Abstract
In this study, we evaluated the effects of autologous serum collected after two types of exercise on the in vitro inflammatory profile and T cell phenotype of resting peripheral blood mononuclear cells (PBMCs) in obese men. Serum samples and PBMCs were obtained from eight obese men who performed two exercise bouts—high intensity interval exercise (HIIE) and exhaustive exercise session to voluntary fatigue—in a randomized cross-over trial. Pre-exercise PBMCs were incubated with 50% autologous serum (collected before and after each exercise bout) for 4 h. In vitro experiments revealed that post-HIIE serum reduced the histone H4 acetylation status and NF-κB content of PBMCs and suppressed the production of both TNF-α and IL-6 by PBMCs, while increasing IL-10 production. Post-exhaustive exercise serum induced histone H4 hyperacetylation and mitochondrial depolarization in lymphocytes and increased TNF-α production. In vitro post-HIIE serum incubation resulted in an increase in the frequencies of CD4 + CTLA-4 + and CD4 + CD25+ T cells expressing CD39 and CD73. Post-exhaustive exercise serum decreased the frequency of CD4 + CD25 + CD73+ T cells but increased CD4 + CD25-CD39 + T cell frequency. Both post-exercise serums increased the proportions of CD4 + PD-1 + and CD8 + PD-1+ T cells. Blood serum factors released during exercise altered the immune response and T cell phenotype. The type of exercise impacted the immunomodulatory activity of the post-exercise serum on PBMCs.
Similar content being viewed by others
Introduction
Acute endurance exercise elicits a transient increase in the number of peripheral blood mononuclear cells (PBMCs), including T cells1. Typically, 70–80% of the mononuclear cells in blood are lymphocytes, which are the main players in the coordination of adaptive immune response2. An augmentation in the number of PBMCs occurs immediately after exercise, mainly due to the increased peripheral frequencies of CD4 + and CD8+ T cells and natural killer (NK) cells1,3. The T cell phenotype is widely influenced by exercise, with a preferential mobilization of memory effector T cells and immunoregulatory cells (i.e., regulatory T cells, Treg cells)4,5. In this sense, exercise induces an increase in the peripheral frequency of senescent T cells (CD4 + and CD8 + cells that are CD28-), effector memory CD4 + CD25-CD39+ T cells, and CD4 + CD25 + CD39 + memory Treg (mTreg) cells6,7. Furthermore, cytokine production by PBMCs (mainly by monocytes, lymphocytes, and NKs) changes in response to exercise8,9.
In recent years, participation of the purinergic system in the regulation of lymphocyte phenotype and function has been observed. The CD4 + CD25+ T cells metabolize adenosine triphosphate (ATP) into adenosine to induce immunomodulatory responses, mainly through Treg cells10,11. Ectonucleoside triphosphate diphosphohydrolase‐1 (ENTPD1, CD39) is an ectoenzyme, expressed in several cells including leukocytes, that hydrolyzes ATP and adenosine diphosphate (ADP) to adenosine monophosphate (AMP). Subsequently, ecto‐5′‐nucleotidase (CD73) converts AMP to adenosine, which is a well‐known immunosuppressive metabolite12,13. Furthermore, CD4+ T cells expressing the surface marker cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have strong inhibitory effects on other leukocytes through cell-to-cell interactions14. Moreover, both CD4 + and CD8+ T cells expressing the programmed cell death protein 1 (PD-1) downregulate the immune response and promote self-tolerance by suppressing T cell function15. In this regard, the increased expression of ectonucleotidases, CTLA-4, and PD-1 is associated with an immunoregulatory, suppressive, and anti-inflammatory profile16.
Modifications in the cell phenotype are also linked to their functional activity. Post-translational histone acetylation-deacetylation events play a pivotal role in chromatin accessibility regulation, promotion of nuclear factor kappa B (NF-κB) recruitment, and increased pro-inflammatory cytokine gene transcription17. Acetylation of histone H4 (H4ac) is especially important for the chromatin structure, gene expression, cell polarization, and cytokine production by myeloid cells18,19. Hence, the activation of NF-κB and production of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-8 are widely dependent on the H4ac status17. Moreover, chromatin remodeling through histone acetylation impacts the immunoregulatory molecules expressed on the cell surface of lymphocytes, such as CTLA-4, CD39, and CD73 in CD4+ T cells, and histone acetyltransferase overexpression increases PD-1 expression in lymphoid cells20,21.
The acute systemic physiological response to exercise involves a wide range of metabolic, biochemical, immunological, and hormonal changes, wherein some mediators released into peripheral blood can have endocrine, autocrine, and paracrine effects in different cells and tissues, including immune sites3. One potential mechanism is that exercise alters systemic molecules (i.e. cytokines, extracellular vesicles, and metabolic factors) and proteins in the peripheral blood that influence the cytokine production by circulating mononuclear cells and the leukocyte phenotype22,23. It was demonstrated that acute exercise induces several systemic proteins, including apolipoproteins and immunological mediators, that may be involved in immunosurveillance during and after exercise bouts24. However, some exercise modalities, such as exhaustive exercise that induces higher degrees of muscle damage and systemic stress response, may induce a greater pro-inflammatory response and activation of peripheral leukocytes that become deregulated and detrimental to health9,25.
Different modes of exercise impact the T cell phenotype during and after exercise. Acute high-intensity exercise mobilizes Treg cells and T cells with activated and senescent phenotypes, with subsequent redistribution of these cells to inflamed exercised-tissues (i.e. muscle tissue)5,6,26. Furthermore, acute high-intensity, but not moderate-intensity exercise, induces a switch in intracellular transcription factor expression in T cells, favoring a type 2 inflammatory response mediated by the GATA3-IL-4 axis27,28. In contrast, exhaustive exercise increases intracellular NF-κB activation in peripheral lymphocytes from healthy men29. However, how the systemic factors released as a result of exercise (i.e. cytokines, hormones, and metabolic molecules) modulate the epigenetic events, inflammatory status, and phenotype of human circulating T cells and influence of the type and intensity of the bout in these immunoregulatory mechanisms, remains to be elucidated.
Conceptually, it is believed that exercise may mediate its effects on immune function by regulating systemic mediators released during each bout30. Chronic exercise training consists of frequent repetitions of acute bouts of exercise over time followed by recovery periods. In this regard, acute exercise and long-term exercise training represent two distinct situations with different physiological and immune alterations. The systemic physiological stress of acute exercise alters the blood composition and phenotype of immune cells by adjustments in cardiovascular parameters, neuroendocrine response, and metabolic dynamics31. In contrast, the effects of exercise training, i.e., improved fitness level, reduced adiposity, and enhanced muscle mass, can lead to the lowering of the circulating metabolic and inflammatory parameters that are recognized to alter PBMC phenotype and function32. While the adaptations to chronic exercise training and the physiological response to acute exercise have plausible mechanisms to improve immune function, so far, only a few studies have assessed the potential changes in blood serum composition induced by acute exercise concerning the immunological parameters22,23,33,34. Therefore, it is believed that intermediate metabolites released during exercise, such as lactate, may regulate the immune function during and after each bout. Incubation of PBMCs with lactate at concentrations similar to those occurring in circulation after moderate exercise (3.5 mM) reduced lipopolysaccharide (LPS)-induced inflammatory cytokine production35.
In fact, the blood composition directly affects the phenotype and the function of several cell lines36,37,38,39. Serum from humans under calorie restricted diets showed increased heat- and redox-induced stress resistance and upregulated genes related to longevity (i.e. Sirt1)38,39. Using Jurkat cells, an immortalized human T-lymphocyte cell line, Radom-Aizik et al.34 reported that the incubation of 10% of the serum from healthy men obtained 30 min after high-intensity exercise (80% of the individual peak work rate) resulted in the suppression of IL-2 and TNF-α production by those cells. As such, our study evaluated the impact of autologous serum collected after two types of exercise on cytokine production, NF-κB activity, epigenetic markers (H3ac and H4ac status), redox state of PBMCs, and changes in the phenotype of circulating CD4 + and CD8+ T cells of obese men. We hypothesized that a) the incubation of resting PBMCs from obese individuals with autologous serum collected after exercise results in changes in the T cell phenotype and cytokine production, epigenetic markers, and the NF-κB content and b) an immunoregulatory profile of PBMCs would be induced by autologous serum collected after high-intensity interval exercise (HIIE).
Results
Experimental study design
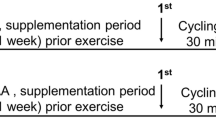
We performed a two-phase study to address the study aim. One week after the preliminary trial, participants engaged in the first study phase: two isocaloric exercise trials with a one-week interval between. The HIIE session consisted of a warm‐up (5 min) at a workload eliciting 40% maximum velocity (MAV) followed by 10 bouts of 60 s (85–90% MAV) intercepted by 75 s of active recovery (50% MAV) on a motorized treadmill. The exhaustive exercise consisted of stepping up and down from a step. The stepping rhythm was paced acoustically at 60 beats per min with the same periods (1 s) for stepping up and down, respectively. When the participants were not able to maintain the required stepping rhythm, a 30 s recovery period was given. Blood samples were collected before and immediately after each exercise session to analyze blood biochemical markers, including systemic cytokines, hormones, and oxidative stress variables, and the peripheral frequency of the T cell phenotype.
In phase 2 of the study, PBMCs acquired pre-exercise (“resting PBMCs”) from each participant were incubated with autologous serum (50%) collected before and after exercise bouts (HIIE and exhaustive) for 4 h in culture. Analyses of the cytokine production in the supernatant, T cell phenotype, histone H3/H4 acetylation status, NF-κB content, and redox state in PBMCs were conducted after the incubation time (Fig. 1).
Physiological response to exercise and systemic serum biomarkers
Both protocols were well tolerated, and all participants completed all the bouts. There were no differences in energy expenditure between HIIE and exhaustive exercise (282 ± 14.2 kcal vs. 269 ± 20.01 kcal; p > 0.05). However, significant differences between groups were observed in relation to exercise duration (HIIE, 1800 ± 128 s; exhaustive exercise, 1232 ± 281 s; p < 0.001) and the average heart rate (HIIE, 148.18 ± 7.12 bpm; exhaustive exercise, 165.19 ± 5.01 bpm; p = 0.01).
Exercise significantly increased several serum biomarkers immediately after bouts (Table 1). Acute HIIE increased the systemic levels of IL-6 (p = 0.03; effect sizes (ES) = 1.13), IL-10 (p = 0.03; ES = 1.81), IGF-1 (p = 0.02; ES = 2.5), cortisol (p = 0.02; ES = 1.28), lactate (p < 0.001; ES = 4.01), uric acid (p = 0.04; ES = 1.59), and thiobarbituric acid reactive substances (TBARS) (p = 0.04; ES = 2.53) in the serum of the participants. A single bout of exhaustive exercise increased the serum concentrations of IL-6 (p = 0.03; ES = 2.18), TNF-α (p = 0.04; ES = 1.46), IL-17a (p = 0.04; ES = 0.74), IGF-1 (p = 0.01; ES = 2.29), cortisol (p = 0.02; ES = 1.69), lactate (p < 0.01; ES = 3.98), uric acid (p = 0.04; ES = 0.40), and TBARS (p < 0.01; ES = 4.24). Interestingly, TBARS levels were higher after exhaustive exercise compared to HIIE (p = 0.03; ES = 4.47). The serum leptin concentrations decreased immediately after HIIE (p = 0.03; ES = 0.94), without changes after exhaustive exercise (p > 0.05).
Acute T cell mobilization in response to exercise bouts
Lymphocytosis was induced immediately after HIIE, characterized by increased peripheral frequencies of CD4 + and CD8+ T cells (p = 0.03, ES = 0.54; p < 0.001, ES = 0.88, respectively) (Table 2). Exhaustive exercise mobilized higher proportions of CD8+ T cells (p < 0.001; ES = 2.01), without difference in CD4 + T cell frequency (p > 0.05). The peripheral frequencies of CD4+ T cells expressing ectonucleotidases, CTLA-4, and PD-1 differentially changed in response to exercise protocols. Acute HIIE increased the peripheral frequency of CD4 + CD25-CD39 + (p = 0.03; ES = 0.79), CD4 + CD25 + CD39 + (p = 0.04; ES = 0.88), CD4 + CD25 + CD73 + (p = 0.04; ES = 0.36), CD4 + CTLA-4 + (p = 0.02; ES = 0.32), CD4 + PD-1 + (p = 0.03; ES = 0.78), and CD8 + PD-1 + (p = 0.03; ES = 0.70) without differences in the frequencies of CD4 + CD25-CD73 + and CD4 + CD25+ T cells co-expressing CD39 + CD73 + (p > 0.05). Conversely, acute exhaustive exercise increased the peripheral proportions of CD4 + CD25-CD39 + (p = 0.03; ES = 1.86), CD4 + PD-1 + (p = 0.03; ES = 0.39), and CD8 + PD-1 + (p = 0.04; ES = 1.0), but decreased the frequencies of CD4 + CD25-CD73 + (p = 0.02; ES = 1.68), CD4 + CD25 + CD73 + (p = 0.04; ES = 0.69), and CD4 + CTLA-4 (p = 0.04; ES = 0.38) (Table 2).
Autologous serum collected after exercise bouts changes the redox state and inflammatory profile of PBMCs
Both post-exercise serums collected post-HIIE and post-exhaustive exercise protocols changed the PBMC redox state of resting obese individuals. The in vitro treatment of PBMCs from obese individuals with serum collected after HIIE or exhaustive exercise-induced significant lipid peroxidation (p = 0.01, ES = 1.97 for interval exercise; p = 0.01, ES = 3.91 for exhaustive exercise; Fig. 2A) and reactive oxygen species (ROS) production (p = 0.03, ES = 2.06 for HIIE; p = 0.02, ES = 5.03 for exhaustive exercise; Fig. 2B) without changes in total thiol content of treated cells (p > 0.05; Fig. 2C). The serum from post-exhaustive exercised men induced higher PBMC ROS production compared to serum from post-HIIE exercised men (p = 0.04; ES = 1.93; Fig. 2B). Furthermore, mitochondrial depolarization was higher in cells incubated with serum acquired from post-exhaustive exercised men (p = 0.04; ES = 2.0; Fig. 2D).
The redox state of mononuclear cells incubated with autologous serum from before (white bars) and after (black bars) two types of exercise. Lipid peroxidation (A), ROS production (B), total thiols content (C), and mitochondrial membrane depolarization (D) of PBMC incubated with serum collected before and immediately after high-intensity interval exercise (HIIE) or exhaustive exercise. (E) is a representative histogram of mitochondrial membrane potential of PBMC incubated with serum collected before and after each exercise protocol. Statistical analysis conducted by two-way repeated measurements ANOVA with Bonferroni’s post hoc (p < 0.05).
Incubation of PBMCs of resting obese individuals with serum collected post-HIIE decreased the levels of global H4ac (p = 0.03; ES = 1.18, Fig. 3B), while treatment with serum from the exhaustive exercise session increased H4ac (p = 0.025; ES = 3.83). However, no differences were identified in H3ac after incubation with post-exercise serum of HIIE or exhaustive exercise bouts (p > 0.05; Fig. 3A). In addition, the post-HIIE serum decreased the NF-κB p65 total content in nuclear fractions of PBMCs of obese men (p = 0.03; ES = 1.73), while there were no changes in post-exhaustive exercise serum treatment (p > 0.05) (Fig. 3C). Incubation of PBMCs with post-HIIE serum decreased IL-6 (p = 0.02, ES = 1.35; Fig. 3D) and TNF-α (p = 0.04, ES = 0.71; Fig. 3F) secretion. In contrast, incubation of PBMCs with serum collected post-exhaustive exercise increased TNF-α production (p = 0.03, ES = 1.62; Fig. 3F). Moreover, higher IL-10 production was found in PBMCs stimulated with post-HIIE serum (p = 0.02; ES = 2.40; Fig. 3E), with no differences in post-exhaustive exercise serum incubation (p > 0.05).
Epigenetic markers, NF-κB content and cytokine production in peripheral blood mononuclear cells incubated in vitro with autologous serum from before (white bars) and after (black bars) two types of exercise. Intracellular content of global H3ac (2A), H4ac (2B), Nuclear factor kappa B (2C) in PBMC, and the production of IL-6 (2D), IL-10 (2E) and TNF-α (2F) by PBMC incubated with serum collected before and immediately after high-intensity interval exercise (HIIE) or exhaustive exercise. Statistical analysis conducted by two-way repeated measurements ANOVA with Bonferroni’s post hoc (p < 0.05).
Autologous serum collected after exercise bouts modulate the frequency of CD4+ T cells expressing CD39, CD73, CTLA-4, and PD-1
As PBMCs are composed mainly of lymphocytes (approximately 85%), and T cells are more responsive than B cells to exercise, we analyzed the effects of autologous serum from before and after the two types of exercise on CD4 + and CD8 + T cell expressing regulatory molecules (Fig. 4). Autologous post-HIIE serum increased the frequencies of CD4 + CD25 + CD39 + (p = 0.02, ES = 1.40; Fig. 4C), CD4 + CD25 + CD73 + (p = 0.03, ES = 0.51; Fig. 4D), and CD4 + CD25 + CD39 + CD73+ T cells (p = 0.03, ES = 0.54; Fig. 4E) compared to the incubation model with pre-HIIE serum. Conversely, incubation of lymphocytes with serum acquired post-exhaustive exercise increased the frequency of CD4 + CD25-CD39 + (p = 0.02, ES = 0.86; Fig. 4A), but decreased the percentage of CD4 + CD25-CD73 + (p = 0.02, ES = 0.73; Fig. 4B). CD4 + CD25-CD39+ T cells were higher in post-exhaustive exercise serum incubation compared to post-HIIE serum incubation (p = 0.04, ES = 0.61; Fig. 4A). In addition, the frequencies of CD4 + CD25 + CD39 + (p = 0.03, ES = 1.24; Fig. 4C) and CD4 + CD25 + CD39 + CD73+ T cells (p = 0.04, ES = 0.15; Fig. 4E) were higher in PBMCs incubated with post-HIIE serum in relation to the post-exhaustive exercise serum incubation model.
CD4+ T cells expressing CD39 and CD73 ectonucleotidases following in vitro incubation with autologous serum from before (white bars) and after (black bars) two types of exercise. The frequencies of CD4 + CD25-CD39 + (3A), CD4 + CD25-CD73 + (3B), CD4 + CD25 + CD39 + (3C), CD4 + CD25 + CD73 + (3D), CD4 + CD25 + CD39 + CD73 + (3E) T cells were analyzed by flow cytometry after in vitro PBMC stimulation with serum collected before and immediately after high-intensity interval exercise (HIIE) or exhaustive exercise. Statistical analysis conducted by two-way repeated measurements ANOVA with Bonferroni’s post hoc (p < 0.05).
Autologous serum collected after exercise bouts modulates the frequency of CD4 + CTLA-4 + and CD8 + PD-1+ T cells
We also evaluated the effect of in vitro incubation of PBMCs with post-exercise serum on CD4 + and CD8+ T cells expressing the inhibitory molecules CTLA-4 (in CD4+ T cells) and PD-1 (in CD4 + and CD8+ T cells). Lymphocytes incubated with autologous serum collected post-HIIE showed an increased frequency of CD4 + CTLA-4 + (p = 0.04, ES = 1.64; Fig. 5A) compared to the incubation model with serum collected pre-HIIE. The frequency of CD8 + PD-1+ T cells was higher in both post-exercise serum incubation models (HIIE: p = 0.04, ES = 0.89; exhaustive exercise: p = 0.04, ES = 0.77; Fig. 5C). No statistical difference was found in CD4 + PD-1+ T cells in both groups (Fig. 5B; p > 0.05).
CD4 + and CD8+ T cells expressing PD-1 or CTLA-4 following in vitro incubation with autologous serum from before (white bars) and after (black bars) two types of exercise. The frequencies of CD4 + CTLA-4 + (4A), CD4 + PD-1 + (4B) and CD8 + PD-1 + (4C) T cells were analyzed by flow cytometry after in vitro PBMC stimulation with serum collected before and immediately after high-intensity interval exercise (HIIE) or exhaustive exercise. Statistical analysis conducted by two-way repeated measurements ANOVA with Bonferroni’s post hoc (p < 0.05).
Discussion
This report provides the first evidence that systemic factors released into the peripheral blood during different types of exercise bouts differentially change several parameters related to the immune function in the resting PBMCs of obese men. We observed that the inflammatory response and lymphocyte phenotypical markers in PBMCs incubated with post-exercise serum were dependent on the exercise type: (a) post-HIIE serum incubation increased the frequencies of CD4 + CD25 + CD39 +, CD4 + CD25 + CD73 +, CD4 + CD25 + CD39 + CD73 +, CD4 + CTLA-4 +, and CD8 + PD-1+ T cells and the production of IL-10 concomitant to the decreased NF-κB content, H4ac levels, TNF-α, and IL-6 production in PBMCs and (b) higher TNF-α production and H4ac levels, increased frequencies of CD4 + CD25-CD39 + and CD8 + PD-1+ T cells, and lower proportions of CD4 + CD25-CD73+ T cells occurred after the incubation of PBMCs with autologous serum collected after exhaustive exercise. Higher lipid peroxidation and ROS production were observed in PBMCs incubated with serum collected after both HIIE and exhaustive exercise. Moreover, the incubation of PBMCs with serum collected after the exhaustive bout led to significant mitochondrial membrane depolarization. Thus, the changes in the PBMC parameters were dependent on the type of the exercise bout, since opposite effects were observed on the H4ac levels, inflammatory response, and T cell phenotype in serum obtained after HIIE and exhaustive exercise.
First, we evaluated some systemic biochemical and immunological parameters in the same obese men who were subjected to an acute HIIE session and an acute exhaustive exercise session. The systemic and physiological responses to exercise are complex and not well understood but involve a wide range of metabolic, immunological, and neuroendocrine changes24. Here, we analyzed serum biomarkers that are commonly modulated by acute exercise, including cytokines (IL-6, IL-10, IL-17a, IFN-γ, and TNF-α), adipokines (leptin), blood hormones (cortisol and IGF-1), metabolic molecules (glucose and lactate), and oxidative stress markers (TBARS and uric acid). Changes in these above-mentioned parameters are dependent on the type, volume, and intensity of the bout. A similar increase in the systemic levels of IL-6, IGF-1, cortisol, lactate, uric acid, and TBARS were observed after both HIIE and exhaustive exercise. However, only HIIE induced a significant increase in IL-10 plasma levels and decrease in leptin concentrations. Exhaustive exercise enhanced the systemic TNF-α and IL-17a serum concentrations. These results denote that while both exercise protocols lead to systemic metabolic perturbations, the cytokine response is dependent on the type and mode of the exercise protocol. In fact, we and others demonstrated the role of HIIE in the increase of systemic IL-10 and other immunoregulatory cytokines, such as IL-1Ra and TGF-β, in a variety of subjects40,41,42,43. In addition, de Souza et al. also identified lower levels of leptin after interval exercise, which may indicate an anti-inflammatory effect of exercise by a decrease in the release of adipokine from adipose tissue41. Conversely, the strong pro-inflammatory response may be linked to the higher muscle damage degree observed in exhaustive exercise44. Suzuki and coworkers45 found increased concentrations of systemic anti-inflammatory (IL-1ra and IL-10) and pro-inflammatory (IL-8) cytokines, associated with rises in hormones (cortisol and GH) and markers of muscle damage in the serum of athletes after a marathon race. The increases in the concentrations of immunoregulatory cytokines after strenuous exercise may be an attempt to counter the uncontrolled inflammatory response induced by continuous hormone stimulation and increased muscle damage after the bout.
It is well known that the systemic physiological perturbations, such as microbial translocation, muscle damage, sympathetic discharge, immunometabolism, and increased myokine expression, can directly alter the leukocyte phenotype and function during and after exercise4,22,23,24,46. Previous studies examined the immunomodulatory effects of serum obtained after endurance exercise on PBMCs22,23. PBMCs treated with 50% autologous post-exercise serum for 18 h increased HLA-DR expression in total monocytes and CD14 + CD16 + pro-inflammatory monocytes23. Moreover, PBMCs from healthy men incubated with post-exercise serum obtained after 1 h of exercise increased NK cytotoxic activity, which inversely correlated with changes in serum cortisol levels after the bout22.
In this sense, we believed that systemic factors released into peripheral blood during exercise may directly influence immune function and the T cell phenotype of resting PBMCs. Our study hypotheses were partially confirmed. Here, we observed that acute HIIE induces significant mobilization of CD4 + CD25-CD39 +, CD4 + CD25 + CD39 +, CD4 + CD25 + CD73 +, CD4 + CTLA-4 +, CD4 + PD-1 + and CD8 + PD-1+ T cells into peripheral blood. Interestingly, most of these T cell subsets changed in our in vitro PBMC incubation with post-exercise autologous serum. In this regard, in vitro PBMC incubation with post-HIIE autologous serum increased the frequencies of CD4+ T cells expressing CD39/CD73 ectonucleotidases, CTLA-4, and PD-1, which may represent the immunoregulatory effects of HIIE in obese individuals. In fact, all these aforementioned molecules are now recognized as immune checkpoints that hamper the inflammatory response47,48. The CD39/CD73 axis metabolizes ATP to adenosine, and downregulates NF-κB expression in immune cells13. In addition, CTLA-4 exerts cell-to-cell immunosuppressive actions by interacting with CD80/CD86 of antigen-presenting cells (APCs), thus inhibiting the induction of the adaptive immune response by lowering CD28 expression14,49. Regarding the ectonucleotidases, we previously showed that HIIE increases the frequencies of CD4 + CD25 + CD39 + CD73+ T cells, a memory Treg (mTreg) phenotype, in sedentary and highly active men6.
Indeed, PD-1 is a key tolerogenic T cell molecule that is highly expressed on memory T cells and involved in modulating cell activation, differentiation, and migration to inflamed tissues by dampening T cell receptor (TCR) signaling and decreasing T cell functions, such as cytokine production, T cell proliferation, and cell migration15,50. Gustafsson et al.51 recently reported changes in peripheral CD4 + and CD8+ T cells expressing PD-1 after 45 min of maximal cycling exercise. Furthermore, HIIE seems to be superior in increasing the peripheral proportions of central memory and effector memory CD8+ T cells expressing PD-1 than moderate continuous exercise. The increased PD-1 expression on these cells after HIIE may be involved in the maintenance of homeostasis, decrease in the activation, differentiation, and redistribution of less differentiated memory phenotypes, such as central memory T cells, in a physiological condition52. Thus, HIIE mobilizes T cells expressing checkpoints that may be linked to the anti-inflammatory effects of exercise. Our data highlight the effect of exercise-induced blood factors on the upregulation of immune checkpoint molecules that can have an impact on the inflammatory profile observed in obese individuals.
Although exercise modifies a milieu of molecules in the blood that alter immune function, we identified a significant decrease in systemic leptin levels concomitant with higher lactate levels after HIIE. Leptin activates T cells and reduces the Treg phenotype as well as CTLA-4 expression by stimulating downstream pathways, phosphatidylinositol-3 kinase (PI3K), and protein kinase A (PKA)53,54. Moreover, the synthesis and expression of PD-1 by memory CD4 + and CD8+ T cells are stimulated by higher glycolytic activity and lactate consumption by T cells55. Hence, metabolic changes in T cells may be induced by blood factors released during exercise that together may favor a homeostatic status. Circulating lactate concentrations increase during exercise, and exhaustive exercise activities can rise to 100-fold on active muscles, with a concomitant increase of more than tenfold in blood serum56. Moreover, PBMC incubation with lactate at moderate-intensity concentrations (3.5 mM) blunted the LPS-induced pro-inflammatory cytokine production35. However, exercise induces the secretion of a wide range of metabolic products that could affect the immune function, and in vitro PBMC incubation with post-exercise serum does not allow for analyzing the individual effect of each molecule found in the serum on leukocyte function and phenotype.
The in vitro incubation of PBMCs with autologous serum obtained after exhaustive exercise increased the frequencies of CD4 + CD25-CD39 + memory effector T cells (mTeff) and CD8 + PD-1+ T cells, and decreased the frequency of CD4 + CD25-CD73+ T cells. Conversely, the peripheral blood frequencies of CD4 + CD25-CD39 +, CD4 + PD-1 +, and CD8 + PD-1+ T cells increased after exhaustive exercise, concomitant with a decrease in the proportions of CD4 + CD25-CD73 +, CD4 + CD25 + CD73 +, and CD4 + CTLA-4+ T cells. Therefore, changes in blood factor composition after exhaustive exercise may not explain all T cell phenotype changes observed after the bout.
Interestingly, CD39 expression is a marker of T cell activation, and CD4 + CD25-CD39 + mTeff cells produce higher levels of IL-17a and IFN-γ57. The CD4 + CD25-CD39 + T cell phenotype presents signs of metabolic stress (i.e. alterations in redox state) and high susceptibility to apoptosis58, and these cells were previously related to lower immune tolerance59. It was previously reported that exhaustive exercise leads to higher CD4 + Th17 phenotype and lowered Treg counts in the peripheral blood of highly trained men60. Furthermore, in vitro incubation of resting PBMCs with post-exhaustive exercise serum in a study by Perry et al.60 led to decrease in CD4 + CD25 + FoxP3 + Treg frequency. Thus, the higher systemic stress induced by exhaustive exercise may induce a strong inflammatory response through the induction of CD25-CD39 + phenotype and lower CTLA-4 expression in CD4+ T cells. Moreover, the decreased CD4 + CD25-CD73 + T cell proportions in PBMCs incubated with post-exhaustive exercise autologous serum may be linked with a hampered ability to metabolize ATP by CD4+ T cells.
Here, we shed light on the exercise-induced epigenetic changes in immune cells mediated by systemic factors released into the peripheral blood. The observations that a differential response of global H4ac in PBMCs stimulated with post-exercise serum from HIIE or exhaustive exercise are in line with previous studies that demonstrate that exercise changes epigenetic machinery in immune cells9,61. Our data revealed that the incubation of post-HIIE serum reduced the H4ac status and NF-κB content in the nucleus of PBMCs. In fact, data from our group demonstrated that an acute bout of HIIE increased the intracellular global histone deacetylase (HDAC) activity in PBMCs, which is responsible for removing the acetyl group from the N-terminal tail of the lysine site of histones, in association with higher plasma IL-10 and TGF-β concentrations62. The decreased NF-κB content in the nucleus of PBMCs identified after the incubation with post-HIIE serum may be related to the downregulation of Toll-like receptors (TLR) on the cell surface of lymphocytes and monocytes63. Furthermore, acute high-intensity exercise releases pro-resolving lipid mediators such as resolvins and maresins into peripheral blood that contribute to the resolution of inflammation through receptor-mediated actions on leukocytes64. Exposure of naïve bone marrow-derived macrophages to plasma isolated from exercised mice promotes the resolution of acute inflammation by stimulating macrophage phagocytosis and lowering inflammatory cytokine expression through enhanced pro-resolving pathways65. Incubation of Jurkat cells with 10% of post-high-intensity exercise (80% of maximal work rate) serum decreased TNF-α production34. Collectively, these results may indicate that serum factors released during HIIE induce a condensed state of chromatin and lower NF-κB activity to inflammatory gene transcription in peripheral leukocytes.
The increases in IL-10 production by PBMCs stimulated with post-HIIE serum are in line with the immunoregulatory phenotype associated with the higher proportions of CD4+ T cells expressing CD39/CD73 and CTLA-4 induced by post-exercise serum factors. IL-10 blunts IκB kinase and NF-κB DNA binding activity, suppressing the pro-inflammatory gene expression66. In contrast, serum collected post-exhaustive exercise increased TNF-α production and the H4ac status of PBMCs. In fact, the higher muscle damage, systemic endotoxin levels, and ROS generation were associated with increased TLR expression on monocyte cell surface and NF-κB phosphorylation after exhaustive exercise67. In a rat model study, Goutianos et al36 showed that intravenous administration of plasma collected from exhaustive exercise trained rats increased inflammatory cytokine expression (IL-2, IL-6, IL-8, TNF-α, and C-reactive protein) measured in plasma samples, skeletal muscle, and adipose tissue of non-exercised rats. We previously demonstrated that exhaustive exercise-induced a state of histone H4 hyperacetylation status and increased TNF-α and IL-8 production by LPS-stimulated PBMCs in obese individuals9. Thus, exhaustive exercise induces the release of both danger and pathogen associated molecular patterns to the peripheral blood that are recognized by TLRs and other pattern recognition receptors that increase the intracellular inflammatory signaling cascade in mononuclear cells.
Here, we found similar changes in redox states in PBMCs after exposure to serum collected after HIIE or exhaustive exercise, as observed by higher ROS production and TBARS content in the cell lysate. Higher levels of lipid peroxidation observed in PBMCs after exercise are related to increased metabolic process and mitochondrial respiration of these cells during the bout68,69,70,71. In contrast, the incubation of zymosan-stimulated phagocytes with post-marathon race plasma leads to decreased ROS production33. This contradictory finding may be related to the some experimental conditions in the study of Katsuhiko et al33, such as the antigenic stimulation of myeloid cells (monocytes and granulocytes) and the highly trained study population. In fact, it was suggested that biochemical adaptations in the athletic population induce higher antioxidant protection, which can quickly control acute ROS elevations after exhausting exercise72. On the other hand, obese individuals present low antioxidant enzymes in both serum and PBMC, and maximal exercise leads to a higher imbalance in the redox state in lymphocytes of obeses73,74. Therefore, in our study it is possible that ROS elevation in PBMC incubated with post-exercise serum may be related to lower systemic antioxidant mediators in the serum of obese individuals after the bouts. The thiobarbituric acid (TBA) reactivity test is a reliable estimator for identifying several end products formed through the decomposition of lipid peroxidation products75. However, a variety of other lipid compounds, such as oxidized lipids and saturated/unsaturated aldehydes, interfere in the assay causing overestimation of malondialdehyde (MDA) concentration76. In this respect, we recognize that TBARS analysis is one of the main limitations of our study for its low sensitivity and selectivity since many MDA-unrelated molecules can react with TBA, and some artifactual generation of MDA during the assay has been raised75.
In addition, post-exhaustive exercise serum incubation leads to significant increase in mitochondrial membrane depolarization of lymphocytes. Mitochondrial membrane depolarization leads to cytochrome c leakage and precedes the activation of caspases that induces degradation of several proteins, and is involved in events of cell death, such as DNA fragmentation and apoptosis77,78,79. Hence, the increased TNF-α, mitochondrial membrane depolarization, and senescent T cell phenotype induced by autologous post-exhaustive exercise serum stimulation may be associated with the induction of cell death by systemic mediators released during the bout into peripheral circulation.
This study demonstrated that systemic factors released in response to exercise modify the PBMC H4ac status, NF-κB levels in the nucleus of PBMCs, cytokine production and CD4 + and CD8+ T cells expressing ectonucleotidases, CTLA-4, and PD-1. Although the precise signaling systemic molecules involved in post-translational histone acetylation remain to be elucidated, our data suggest that a differential response to HIIE and exhaustive exercise may alter the immune and epigenetic status of PBMCs. Our study hypothesis was partially confirmed, and the effects of the two exercise types on the immune parameters analyzed in the peripheral blood, and after the in vitro incubation of resting PBMCs with autologous serum collected before and after exercise bouts are qualitatively summarized in Table 3. Collectively, the data suggests an influence of systemic factors released during exercise in the immunomodulatory effects promoted by exercise.
Methods
Ethical statements and participants
This study was approved by the Research Ethics Committee of the Universidade Federal de Ciências da Saúde de Porto Alegre (protocol 1.973.432) and all experimental procedures were performed according to the Declaration of Helsinki. All study subjects were informed about the study and signed the informed consent form. Eight sedentary normoglycemic obese men (age 28.42 ± 4.19 years; height 1.75 ± 0.03 m; body mass 99.8 ± 3.5 kg; body mass index 32.81 ± 2.19 kg/m2; waist circumference 105.12 ± 8.14 cm; body fat 32.2 ± 3.9%; fasting glucose 88.29 ± 9.21 mg/dL; maximal oxygen consumption 40.12 ± 4.28 mL/kg/min) participated in this study. Exclusion criteria included autoimmune, cardiac, endocrine or metabolic diseases, acute and chronic infections, and under current pharmacological drugs known to affect the metabolic (i.e., hypoglycemic drugs, statins, and other lipid-lowering drugs) or immune system (i.e., steroidal and non-steroidal anti-inflammatory drugs and immunomodulatory medications), or dietary supplements with recognized impact on the immune system.
Preliminary trial
One week before the experimental trial, all participants went to the Human Movement Laboratory of UFCSPA (Porto Alegre/Brazil) to estimate their body composition and cardiorespiratory fitness. Body mass (kg) and height (meters) were determined by a semi-analytical scale with a stadiometer attached (Welmy, Santa Barbara D’Oeste, Brazil). Waist circumferences (WC, cm) were measured through an inelastic measuring tape. Body fat percent was measured as previously reported9. The cardiopulmonary exercise testing was performed to determine the intensity of exercise sessions using a ramp protocol80. The exercise test started at 4.5 km/h, with no slope for all study participants. Then, the load (speed and slope) was individually increased for each participant considering their physical condition and tolerance to obtain maximal oxygen consumption (VO2Max) within 8–12 min. The test was terminated after verification of the following circumstances: (a) request of the participant due to extreme tiredness and/or perception of the intense sensation of dyspnea; (b) attained the maximum heart rate (HR) predicted by age (HRmax) ≥ 85%; (c) peak respiratory exchange ratio RER > 1.1; (d) VO2 plateau was reached even with increasing workload. Ventilatory and metabolic parameters were collected by respiration using Metalyzer 3B (Cortex, Leipzig, Germany), and were analyzed after the mean of the data in eight respiratory cycles. The VO2 values were analyzed with averages of the last 10 s of each stage and the VO2Max was assumed as the stabilization of the highest average value attained during the test, while the highest sustained velocity during the entire stage was assumed to be MAV.
Main trials and blood collection
All subjects were subjected to both protocols (HIIE and exhaustive exercise) with an interval of at least one week between sessions. Both trials were conducted in the Human Movement Laboratory of UFCSPA (Porto Alegre/Brazil). Blood samples (15 mL) were collected before and immediately after each exercise session (pre and post each bout) into tubes without anticoagulant, and with ethylenediaminetetraacetic acid (EDTA). Tubes without anticoagulant were centrifuged (2000 g, 10 min) and the serum was aliquoted and frozen (-80ºC) for cell culture experiments. The HIIE session consisted of a warm‐up (5 min) at a workload eliciting 40% HRmax. The HIIE consisted of 10 bouts of 60 s (85–90% MAV) alternated with 75 s of recovery (50% MAV) on a motorized treadmill40. The exhaustive exercise consisted of stepping up and down from a step adjusted to the height of each subject’s femoral condyles9,81. The stepping rhythm was paced acoustically at 60 beats per min with the same periods (1 s) for stepping up and down, respectively. When the participants were not able to maintain the required stepping rhythm, a 30 s recovery period was given.
During exercise, gas analysis data were monitored online using Metalyzer 3B (Cortex, Leipzig, Germany), and HR by telemetry. We equalized the exercise sessions by the energy expenditure (EE) of HIIE. Thus, the EE of exhaustive exercise should be equal to those achieved during HIIE. The EE during exercise was calculated based on the metabolic equivalent method82. The EE was calculated at the end of every minute in both sessions, and exhaustive exercise stopped once the target EE had been attained.
Biochemical analysis of the peripheral blood
Biochemical analysis was conducted in the serum from the participants collected before and after each exercise bout. Systemic leptin (Peprotech Inc, USA) and insulin growth factor-1 (IGF-1) (BOSTER, USA) levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Cortisol concentrations were measured using an Immulite immunoassay system (Siemens, USA). Blood glucose, lactate, and uric acid levels were evaluated through automated biochemical assay using Bioclin BS120 (BioClin, Brazil).
PBMC isolation and stimulation
The PBMCs of each participant was isolated before and after each exercise bout. Briefly, PBMCs were isolated from peripheral blood from participants using histopaque gradient solution, Histopaque 1077 (Sigma‐Aldrich, St Louis, MO, USA) as previously described9. PBMC viability was determined by trypan blue exclusion and the viability was always more than 95%. Then, the cells were washed and suspended in Roswell Park Memorial Institute‐1640 medium (Sigma‐Aldrich, USA) supplemented with 2 g/L sodium bicarbonate, 2% glutamine, and 100 U/mL penicillin – 0.1 mg/mL streptomycin (Sigma‐Aldrich, USA).
To determine the contribution of exercise-induced systemic mediators on lymphocyte phenotype and inflammatory profile, PBMCs from each participant acquired pre-exercise condition were incubated with 50% of their own serum collected before and immediately after each exercise protocol. This concentration of serum approximates to the proportion of serum in human blood and has been used in other works22,23. Cells (1 × 106/mL) were incubated in RPMI-1640 with 50% of serum collected before and after HIIE and exhaustive exercise bout for 4 h (5% CO2, 37 °C) in 24-well plates in a final volume of 1 mL. Then, the culture supernatants were collected, centrifuged (400 g, 25 °C, 10 min), and kept at − 80 °C for cytokine quantification. PBMCs were collected, washed with phosphate-saline buffer 1x, and frozen at − 80 °C in a solution containing 90% fetal bovine serum and 10% dimethyl sulfoxide (DMSO) for epigenetic analysis. For T cell phenotyping, PBMCs were collected, immediately stained with antibody-fluorochrome, and analyzed with flow cytometry.
Histone H3 and H4 acetylation status and NF-κB activity in PBMCs
Histone extracts from PBMCs were prepared using a total histone extraction kit (EpiGentek, USA) according to the manufacturer’s protocol. H4ac and H3ac levels in PBMCs were determined using the Global Histone H4 and Global Histone H3 Acetylation Assay Kits (Colorimetric Detection, EpiQuik, USA) according to the manufacturer’s instructions and expressed as ng/mg protein. The nuclear fraction of the isolated PBMCs was extracted using a commercially available nuclear extraction kit (Epiquik Nuclear Extraction Kit, USA) according to the manufacturer’s protocol. The levels of total NFκB p65 in the nuclear fraction, which had been isolated from lysed cells by centrifugation, were measured using an ELISA commercial kit from Invitrogen (NFkB p65Total Human InstantOne ELISA Kit, ThermoFisher, USA). The nuclear protein concentration was determined using a Bradford assay83, and the activated NF-kB p65 level was expressed as arbitrary units per mg of protein (AU/mg of nuclear protein).
Assessment of cytokine levels and oxidative stress markers
The concentrations of TNF-α, IL-6, IL-10, IL-17a and interferon-gamma (IFN-γ) (all from eBioscience, USA) in the supernatants of serum-stimulated cells and serum from participants were quantified by ELISA kits, according to the manufacturer’s protocols. Lipid peroxidation was measured through the TBARS in PBMCs following a previously described protocol69 and expressed in MDA nmol/mL. Total thiol concentrations were measured in PBMCs using 5–5′-dithio-bis(2-nitrobenzoic acid) reagent9. ROS production was evaluation in the cell lysate through the fluorescence intensity of the redox-sensitive dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFH, 100 µM, Sigma-Aldrich) (excitation and emission wavelengths of 480 and 535 nm, respectively) using SpectraMax M2e (Molecular Devices, USA).
Lymphocyte cell stanning and flow cytometry evaluation
The phenotype of freshly isolated PBMCs and PBMCs cultured in vitro with autologous serum collected before and after exercise were evaluated by flow cytometry. Briefly, 2 × 105 PBMCs were stained with monoclonal antibodies (all anti-human) conjugated with specific fluorochromes: CD4 FITC, CD8 PerCP, CD25 Pe, CD39 PerCP-7, CD73 APC (all from Ebioscience, USA); CD125 (CTLA-4) Pe, CD279 (PD-1) Pe (all from Biogems, USA). Cell phenotype was acquired using CELLQuest Pro Software (BD Bioscience, USA) on a FACSCalibur flow cytometer (BD Bioscience, USA). A minimal of 30,000 events/tubes were acquired, and lymphocyte were identified and gated according to each forward scatter (FSC) and side scatter (SSC) profile. The frequencies of CD4 + CD25-CD39 +, CD4 + CD25-CD73 +, CD4 + CD25 + CD39 +, CD4 + CD25 + CD73 +, CD4 + CD25 + CD39 + CD73 +, CD4 + CTLA-4 +, CD4 + PD-1 +, and CD8 + PD-1+ T cells were evaluated. Single color control tubes and negative control were used in each assay to account for spectral overlap.
The mitochondrial membrane potential (ΔΨm) was quantified according to a method previously described84, using the fluorescent dye rhodamine 123 (Rh 123, Sigma-Aldrich). The analysis was performed using a BD FACSCalibur (Becton–Dickinson, Rutherford, NJ, USA) flow cytometer and CellQuest Pro software (Joseph Trotter, Scripps Research Institute, La Jolla, CA, USA), using the blue argon-ion 488 nm laser with the FL1filter channel. A total of 30,000 events were acquired in the region that corresponded to the lymphocytes.
Statistical analysis
Data were analyzed using SPSS 20.0 (SPSS Inc, IBM, Chicago, IL, USA). After the normality test by Shapiro‐Wilk, all values were presented as mean ± standard deviation (SD). Two‐way repeated measurement analysis of variance (ANOVA), taking time and exercise as factors, were performed followed by Bonferroni’s post hoc for multiple comparisons. p ≤ 0.05 was adopted in all analysis. In addition, Cohen’s d ES were calculated using G*Power 3.1.9 for significant results.
Data availability
The data will be available when requested.
References
Simpson, R. J., Kunz, H., Agha, N. & Graff, R. Exercise and the Regulation of Immune Functions. Progress in Molecular Biology and Translational Science Vol. 135 (Elsevier Inc., Amstredam, 2015).
Kleiveland, C. & Kleiveland, C. Peripheral blood mononuclear cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models (2015). https://doi.org/10.1007/978-3-319-16104-4_15.
Campbell, J. P. & Turner, J. E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. https://doi.org/10.3389/fimmu.2018.00648 (2018).
Graff, R. M. et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. https://doi.org/10.1016/j.bbi.2018.08.017 (2018).
Krueger, K. et al. Apoptosis of T-Cell subsets after acute high-intensity interval exercise. Med. Sci. Sports Exerc. 48(10), 2021–2029 (2016).
Dorneles, G. P., da Silva, I. M., Peres, A. & Romão, P. R. T. Physical fitness modulates the expression of CD39 and CD73 on CD4+ CD25− and CD4+ CD25+ T cells following high intensity interval exercise. J. Cell. Biochem. https://doi.org/10.1002/jcb.28364 (2019).
Simpson, R. J. et al. Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exerc. Immunol. Rev. (2010).
Antunes, B. M. et al. Anti-inflammatory response to acute exercise is related with intensity and physical fitness. J. Cell. Biochem. https://doi.org/10.1002/jcb.27810 (2018).
Dorneles, G. P. et al. Acute strenuous exercise induces an imbalance on histone H4 acetylation/histone deacetylase 2 and increases the proinflammatory profile of PBMC of obese individuals. Oxid. Med. Cell. Longev. 2017 (2017).
Mandapathil, M. et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25 high FOXP3+ regulatory T Cells. J. Biol. Chem. https://doi.org/10.1074/jbc.M109.047423 (2010).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. https://doi.org/10.1084/jem.20062512 (2007).
Linden, J. & Cekic, C. Regulation of lymphocyte function by adenosine. Arterioscler. Thromb. Vasc. Biol. https://doi.org/10.1161/ATVBAHA.111.226837 (2012).
Antonioli, L., Pacher, P., Vizi, E. S. & Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. https://doi.org/10.1016/j.molmed.2013.03.005 (2013).
Oderup, C., Cederbom, L., Makowska, A., Cilio, C. M. & Ivars, F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology https://doi.org/10.1111/j.1365-2567.2006.02362.x (2006).
Dermani, F. K., Samadi, P., Rahmani, G., Kohlan, A. K. & Najafi, R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J. Cell. Physiol. https://doi.org/10.1002/jcp.27172 (2019).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. Cancer Clin. Trials https://doi.org/10.1097/COC.0000000000000239 (2016).
Lee, J. Y., Kim, N. A., Sanford, A. & Sullivan, K. E. Histone acetylation and chromatin conformation are regulated separately at the TNF-alpha promoter in monocytes and macrophages. J. Leukoc. Biol. https://doi.org/10.1189/jlb.1202618 (2003).
Zhang, Z., Song, L., Maurer, K., Bagashev, A. & Sullivan, K. E. Monocyte polarization: the relationship of genome-wide changes in H4 acetylation with polarization. Genes Immun. https://doi.org/10.1038/gene.2011.17 (2011).
Schübeler, D. et al. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. https://doi.org/10.1101/gad.1198204 (2004).
Doñas, C. et al. Trichostatin a promotes the generation and suppressive functions of regulatory T cells. Clin. Dev. Immunol. https://doi.org/10.1155/2013/679804 (2013).
Bally, A. P. R., Austin, J. W. & Boss, J. M. Genetic and epigenetic regulation of PD-1 expression. J. Immunol. https://doi.org/10.4049/jimmunol.1502643 (2016).
Gupta, P., Bigley, A. B., Markofski, M., Laughlin, M. & LaVoy, E. C. Autologous serum collected 1 h post-exercise enhances natural killer cell cytotoxicity. Brain. Behav. Immun. https://doi.org/10.1016/j.bbi.2018.04.007 (2018).
Booth, S. et al. The impact of acute strenuous exercise on TLR2, TLR4 and HLA.DR expression on human blood monocytes induced by autologous serum. Eur. J. Appl. Physiol. 1, 2. https://doi.org/10.1007/s00421-010-1616-2 (2010).
Krumholz-Wagner, I. et al. Effects of acute endurance exercise on plasma protein profiles of endurance-trained and untrained individuals over time. Mediat. Inflamm. 2016, (2016).
Slusher, A. L., Zúñiga, T. M. & Acevedo, E. O. Maximal exercise alters the inflammatory phenotype and response of mononuclear cells. Med. Sci. Sports Exerc. https://doi.org/10.1249/MSS.0000000000001480 (2018).
Krüger, K., Lechtermann, A., Fobker, M., Völker, K. & Mooren, F. C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain. Behav. Immun. https://doi.org/10.1016/j.bbi.2007.08.008 (2008).
Ulven, S. M. et al. An acute bout of exercise modulate the inflammatory response in peripheral blood mononuclear cells in healthy young men. Arch. Physiol. Biochem. 121, 41–49 (2015).
Xiang, L., Rehm, K. E. & Marshall, G. D. Effects of strenuous exercise on Th1/Th2 gene expression from human peripheral blood mononuclear cells of marathon participants. Mol. Immunol. 60, 129–134 (2014).
Vider, J. et al. Physical exercise induces activation of NF-κB in human peripheral blood lymphocytes. Antioxidants Redox Signal. https://doi.org/10.1089/152308601317203639 (2001).
Krüger, K., Mooren, F.-C. & Pilat, C. The immunomodulatory effects of physical activity. Curr. Pharm. Des. https://doi.org/10.2174/1381612822666160322145107 (2016).
Walsh, N. P. et al. Position statement part one: Immune function and exercise. Exerc. Immunol. Rev. (2011).
Walsh, N. P. et al. Position statement part two: Maintaining immune health. Exerc. Immunol. Rev. (2011).
Suzuki, K. et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. https://doi.org/10.1249/01.MSS.0000048861.57899.04 (2003).
Radom-Aizik, S., Leu, S. Y., Cooper, D. M. & Zaldivar, F. Serum from exercising humans suppresses t-cell cytokine production. Cytokine https://doi.org/10.1016/j.cyto.2007.08.008 (2007).
Ratter, J. M. et al. In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front. Immunol. https://doi.org/10.3389/fimmu.2018.02564 (2018).
Goutianos, G. et al. Plasma from exercised rats administered to sedentary rats induces systemic and tissue inflammation. Physiol. Rep. https://doi.org/10.14814/phy2.13087 (2016).
Goutianos, G. et al. Chronic administration of plasma from exercised rats to sedentary rats does not induce redox and metabolic adaptations. J. Physiol. Sci. https://doi.org/10.1186/s12576-020-00737-2 (2020).
Allard, J. S. et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE 3, e3211 (2008).
Omodei, D., Licastro, D., Salvatore, F., Crosby, S. D. & Fontana, L. Serum from humans on long term calorie restriction enhances stress resistance in cell culture. Aging (Albany NY). 5, 599–606 (2013).
Dorneles, G. P. et al. High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight-obese individuals. Cytokine https://doi.org/10.1016/j.cyto.2015.10.003 (2016).
de Souza, D. C. et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front. Physiol. 9, 567 (2018).
Cabral-Santos, C. et al. Inflammatory cytokines and BDNF response to high-intensity intermittent exercise: effect the exercise volume. Front. Physiol. 7, 1–8 (2016).
Wadley, A. J., Chen, Y.-W., Lip, G. Y. H., Fisher, J. P. & Aldred, S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J. Sports Sci. https://doi.org/10.1080/02640414.2015.1035666 (2015).
Pedersen, B. K., Ostrowski, K., Rohde, T. & Bruunsgaard, H. The cytokine response to strenuous exercise. Can. J. Physiol. Pharmacol. https://doi.org/10.1139/y98-055 (1998).
Suzuki, K. et al. Circulating sytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. https://doi.org/10.1007/s004210050044 (2000).
Zwaag, J. et al. Involvement of lactate and pyruvate in the anti-inflammatory effects exerted by voluntary activation of the sympathetic nervous system. Metabolites https://doi.org/10.3390/metabo10040148 (2020).
Vigano, S. et al. Targeting adenosine in cancer immunotherapy to enhance T-Cell function. Front. Immunol. https://doi.org/10.3389/fimmu.2019.00925 (2019).
Collin, M. Immune checkpoint inhibitors: a patent review (2010–2015). Expert Opin. Ther. Pat. https://doi.org/10.1080/13543776.2016.1176150 (2016).
Ohta, A. et al. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 3, 1–12 (2012).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. https://doi.org/10.1146/annurev.immunol.26.021607.090331 (2008).
Gustafson, M. P. et al. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J. Immunother. Cancer https://doi.org/10.1186/s40425-017-0231-8 (2017).
Wadley, A. J. et al. High intensity interval exercise increases the frequency of peripheral PD-1+ CD8+ central memory T-cells and soluble PD-L1 in humans. Brain Behav. Immun. Health https://doi.org/10.1016/j.bbih.2020.100049 (2020).
Gerriets, V. A. et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. https://doi.org/10.1002/eji.201545861 (2016).
Orlova, E. G. & Shirshev, S. V. Role of PKA and PI3K in leptin and ghrelin regulation of adaptive subpopulations of regulatory CD4+ T-lymphocyte formation. Biochemistry https://doi.org/10.1134/S0006297917090103 (2017).
Renner, K. et al. Restricting glycolysis preserves T Cell effector functions and augments checkpoint therapy. Cell Rep. https://doi.org/10.1016/j.celrep.2019.08.068 (2019).
Goodwin, M. L., Harris, J. E., Hernández, A. & Gladden, L. B. Blood lactate measurements and analysis during exercise: a guide for clinicians. J. Diabetes Sci. Technol. https://doi.org/10.1177/193229680700100414 (2007).
Dwyer, K. M. et al. Expression of CD39 by human peripheral blood CD4+CD25+T cells denotes a regulatory memory phenotype. Am. J. Transpl. https://doi.org/10.1111/j.1600-6143.2010.03291.x (2010).
Fang, F. et al. Expression of CD39 on activated T cells impairs their survival in older individuals. Cell Rep. https://doi.org/10.1016/j.celrep.2016.01.002 (2016).
McRae, J. L., Chia, J. S., Pommey, S. A. & Dwyer, K. M. Evaluation of CD4 + CD25+/− CD39 + T-cell populations in peripheral blood of patients following kidney transplantation and during acute allograft rejection. Nephrology 22, 505–512 (2017).
Perry, C. et al. Endurance exercise diverts the balance between Th17 cells and regulatory T cells. PLoS ONE 8, 1–8 (2013).
Ferioli, M. et al. Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J. Cell. Physiol. https://doi.org/10.1002/jcp.28304 (2019).
Dorneles, G. P. et al. High intensity interval exercise enhances the global HDAC activity in PBMC and anti-inflammatory cytokines of overweight-obese subjects. Obes. Med. 2, (2016).
Durrer, C., Francois, M., Neudorf, H. & Little, J. P. Acute high-intensity interval exercise reduces human monocyte Toll-like receptor 2 expression in type 2 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R529–R538 (2017).
Markworth, J. F., Maddipati, K. R. & Cameron-Smith, D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. (2016).
Zheng, J.-J., Pena Calderin, E., Hill, B. G., Bhatnagar, A. & Hellmann, J. Exercise promotes resolution of acute inflammation by catecholamine-mediated stimulation of resolvin d1 biosynthesis. J. Immunol. https://doi.org/10.4049/jimmunol.1900144 (2019).
Schottelius, A. J. G., Mayo, M. W., Balfour Sartor, R. & Baldwin, A. S. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J. Biol. Chem. https://doi.org/10.1074/jbc.274.45.31868 (1999).
Cavalcante, P. A. M., Gregnani, M. F., Henrique, J. S., Ornellas, F. H. & Araújo, R. C. Aerobic but not resistance exercise can induce inflammatory pathways via toll-like 2 and 4: a systematic review. Sports Med. Open https://doi.org/10.1186/s40798-017-0111-2 (2017).
Moir, H. et al. AMPK inactivation in mononuclear cells: a potential intracellular mechanism for exercise-induced immunosuppression. Appl. Physiol. Nutr. Metab. 33, 75–85 (2008).
Tossige-Gomes, R. et al. Lymphocyte redox imbalance and reduced proliferation after a single session of high intensity interval exercise. PLoS ONE 11, 1–18 (2016).
Turner, J. E., Bosch, J. A., Drayson, M. T. & Aldred, S. Assessment of oxidative stress in lymphocytes with exercise. J. Appl. Physiol. https://doi.org/10.1152/japplphysiol.00051.2011 (2011).
Estruel-Amades, S., Camps-Bossacoma, M., Massot-Cladera, M., Pérez-Cano, F. J. & Castell, M. Alterations in the innate immune system due to exhausting exercise in intensively trained rats. Sci. Rep. 10, 1–12 (2020).
Powers, S. K. & Jackson, M. J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276 (2008).
Peres, A. et al. DNA damage in mononuclear cells following maximal exercise in sedentary and physically active lean and obese men. Eur. J. Sport Sci. https://doi.org/10.1080/17461391.2020.1801850 (2020).
Sindhu, S. et al. Increased expression of the innate immune receptor TLR10 in obesity and type-2 diabetes: association with ROS-mediated oxidative stress. Cell. Physiol. Biochem. 45, 572–590 (2018).
Dasgupta, A. & Klein, K. Methods for Measuring Oxidative Stress in the Laboratory. In: Antioxidants in Food, Vitamins and Supplements (2014). https://doi.org/10.1016/b978-0-12-405872-9.00002-1.
Catalá, A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. https://doi.org/10.1016/j.biocel.2006.02.010 (2006).
Suen, D. F., Norris, K. L. & Youle, R. J. Mitochondrial dynamics and apoptosis. Genes Dev. https://doi.org/10.1101/gad.1658508 (2008).
Brunelle, J. K. & Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. https://doi.org/10.1242/jcs.031682 (2009).
Ricci, J. E. et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell https://doi.org/10.1016/j.cell.2004.05.008 (2004).
Balady, G. J. et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122(2), 191–225. https://doi.org/10.1161/CIR.0b013e3181e52e69 (2010).
Saxton, J. M., Claxton, D., Winter, E. & Pockley, A. G. Peripheral blood leucocyte functional responses to acute eccentric exercise in humans are influenced by systemic stress, but not by exercise-induced muscle damage. Clin. Sci. 104, 69 (2003).
Ainsworth, B. E. et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. (2000).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Ferlini, C. & Scambia, G. Assay for apoptosis using the mitochondrial probes, Rhodamine123 and 10-N-nonyl acridine orange. Nat. Protoc. https://doi.org/10.1038/nprot.2007.397 (2007).
Acknowledgements
We are grateful to the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001 for financial support. GPD is supported by postdoctoral fellowship from CAPES. PRTR and AP are grateful to CNPq for the PQ productivity scholarship.
Author information
Authors and Affiliations
Contributions
G.P.D.: Conception and design, acquisition and analysis/interpretation of data, drafting the article; I.M.S.: Conception and design, analysis and interpretation of data, and revising the article critically for important intellectual content; M.A.S.: Revising the article critically for important intellectual content and final approval of the version to be published; V.R.E.: Conception and design and analysis and interpretation of data; S.G.F.: Conception and design and analysis and interpretation of data; A.P.: Conception and design and analysis and interpretation of data; P.R.T.R.: Conception and design, analysis and interpretation of data, and revising the article critically for important intellectual content and final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dorneles, G.P., da Silva, I.M., Santos, M.A. et al. Immunoregulation induced by autologous serum collected after acute exercise in obese men: a randomized cross-over trial. Sci Rep 10, 21735 (2020). https://doi.org/10.1038/s41598-020-78750-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78750-z
- Springer Nature Limited