nematodes, the involvement of butyrylcholinesterase as a front line of defense was proposed because it may protect acetylcholinesterase from an early attack by its inhibitors39. Results of our study and some other reports suggest that several EPNs are compatible with many insecticides and hence can be used in combinations or mixtures in pest management programs11,12,13,21,38.
Synergistic, additive, and antagonistic interactions have been reported when some conventional and novel chemical insecticides were tested in combination with S. carpocapsae and H. indica after 48 h of treatment exposures. However, with increased exposure time to 96 h, the interactions of EPNs and insecticides turned antagonistic25. The increased exposure period to the EPNs alone and in combination with insecticides have proven to increase the mortality of insect larvae11,12,38. Similarly, an increase in rates of H. indica and S. glaseri, resulted in higher mortalities in S. litura under lab conditions, whereas H. indica demonstrated greater efficacy in greenhouse-pot-culture conditions14. Increased rates of H. indica and S. carpocapsae also produced greater mortality of S. litura larvae after 48 and 96 h of exposure12,24.
Combinations of H. bacteriophora with chlorantraniliprole and imidacloprid demonstrated synergistic or additive interactions in second-instar larvae of Holotrichia oblita and produced faster mortality than the EPNs or insecticide alone. Combination of S. carpocapsae with chlorantraniliprole or spinetoram has been suggested as a least toxic control strategy against the fall armyworm, S. frugiperda15.
It can be inferred from our results that all insecticides proved toxic alone and when applied in mixtures with EPNs against S. litura larvae. We suggest that more EPNs should be investigated for their compatibility with insecticides against certain insect pests of agricultural economic significance.
Conclusions
Several reports describe the development of insecticide resistance in the armyworm S. litura against many insecticides included in pest management programs in many countries. Growers often apply huge volumes of insecticide to control resistant strains of pests without knowing their risks to the environment and to public health. Efforts are being made to investigate alternative methods to be used either alone or in combination with chemical insecticides for mitigation of this notorious pest. Entomopathogenic nematodes are among useful options for inclusion in IPM programs, used alone or in combination with chemical insecticides. The experiments conducted in the present study on compatibility of two EPN spp. with reduced doses of some novel reduced-risk chemical insecticides show significant potential for armyworm management. EPN mixtures with reduced volumes of insecticides will also help in lowering the risks of environmental pollution and to public health.
Data availability
Statistically nalysed data are presented in the manuscript. Observations recorded during the experiments are acquiesced to the Department of Entomology, University of Agriculture, Faisalabad, Pakistan and hence, the consent of authorities is required for obtaining the data.
References
Shad, S. A. et al. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). J. Pest. Sci. 85, 153–162 (2012).
Ahmad, M. & Gull, S. Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. Can. Entomol. 149, 649–661 (2017).
Ahmad, M., Farid, A. & Saeed, M. Resistance to new insecticides and their synergism in Spodoptera exigua (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 107, 79–86 (2018).
Sisay, B., Tefera, T., Wakgari, M., Ayalew, G. & Mendesil, E. The efficacy of selected synthetic insecticides and botanicals against fall armyworm, Spodoptera frugiperda, in maize. Insects 10, 45, https://doi.org/10.3390/insects10020045 (2019).
Ishtiaq, M. et al. Stability, cross-resistance and fitness costs of resistance to emamectin benzoate in a re-selected field population of the beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae). Crop Prot. 65, 227–231 (2014).
Khan, R. R., Al-Ghafri, T. H. A., Al-Khatri, S. A. H., Al-Mazidi, I. S. S. & Al-Rawahi, F. G. Resistance to deltamethrin and fenitrothion in dubas bug, Ommatissus lybicus de Bergevin (Homoptera: Tropiduchidae) and possible biochemical mechanisms. Sci. Rep. 10, 13220. https://doi.org/10.1038/s41598-020-70150-7 (2020).
Negrisoli, A. S., Garcia, M. S., Barbosa Negrisoli, C. R. C., Bernardi, D. & da Silva, A. Efficacy of entomopathogenic nematodes (Nematoda: Rhabditida) and insecticide mixtures to control Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae) in corn crops. Crop Prot. 29, 677–683 (2010).
Saeed, Q., Saleem, M. A. & Ahmad, M. Toxicity of some commonly used synthetic insecticides against Spodoptera exigua (Fab) (Lepidoptera: Noctuidae). Pak. J. Zool. 44, 1197–1201 (2012).
Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 8, 235. https://doi.org/10.3390/agronomy8110235 (2018).
Antwi, F. B. & Reddy, G. V. P. Efficacy of entomopathogenic nematodes and sprayable polymer gel against crucifer flea beetle (Coleoptera: Chrysomelidae) on canola. J. Econ. Entomol. 109, 1706–1712 (2016).
Yadav, S., Patil, J. & Sharma, H. K. Bio-efficacy of Steinernema carpocapsae against Spodoptera litura under laboratory condition. J. Pure Appl. Biosci. 5, 165–172 (2017).
Khan, R. R. et al. Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) and the biocide, spinosad for mitigation of the armyworm, Spodoptera litura (F.) (Lepidoptera: Noctuidae). Egypt J. Biol. Pest Control 28, 1–6 (2018).
Khan, B. et al. Potential of entomopathogenic nematode (Steinernema kraussei) against last instar larvae of different lepidopteran insect pests. Pak. J. Zool. 52, 1275–1281 (2020).
Radhakrishnan, S. & Shanmugam, S. Bioefficacy of entomopathogenic nematodes against Spodoptera litura (Lepidoptera: Noctuidae) in Bhendi. Int. J. Curr. Microbiol. Appl. Sci. 6, 2314–2319 (2017).
Viteri, D. M., Linares, A. M. & Flores, M. Use of the entomopathogenic nematode Steinernema carpocapsae in combination with low-toxicity insecticides to control fall armyworm (Lepidoptera: Noctuidae) larvae. Fl. Entomol. 101, 327–329 (2018).
Kamaliya, R. P., Jethva, D. M., Kachhadiya, N. M., Bhut, J. B. & Ahir, V. R. Bio-efficacy of entomopathogenic nematode Heterorhabditis indica against Spodoptera litura (Fabricius). J. Pharmocogn. Phytochem. 8, 1563–1567 (2019).
Somvanshi, V. S., Ganguly, S. & Paul, A. V. N. Field efficacy of the entomopathogenic nematode Steinernema thermophilum Ganguly and Singh (Rhabditida: Steinernematidae) against diamondback moth (Plutella xylostella L.) infesting cabbage. Biol. Control 37, 9–15 (2006).
Sunanda, B. S., Jeyakumar, P. & Jacob, V. V. Bioefficacy of different formulations of entomopathogenic nematode Steinernema carpocapsae against diamondback moth (Plutella xylostella) infesting cabbage (Brassica oleracea var. capitata). J. Biopest. 7, 210–215 (2014).
Garcia-del-Pino, F., Alabern, X. & Morton, A. Efficacy of soil treatments of entomopathogenic nematodes against the larvae, pupae and adults of Tuta absoluta and their interaction with the insecticides used against this insect. Biocontrol 58, 723–731 (2013).
Amizadeh, M., Hejazi, M. J., Niknam, G. & Askari-Saryazdi, G. Interaction between the entomopathogenic nematode, Steinernema feltiae and selected chemical insecticides for management of the tomato leaf miner, Tuta absoluta. Biocontrol 64, 709–721 (2019).
Sabino, P. H. S. et al. Combined application of entomopathogenic nematodes and insecticides in the control of leaf-miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on tomato. Neotrop. Entomol. 48, 314–322 (2019).
Koppenhöfer, A. M. & Fuzy, E. M. Early timing and new combinations to increase the efficacy of neonicotinoid–entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae). Pest Manag. Sci. 64, 725–735 (2008).
Liesch, P. J. & Williamson, R. C. Evaluation of chemical controls and entomopathogenic nematodes for control of Phyllophaga white grubs in a Fraser fir production field. J. Econ. Entomol. 103, 1979–1987 (2010).
Guo, W., Yan, X., Zhao, G. & Han, R. Increased efficacy of entomopathogenic nematode–insecticide combinations against Holotrichia oblita (Coleoptera: Scarabaeidae). J. Econ. Entomol. 110, 41–51 (2017).
Negrisoli, A. S., Garcia, M. S. & Barbosa Negrisoli, C. R. C. Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) with registered insecticides for Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae) under laboratory conditions. Crop Prot. 29, 545–549 (2010).
Can Ulu, T., Sadic, B. & Susurluk, I. A. Effects of different pesticides on virulence and mortality of some entomopathogenic nematodes. Invertebr. Surviv. J. 13, 111–115 (2016).
White, G. F. A method for obtaining infective nematode larvae from cultures. Science 66, 302–303 (1927).
Khan, R. R., Ashfaq, M., Ahmed, S. & Sahi, S. T. Mortality responses in Bracon hebetor (Say) (Braconidae: Hymenoptera) against some new chemistry and conventional insecticides under laboratory conditions. Pak. J. Agric. Sci. 46, 30–33 (2009).
Ashfaq, M., Khan, R. R. & Farooq, M. A. Refinement of rearing technique of a potent larval parasitoid Bracon hebetor (Say), (Braconidae: Hymenoptera). Proc. Pak. Acad. Sci. 48, 83–88 (2011).
Ali, M.A. A handbook for agriculture extension agents on the pesticides registered with recommendations for safe handling and use in Pakistan. ISBN 9251920842. http://www.parc.gov.pk/files/parc_pk/2018/General%20Files/Pesticide-Book--8-04-18.pdf (2018).
Khan, R. R., Ahmed, S. & Nisar, S. Mortality responses of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae) against some conventional and new chemistry insecticides under laboratory conditions. Pak. Entomol. 33, 147–150 (2011).
de Morais, M. R., Zanardi, O. Z., Rugno, G. R. & Yamamoto, P. T. Impact of five insecticides used to control citrus pests on the parasitoid Ageniaspis citricola Longvinovskaya (Hymenoptera: Encyrtidae). Ecotoxicology 25, 1011–1020 (2016).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267 (1925).
Khan, R. R. et al. Susceptibility survey of Ommatissus lybicus (de Bergevin) populations against deltamethrin and fenitrothion in Oman. Sci. Rep. 9, 11690. https://doi.org/10.1038/s41598-019-48244-8 (2019).
Yan, X., Moens, M., Han, R., Chen, S. & De Clercq, P. Effects of selected insecticides on osmotically treated entomopathogenic nematodes. J. Plant Dis. Prot. 119, 152–158 (2012).
Yan, X. et al. Efficacy of entomopathogenic nematodes against the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 113, 64–72 (2020).
Gutiérrez, C., Campos-Herrera, R. & Jiménez, J. Comparative study of the effect of selected agrochemical products on Steinernema feltiae (Rhabditida: Steinernematidae). Biocontrol Sci. Technol. 18, 101–108 (2008).
Monteiro, C. M. O. et al. Compatibilidade de Heterorhabditis amazonensis (Rhabditida: Heterorhabditidae) isolado RSC-5 com diferentes carrapaticidas utilizados no controle de Rhipicephalus microplus (Acari: Ixodidae). Arq. Inst. Biol. (Sao Paulo) 81, 03–08 (2014).
Selkirk, M. E., Henson, S. M., Russell, W.S. & Hussein, A. S. Acetylcholinesterase secretion by nematodes. in Parasitic Nematodes—Molecular Biology, Biochemistry and Immunology (ed. Kennedy, H.) 211–228 (CABI Publishing, 2001).
Acknowledgements
The authors sincerely thank Dr. Murray B. Isman, University of British Columbia, for reviewing and improving the manuscript. Authors are also thankful to the Department of Entomology, University of Agriculture, Faisalabad, Pakistan for providing the laboratory as well as other logistic facilities.
Author information
Authors and Affiliations
Contributions
R.R.K. and M.A. conceived and designed research. A.A. carried out field surveys and collected insects. M.A. and A.A. conducted experiments. R.R.K. analyzed the data. R.R.K. and M.A. wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, R.R., Arshad, M., Aslam, A. et al. Additive interactions of some reduced-risk biocides and two entomopathogenic nematodes suggest implications for integrated control of Spodoptera litura (Lepidoptera: Noctuidae). Sci Rep 11, 1268 (2021). https://doi.org/10.1038/s41598-020-79725-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79725-w
- Springer Nature Limited