Abstract
Masticatory muscle thickness provides objective measurements of the oral motor function, which may change in patients with oral myofascial pain. In this study, we aimed to establish a reliable ultrasound (US) protocol for imaging the superficial and deep masticatory muscles and to identify the potential influencers of the measurements. Forty-eight healthy participants without orofacial pain were enrolled. The intra-and inter-rater reliabilities of US measurements for masseter, temporalis, and lateral pterygoid muscles were assessed. Intraclass correlation coefficients for all muscles were greater than 0.6. The generalised estimating equation was used to analyse the impact of age, gender, laterality, and body mass index on the measurements, whereby age and body mass index were likely to be associated with an increase in masticatory muscle thickness. The thickness tended to be lesser in females. Laterality seemed to exert minimal influence on masticatory muscle thickness. Our study shows acceptable reliability of US in the evaluation of superficial and deep masticatory muscle thickness. Future studies are warranted to validate the usefulness of US imaging in patients with oral myofascial pain syndrome.
Similar content being viewed by others
Introduction
Temporomandibular disorder (TMD) is frequently observed in patients seeking dental treatment. It comprises various findings concerning the stomatognathic system, involving the temporomandibular joints, masticatory muscles, teeth, and ears1,2. The prevalence of TMDs in the general population ranges from 34.9 to 42.4% across different studies3,4, and the incidence is more prevalent in females5. Of note, oral myofascial pain is commonplace in about 10–15% of patients with TMD6.
The aetiology of oral myofascial pain is still controversial. The proposed mechanisms include overuse of the masticatory muscles and a decrease in its pain threshold following central sensitization7. While overuse might lead to hypertrophy of the masticatory muscles in the early stages, persistent pain could result in disuse atrophy in chronic cases8. In this sense, masticatory muscle thickness provides objective measurements of the oral motor function9, which is supposed to change in patients suffering from oral myofascial pain.
Several imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) have been used to image the masticatory muscles. CT allows precise delineation of the skull bone where the masticatory muscles attach. However, radiation and poor resolution of the soft tissues make it unsuitable for muscle thickness measurements. Nowadays, MRI serves as the gold standard for depicting soft tissue pathologies10, but it is limited by its high cost and contraindications in patients with metal implants. US has various advantages over CT and MRI, i.e. real-time evaluation, zero radiation, cost-effectiveness, portability, and ease of dynamic examination11. Further, it is capable of delineating muscle fibres—making it highly effective for imaging muscle trauma and neuromuscular diseases12,13,14,15.
Although several studies16,17 have assessed masticatory muscle thickness using US, only a few of them considered the influence of potential confounders, such as laterality, oral posture, age, gender, weight and height18,19. Additionally, previous research focused only on the superficial masticatory muscles but not the deep ones, which would be equally important for the oral motor function. Therefore, the present study aimed to develop a reliable US protocol for imaging the superficial and deep masticatory muscles and to identify the potential influencers of muscle thickness measurements.
Materials and methods
Participants
This pilot study included healthy adults (24 males and 24 females) aged more than 20 years who did not experience any oral myofascial pain, TMD, and sleep bruxism. All the subjects underwent a physical examination in accordance with the Diagnostic Criteria for Temporomandibular Disorders1 and were not allowed to have pain in the jaw, temple, and auricular region. Subjects without complete dentition were excluded. All participants were required to submit an informed consent and the study protocol was approved by the institutional review board of National Taiwan University Hospital, Taipei, Taiwan. (201804014RINA). All methods were carried out in accordance with relevant guidelines and regulations (the Declaration of Helsinki).
Ultrasound measurements
The BenQ Ultrasound System (T3300, BenQ Medical Technology Corp., Taipei, Taiwan) was used for the measurements. The superficial masticatory muscles were assessed using a linear probe (L154BH, 4–15 MHz), while a curved probe (C62B, 2–6 MHz) was employed to obtain the images of the lateral pterygoid muscle due to its deep location. The scanning depth was set at 40 mm for measuring the masseter and the temporalis muscles, and at 60 mm for surveying the lateral pterygoid muscle. The frame rate was set at 300 frames per second.
The subjects were seated on a chair with back support while their heads were kept in a neutral position. The hands were comfortably rested on the knees. All the examinations were repeated three times for each muscle at each position, and the average values were used for the analyses. The measurements were conducted offline by using the stored US pictures and imaging processing software, Image J (National Institutes of Health, Rockville Pike, Bethesda, MD).
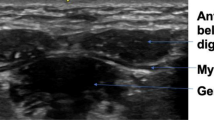
The thickness of the masseter muscle was measured at its upper, middle, and lower parts. To visualize the upper part, the transducer was first placed along the zygomatic arch and then slightly moved towards the chip until the zygomatic arch became invisible. It was then relocated towards the chin parallel to the long axis of the mandibular body, near the angular portion of the mandibular ramus to inspect the lower masseter (Fig. 1). The transducer was then rotated 90 degrees to locate the tip of the condylar notch, following which it was pivoted back to the plane parallel to the long axis of the mandibular body using the above-mentioned point as the centre of rotation to measure its middle part (Fig. 2). The muscle thickness was defined as the maximal distance between the outer and inner fasciae. The muscle was measured bilaterally during relaxation, maximal jaw clenching, and maximal mouth opening. During clenching, we asked our participants to bite as forceful as possible without feeling pain over the teeth. During maximal mouth opening, we required our participants to open the mouths as wide as possible without have discomfort over the temporomandibular joints.
For the temporalis muscle, the linear probe was placed on the upper border of the zygomatic arch and slightly moved cranially and parallel to the short axis of the zygomatic arch until the temporalis muscle was shown on the screen (Fig. 3). The muscle thickness was defined as the maximal distance between the outer and inner fasciae of the temporalis muscle. The muscle was also measured bilaterally during relaxation, maximal jaw clenching, and maximal mouth opening.
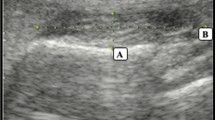
For the lateral pterygoid muscle, the transducer was placed along the zygomatic arch then relocated caudally to the mandibular notch in the horizontal plane, where a hypoechoic gap can be identified between the coronoid and condylar processes of the mandible20. After opening the mouth, the lateral pterygoid muscle was fully observed as a triangular-shaped muscle attached to the lateral pterygoid plate (Fig. 4). The distance between the outer and inner fasciae in the middle of the lateral pterygoid muscle was defined as its thickness. The scanning protocol of the three muscles was summarized in supplementary Table 1.
Ultrasound imaging (A) when the transducer is placed in the horizontal plane caudal to the zygomatic arch with the mouth closed (B). Ultrasound imaging (C) of the lateral pterygoid muscle (LPM) when the transducer is placed in the horizontal plane caudal to the zygomatic arch with the mouth opened (D). The length of the dashed lines indicates the thickness of the muscle. MM, masseter muscle; CORP, coronoid process; CODP, condylar process; TM, temporalis muscle; LPM, lateral pterygoid muscle.
Study flow
Intra-and inter-rater reliability were assessed in the first 10 participants. The 1st session of muscle measurement was conducted by the primary investigator. The 2nd session was conducted one hour later by another investigator using the same scanning protocol, and the 3rd session was conducted by the primary investigator one week after the 1st session. The data derived from the 1st and 2nd sessions were used for analyses of the inter-rater reliability, whereas those of the 1st and 3rd were used for the intra-rater reliability. Examination of the remaining participants was carried out by the primary investigator. Both investigators were board-certificated dentists with more than one-year training in musculoskeletal US. All the participants were recruited based on their designated age (20–40, 40–60, > 60) and sex (female: male ratio = 1:1) stratification.
Sample size calculation
The sample size was calculated based on a previous study investigating the thickness of masseter muscles in patients with and without temporomandibular joint dysfunction21. We assumed that a variation among certain demographic factors led to a mean difference of 0.9 mm in muscle thickness with a standard deviation of 0.5 mm. The number of participants with that variation was hypothesized to be the same as those without. The alpha level was set at 0.05 with a power of 80%. Considering a drop-out rate of 20%, the total number needed was 38.
Statistical analysis
The data of intra-and inter-rater reliability were analysed by the two-way random effect model and were expressed by the intra-class correlation coefficient (ICC) and its 95% confidence interval (CI). The standard error of measurement (SEM) was calculated using the following formula: SEM = the standard deviation pooled from both evaluations \(\times \sqrt{(1-\mathrm{ICC})}\)22. The minimal detectable change (MDC) was obtained employing the following equation: MDC = 1.96 \(\times \sqrt{2}\)\(\times\) SEM22. The paired t-test compared the mean differences between the evaluations of different sessions. The above-mentioned analyses were conducted by using MedCalc (version 14, Ostend, Belgium).
The generalised estimating equation (GEE) was used to analyse the impact of age, gender, laterality, and body mass index (BMI) on the measurements of masticatory muscle thickness. Compared with either height or weight, BMI provides a simple numeric measure of a person’s thickness or thinness and is associated with skeletal muscle mass23. Therefore, in studies related to evaluation of muscle quantity23,24, BMI is a common parameter used for adjustment. Furthermore, if we put height, weight and BMI in the same model for adjustment, the association of muscle thickness with the body size might not be correctly estimated due to collinearity of weight or height with BMI. The GEE—suitable for managing the clustered or correlated data—was performed using the SPSS (Version 12.0. Chicago, SPSS Inc) software. The participant identification was treated as the clustering variable, whereas laterality (right/left) served as an exchangeable correlation structure. A p value less than 0.05 was considered to be statistically significant.
Ethical approval
All participants were required to submit an informed consent and the study protocol was approved by the institutional review board of National Taiwan University Hospital, Taipei, Taiwan. (201804014RINA). All methods were carried out in accordance with relevant guidelines and regulations (the Declaration of Helsinki).
Results
The average age and body mass index values of the participants were 46.19 ± 15.94 (standard deviation, SD) years and 23.07 ± 3.14 (SD) kg/m2, respectively. Repeated evaluations of the same raters revealed significant differences between the lower masseter thickness measurements during relaxation (0.85 ± 1.85 mm, p = 0.040) and jaw clenching (0.96 ± 2.10 mm, p = 0.039). Similarly, repeated evaluations of different raters revealed significant differences between the thickness measurements of the upper masseters during relaxation (− 0.69 ± 0.35 mm, p = 0.001) and jaw clenching (− 0.73 ± 1.11 mm, p = 0.008); middle masseters during relaxation (− 1.18 ± 1.40 mm, p = 0.001), jaw clenching (− 0.78 ± 1.35 mm, p = 0.018), and maximal mouth opening (− 0.70 ± 1.33 mm, p = 0.029); lower masseters during jaw clenching (1.00 ± 1.96 mm, p = 0.035); and temporalis muscles during maximal mouth opening (0.46 ± 0.78 mm, p = 0.006) (Table 1).
All intra-and inter-rater reliabilities were greater than 0.6, which is considered good according to the Cicchetti’s classification25. The intra-rater SEM and MDC ranged from 0.31 mm (temporalis muscles during maximal mouth opening) to 1.49 mm (lower masseters during jaw clenching) and from 0.85 mm (temporalis muscles during maximal mouth opening) to 4.13 mm (lower masseters during jaw clenching), respectively. The inter-rater SEM and MDC ranged from 0.42 mm (temporalis muscles during maximal mouth opening) to 1.44 mm (lower masseters in relaxation) and from 1.16 mm (temporalis muscle during maximal mouth opening) to 3.98 mm (lower masseters during relaxation), respectively (Table 2).
According to the GEE analysis, age was likely to be positively associated with the masticatory muscle thickness. Statistically significant differences were detected in the lower masseters in all the examined positions (p = 0.017, relaxation; p = 0.003, jaw clenching; and p = 0.010, maximal mouth opening) and only in the maximal mouth opening position (p = 0.006) for the lateral pterygoid muscles. Female sex was found to be inversely associated with masticatory muscle thickness; a statistically significant inverse association was observed for the upper masseters during jaw clenching (p = 0.034) and middle masseters during maximal mouth opening (p = 0.039). The influence of laterality was only seen in the measurement of upper masseters during clenching (p = 0.018) whereby the muscle appeared significantly thicker on the left side. BMI was found to be positively associated with the thickness all masticatory muscles whereby significant differences were detected in the temporalis muscle thickness measurements in all examined positions (p = 0.008, relaxation; p = 0.011, jaw clenching; and p = 0.013, maximal mouth opening) (Table 3).
Discussion
The present study employed a standard US scanning protocol to measure masticatory muscle thickness yielding good intra-and inter-rater reliability. To the authors’ best knowledge, this is the first study exploring the reliability of US measurements for the lateral pterygoid muscle. During the literature search, only a few articles had investigated the influence of age, gender, height and weight on superficial masticatory muscles18,19. In the present research, the multivariate analysis also revealed that age and BMI were likely to be associated with an increase in masticatory muscle thickness, whereas the thickness tended to be lesser in females. Laterality was observed to exert minimal influence on masticatory muscle thickness.
Our research demonstrates that the reliability of US measurements of masticatory muscle thickness, represented by intraclass correlation coefficients (ICCs), ranges from 0.69 to 0.89. Lin et al.26 examined the reliability of MRI measurements for the masseter muscle and found better inter-and intra-rater ICCs (0.996 and 0.997, respectively) when compared with those of ours. However, US provides real-time and dynamic assessment of masticatory muscles as well as a more accessible and cost-effective option than MRI. In 2003, Emshoff et al.27 used US to measure the facial and neck muscles and found ICCs ranging from 0.70 to 0.92. In 2019, Barotsis et al.28 conducted another US study for assessing the masseter thickness and the range of ICCs was between 0.295 and 0.991. While the ICCs from our data are not inferior to those from previous US literature, our results also include muscle thickness measurements in various masticatory postures.
In the present study, we utilized several methods to ensure the reliability of US measurements for the masticatory muscles in different mouth positions. First, bony landmarks, such as the zygomatic arch, mandibular ramus, mandibular body and mandibular notch were used to standardize the site of measurement, which also facilitated the examiner to repeat the scanning process on different participants. Second, the masseter muscles were measured at various segments to minimize the influence of the intramuscular tendon near the insertion on the mandible. In our study, we identified a lower ICC value regarding thickness measurements of the lower masseters during clenching (Table 2). The potential cause might be derived from their muscle origin (the superficial and deep heads). As we did not control the anterior-to-posterior dimension during examination, the variation of contribution from both heads of the masseters might affect the reliability of measurements. Herein, this issue cautions investigators regarding the increased variability observed while measuring the distal parts of the superficial masticatory muscles.
The lateral pterygoid muscle plays a crucial role in controlling protrusion, depression, and unilateral movement of the lower jaw20. While its upper part originates from the greater wing of the sphenoid bone, its lower part originates from the lateral surface of the lateral pterygoid plate. Both parts insert onto the neck of the mandible. Measurement of its thickness using US has never been performed until now. There are three reasons why its thickness measurement is difficult. First, it is deeply located and is not easily appreciated by a linear transducer. Second, it is obscured by the mandibular ramus when the mouth is closed. Third, it is triangular-shaped—challenging the thickness definition. Therefore, we implemented three approaches to make the measurement plausible. First, we used the curved transducer to improve the penetration of US beam for better visualization of the deep structures. Second, the open-mouth view was utilized to avoid the acoustic shadowing of the mandibular ramus. Third, the thickness of the lateral pterygoid muscle was clearly defined by using the midpoint of its superficial fascia. To this end, our study showed that the reliability of thickness measurements for the lateral pterygoid muscle could be as good as those for the superficial masticatory muscles.
As shown in Table 2, the MDC values of the middle and the lower masseter muscles were larger than the other masticatory muscles. A substantial portion of the middle and lower masseter muscles has been evolved to form the tendinous component, causing an increase in variability of muscle thickness across different measurements as well as in the corresponding MDC values. The masseter muscle consists two major heads, superficial and deep29. The superficial head is located more anteriorly and arises from a tendinous aponeurosis. In contrast, the deep head originates from the posterior portion of the zygomatic arch and appears more muscular than the superficial head. The portions of muscle fibers from each head can affect the measurement of the maximal muscle thickness. In this study, the muscle was measured at its thickest portion on the US images without considering possible variations in the anterior-to-posterior dimension, which also accounted for larger MDC values of the middle and lower masseter muscles.
The GEE analysis revealed a likely positive association between the masticatory muscle thickness and age, i.e. statistically significant for the lower masseters and lateral pterygoid muscles. Likewise, a previous US study reported that masticatory muscle thickness gradually increased with age in a population younger than 60 years19. This finding might be attributed to age-related stature changes and hypertrophy due to repeated use.
In the present study, we did not specifically measure the echogenicity of the masticatory muscle. As the present study included measurements of the deep muscles, the gain of ultrasound signals needed to be adjusted dynamically to improve the visibility of the deep muscle fascia. Therefore, the measurements of muscle echogenicity might not be reliable using our study design. However, muscle echogenicity usually increases with aging due to fat replacement30. Isolated thickness measurement was shown to be less informative than muscle echogenicity in patients with neuromuscular disease31. Therefore, future studies can be designed to specifically evaluate the echogenicity of the masticatory muscles, which would be beneficial for exploration of age- or disease-related alternations of muscle texture.
Regarding sex-related differences, males were observed to have thicker masticatory muscles than females, especially for the masseters and lateral pterygoid muscles. Our analysis also revealed a positive association independent of age, body stature, and laterality. One possible explanation would be the relationship between the masticatory muscle thickness and craniofacial morphology, especially for the masseter muscle32,33. Males tend to have greater facial length than females34. Moreover, the diameters of type II muscle fibres are larger than type I35, and there is a higher portion of type II muscle fibres in male masseters as compared to those of female34.
Our study also identified a likely positive association between BMI and masticatory thickness, especially for the temporalis muscle. Since the temporalis muscle travels a longer distance on the skull than the masseter and lateral pterygoid muscles, its size is more dependent on the head volume. Similarly, an antecedent anthropometric study identified a high correlation between brain volume and BMI—partially supporting this issue36.
Our findings uncovered minimal influence of laterality on the masticatory muscle thickness. As this result is consistent with a previous study37 investigating similar issues, it would be interesting to examine whether asymmetry in muscle thickness can be found in patients with masticatory problems in future studies.
There were several limitations that need to be acknowledged. First, the present study used a cross-sectional design. Whether the changes observed in masticatory muscle thickness were associated with long-term health outcomes could not be determined through our analysis. Second, the zygomatic bone is the main obstacle in observing the lateral pterygoid muscle using US. MRI or CT would be needed if the investigators intend to measure its thickness in the closed-mouth position. Third, as the present research aimed to establish the reference standards of masticatory muscle thickness, only asymptomatic volunteers were enrolled. Future studies are needed to explore whether the masticatory muscle thicknesses are altered in patients with TMD or oral myofascial pain syndrome.
In conclusion, using a standardized protocol, US can be employed to evaluate the thickness of superficial and deep masticatory muscles with acceptable reliability. These values may vary in populations with different age range, gender, and BMI values. Future studies are warranted to validate the usefulness of US imaging in patients with common clinical syndromes, such as TMD, malocclusion of teeth, oral motor dysphagia and oral myofascial pain syndrome.
References
Schiffman, E. et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest groupdagger. J. Oral Facial Pain Headache 28, 6–27. https://doi.org/10.11607/jop.1151 (2014).
Greene, C. S. & Laskin, D. M. Long-term evaluation of treatment for myofascial pain-dysfunction syndrome: a comparative analysis. J. Am. Dent. Assoc. 107, 235–238. https://doi.org/10.14219/jada.archive.1983.0246 (1983).
Minghelli, B., Morgado, M. & Caro, T. Association of temporomandibular disorder symptoms with anxiety and depression in Portuguese college students. J. Oral. Sci. 56, 127–133. https://doi.org/10.2334/josnusd.56.127 (2014).
Bertoli, F. M. P. et al. Prevalence of diagnosed temporomandibular disorders: a cross-sectional study in Brazilian adolescents. PLoS ONE 13, e0192254. https://doi.org/10.1371/journal.pone.0192254 (2018).
Bagis, B., Ayaz, E. A., Turgut, S., Durkan, R. & Ozcan, M. Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: a retrospective study on 243 consecutive patients. Int. J. Med. Sci. 9, 539–544. https://doi.org/10.7150/ijms.4474 (2012).
Rantala, M. A. et al. Temporomandibular joint related painless symptoms, orofacial pain, neck pain, headache, and psychosocial factors among non-patients. Acta Odontol. Scand. 61, 217–222. https://doi.org/10.1080/00016350310004089 (2003).
Arangio, G. A., Chen, C., Kalady, M. & Reed, J. F. 3rd. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J. Orthop. Sports Phys. Ther. 26, 238–243. https://doi.org/10.2519/jospt.1997.26.5.238 (1997).
Grunheid, T., Langenbach, G. E., Korfage, J. A., Zentner, A. & van Eijden, T. M. The adaptive response of jaw muscles to varying functional demands. Eur. J. Orthod. 31, 596–612. https://doi.org/10.1093/ejo/cjp093 (2009).
Verri, E. D. et al. Effects of Parkinson’s disease on molar bite force, electromyographic activity and muscle thickness of the masseter, temporal and sternocleidomastoid muscles: a case–control study. J. Oral Rehabil. 46, 912–919. https://doi.org/10.1111/joor.12824 (2019).
Talmaceanu, D. et al. Imaging modalities for temporomandibular joint disorders: an update. Clujul. Med 91, 280–287. https://doi.org/10.15386/cjmed-970 (2018).
Ozcakar, L. et al. Nineteen reasons why physiatrists should do musculoskeletal ultrasound: EURO-MUSCULUS/USPRM recommendations. Am J Phys Med Rehabil 94, e45-49. https://doi.org/10.1097/PHM.0000000000000223 (2015).
Chang, K. V., Wu, W. T. & Ozcakar, L. Ultrasound imaging and rehabilitation of muscle disorders: part 1. Traumatic Injuries. Am. J. Phys. Med. Rehabil. 98, 1133–1141. https://doi.org/10.1097/PHM.0000000000001307 (2019).
Wu, W. T. et al. Basis of shoulder nerve entrapment syndrome: an ultrasonographic study exploring factors influencing cross-sectional area of the suprascapular nerve. Front Neurol. 9, 902. https://doi.org/10.3389/fneur.2018.00902 (2018).
Van Den Engel-Hoek, L., Lagarde, M. & Van Alfen, N. Ultrasound of oral and masticatory muscles: why every neuromuscular swallow team should have an ultrasound machine. Clin. Anat. 30, 183–193. https://doi.org/10.1002/ca.22818 (2017).
Pillen, S., Arts, I. M. & Zwarts, M. J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 37, 679–693. https://doi.org/10.1002/mus.21015 (2008).
Serra, M. D., Duarte-Gaviao, M. B. & dos Santos Uchoa, M.N. The use of ultrasound in the investigation of the muscles of mastication. Ultrasound Med. Biol. 34, 1875–1884. https://doi.org/10.1016/j.ultrasmedbio.2008.05.009 (2008).
Castelo, P. M., Gaviao, M. B., Pereira, L. J. & Bonjardim, L. R. Masticatory muscle thickness, bite force, and occlusal contacts in young children with unilateral posterior crossbite. Eur. J. Orthod. 29, 149–156. https://doi.org/10.1093/ejo/cjl089 (2007).
Park, K. M. et al. The relationship between masseter muscle thickness measured by ultrasonography and facial profile in young Korean adults. Imaging Sci. Dent. 48, 213–221. https://doi.org/10.5624/isd.2018.48.3.213 (2018).
Palinkas, M. et al. Age and gender influence on maximal bite force and masticatory muscles thickness. Arch. Oral Biol. 55, 797–802. https://doi.org/10.1016/j.archoralbio.2010.06.016 (2010).
Chen, Y. J., Chang, P. H., Chang, K. V., Wu, W. T. & Ozcakar, L. Ultrasound guided injection for medial and lateral pterygoid muscles: a novel treatment for orofacial pain. Med. Ultrason. 1, 115–116. https://doi.org/10.11152/mu-1362 (2018).
Imanimoghaddam, M., Davachi, B., Madani, A. S. & Nemati, S. Ultrasonographic findings of masseter muscle in females with temporomandibular disorders. J. Craniofac. Surg. 24, e108-112. https://doi.org/10.1097/SCS.0b013e3182646af0 (2013).
Chang, K. V. et al. Smartphone application with virtual reality goggles for the reliable and valid measurement of active craniocervical range of motion. Diagnostics (Basel) 9, 1. https://doi.org/10.3390/diagnostics9030071 (2019).
Chang, K. V., Yang, K. C., Wu, W. T., Huang, K. C. & Han, D. S. Association between metabolic syndrome and limb muscle quantity and quality in older adults: a pilot ultrasound study. Diabetes Metab. Syndr. Obes. 12, 1821–1830. https://doi.org/10.2147/DMSO.S219649 (2019).
Chang, K. V., Wu, W. T., Huang, K. C., Jan, W. H. & Han, D. S. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: an ultrasound imaging study. Exp. Gerontol. 108, 54–61. https://doi.org/10.1016/j.exger.2018.03.019 (2018).
Cicchetti, D. V., Sharma, Y. & Cotlier, E. Assessment of observer variability in the classification of human cataracts. Yale J. Biol. Med 55, 81–88 (1982).
Lin, C. S. et al. Age- and sex-related differences in masseter size and its role in oral functions. J. Am. Dent Assoc. 148, 644–653. https://doi.org/10.1016/j.adaj.2017.03.001 (2017).
Emshoff, R., Emshoff, I., Rudisch, A. & Bertram, S. Reliability and temporal variation of masseter muscle thickness measurements utilizing ultrasonography. J. Oral Rehabil. 30, 1168–1172. https://doi.org/10.1111/j.1365-2842.2003.01186.x (2003).
Barotsis, N., Tsiganos, P., Kokkalis, Z., Panayiotakis, G. & Panagiotopoulos, E. Reliability of muscle thickness measurements in ultrasonography. Int. J. Rehabil. Res. 43, 123–128. https://doi.org/10.1097/MRR.0000000000000390 (2020).
Guerreschi, P. et al. Masseter muscle termination over the deep surface of the temporal fascia: look out the wrong path. Surg. Radiol. Anat. 33, 863–868. https://doi.org/10.1007/s00276-011-0882-y (2011).
Watanabe, Y. et al. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin. Interv. Aging 8, 993–998. https://doi.org/10.2147/CIA.S47263 (2013).
Simon, N. G., Noto, Y. I. & Zaidman, C. M. Skeletal muscle imaging in neuromuscular disease. J. Clin. Neurosci. 33, 1–10. https://doi.org/10.1016/j.jocn.2016.01.041 (2016).
Bakke, M. et al. Ultrasound image of human masseter muscle related to bite force, electromyography, facial morphology, and occlusal factors. Scand. J. Dent. Res. 100, 164–171. https://doi.org/10.1111/j.1600-0722.1992.tb01734.x (1992).
Raadsheer, M. C., Kiliaridis, S., Van Eijden, T. M., Van Ginkel, F. C. & Prahl-Andersen, B. Masseter muscle thickness in growing individuals and its relation to facial morphology. Arch. Oral. Biol. 41, 323–332. https://doi.org/10.1016/0003-9969(95)00136-0 (1996).
Tuxen, A., Bakke, M. & Pinholt, E. M. Comparative data from young men and women on masseter muscle fibres, function and facial morphology. Arch. Oral. Biol. 44, 509–518. https://doi.org/10.1016/s0003-9969(99)00008-4 (1999).
Meznaric, M. & Cvetko, E. Size and proportions of slow-twitch and fast-twitch muscle fibers in human costal diaphragm. Biomed. Res. Int. 2016, 5946520. https://doi.org/10.1155/2016/5946520 (2016).
Bayat, P.-D., Ghanbari, A., Sohouli, P., Amiri, S. & Sari-Aslani, P. Correlation of skull size and brain volume, with age, weight, height and body mass index of arak medical sciences students. Int. J. Morphol. 30, 157–161 (2012).
Raadsheer, M. C. et al. A comparison of human masseter muscle thickness measured by ultrasonography and magnetic resonance imaging. Arch. Oral. Biol. 39, 1079–1084. https://doi.org/10.1016/0003-9969(94)90061-2 (1994).
Funding
The current research project was supported by (1) National Taiwan University Hospital, Bei-Hu Branch; (2) Ministry of Science and Technology (MOST 106-2314-B-002-180-MY3 and 109-2314-B-002-114-MY3); and (3)Taiwan Society of Ultrasound in Medicine.
Author information
Authors and Affiliations
Contributions
P.H.C., Y.J.C., K.V.C. performed experiments, analyses and interpretation of data and drafted the manuscript. P.H.C., K.V.C. contributed to analyses, drafted the manuscript and supervised the study. Y.J.C., K.V.C., W.T.W, performed experiments and contributed to data analyses. P.H.C., Y.J.C. , K.V.C., W.T. W, L.Ö performed administrative support, contributed to data interpretation and analysis, obtained funding, supervised and designed the study and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, PH., Chen, YJ., Chang, KV. et al. Ultrasound measurements of superficial and deep masticatory muscles in various postures: reliability and influencers. Sci Rep 10, 14357 (2020). https://doi.org/10.1038/s41598-020-71378-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71378-z
- Springer Nature Limited
This article is cited by
-
Comparison of ultrasonography-based masticatory muscle thickness between temporomandibular disorders bruxers and temporomandibular disorders non-bruxers
Scientific Reports (2024)
-
Ultrasonographic examination of masticatory muscles in patients with TMJ arthralgia and headache attributed to temporomandibular disorders
Scientific Reports (2024)
-
The feasibility of visualizing and quantifying muscle changes in postoperative oral cancer patients using Quantitative Muscle Ultrasound (QMUS)
Journal of Ultrasound (2024)
-
Evaluation of the thickness of masticatory muscles in patients with chronic periodontitis by ultrasonography
Oral Radiology (2024)
-
Comments on “Clinical utility of ultrasonography imaging in musculoskeletal conditions: a systematic review and meta-analysis”
Journal of Medical Ultrasonics (2022)