Abstract
Autophagy is a physiological mechanism that can be activated under stress conditions. However, the role of autophagy during oocyte maturation has been poorly investigated. Therefore, this study characterized the role of autophagy on developmental competence and gene expression of bovine oocytes exposed to heat shock (HS). Cumulus-oocyte-complexes (COCs) were matured at Control (38.5 °C) and HS (41 °C) temperatures in the presence of 0 and 10 mM 3-methyladenine (3MA; autophagy inhibitor). Western blotting analysis revealed that HS increased autophagy marker LC3-II/LC3-I ratio in oocytes. However, there was no effect of temperature for oocytes matured with 3MA. On cumulus cells, 3MA reduced LC3-II/LC3-I ratio regardless of temperature. Inhibition of autophagy during IVM of heat-shocked oocytes (3MA-41 °C) reduced cleavage and blastocyst rates compared to standard in vitro matured heat-shocked oocytes (IVM-41 °C). Therefore, the magnitude of HS detrimental effects was greater in the presence of autophagy inhibitor. Oocyte maturation under 3MA-41 °C reduced mRNA abundance for genes related to energy metabolism (MTIF3), heat shock response (HSF1), and oocyte maturation (HAS2 and GREM1). In conclusion, autophagy is a stress response induced on heat shocked oocytes. Inhibition of autophagy modulated key functional processes rendering the oocyte more susceptible to the deleterious effects of heat shock.
Similar content being viewed by others
Introduction
Autophagy is a programmed lysosomal process that degrades damaged or unnecessary cellular proteins, lipids, DNA, RNA, and organelles for recycling amino acids, fatty acids, and nucleosides to act as cellular building blocks for anabolic processes1,2,3. This event is involved in development, differentiation, immunity, aging, and cell death2. The autophagy pathway can be triggered by different stress conditions in order to restore homeostasis as a pro-survival adaptive response to stress. For example, autophagy can be induced by diseases4,5,6 and environmental factors such as nutrient deprivation7,8, high temperature9,10,11, oxidative stress12,13, hypoxia14,15, and toxins16,17,18.
There are three autophagy types described in mammalian cells: chaperone-mediated autophagy, macroautophagy, and microautophagy1. In the chaperone-mediated autophagy, heat shock cognate 70 complex (Hsc70 complex) directs abnormal proteins that have KFERQ-like motifs exposed to lysosome-mediated degradation. On the other hand, during microautophagy the lysosome membrane invaginates, thus capturing a small portion of the cytoplasm to be degraded2. Macroautophagy is the best-understood type of autophagy, which is mediated by double-membrane vesicles that transport abnormal proteins and organelles through the cytoplasm (i.e., autophagosomes). The autophagosome membrane fuses to lysosomes to breakdown the transported material2.
During embryonic development, autophagy is first induced after fertilization to clear sperm-born material that is not necessary for further embryogenesis19,20,21. In turn, autophagy is also associated with abundance of maternal mRNA, such as C-mos and Cyclin B22,23, and it seems to have an important role before mouse and porcine embryo genome activation23,24. Inhibition or induction of autophagy with 3-methyladenine (3MA) and rapamycin, respectively, during culture of porcine embryos from one-cell to the four-cell stage or one-cell to the blastocyst stage affected embryo developmental potential, blastocyst cell number, and apoptosis index. However, the same treatments had no effect from the four-cell to the blastocyst stage23, demonstrating that autophagy is only required for early stages of preimplantation development.
Even though induction of autophagy has been shown to play a role after oocyte activation and during preimplantation development21, little has been demonstrated on the role of autophagy on oocyte maturation25. The autophagy-specific marker microtubule-associated protein 1 light chain-II (LC3-II) known to associate with the autophagosomal membrane was increased in porcine oocytes matured in vitro26. While induction of autophagy during porcine in vitro maturation (IVM) improved oocyte maturation, fertilization, blastocyst development, and quality27, autophagy inhibition disrupted oocyte DNA and mitochondria, increased reactive oxygen species production, apoptosis, and reduced embryonic development28.
There are several environmental stressors that can induce autophagy during oocyte maturation16,17,18. A well-known stressor for oocytes is elevated temperature, to which bovine oocytes are often exposed leading to reduced fertility29,30. The deleterious effects of elevated temperature on bovine oocytes has been well characterized30,31,32,33,34,35,36. However, the molecular mechanisms involved in these processes are not fully understood. As the oocyte has limited ability to respond to stress, understanding the oocyte survival signaling pathways would provide new alternatives to overcome the deleterious effects of heat stress. Therefore, the hypothesis of this study was that autophagy is a stress response activated during maturation of heat-shocked oocytes in order to restore homeostasis and promote survival. The objective was to determine whether autophagy pathway is induced by heat shock and the role of autophagy on developmental competence and gene expression of bovine oocytes exposed to heat shock during IVM.
Materials and methods
Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tissue Culture Medium-199 (TCM-199 containing l-glutamine and phenol red) and fetal bovine serum (FBS) were purchased from GIBCO (Grand Island, NY, USA). Folltropin–V (Follicle-stimulating hormone; FSH) was purchased from Bioniche Animal Health Canada Inc. (Bellevile, Ontario, Canada) and Chorulon (Human chorionic gonadotrophin; hCG) from Intervet Schering Plough (Roseland, NJ, USA). EmbryoMax (KSOM Powdered Media Kit #MR-020P-5F) was purchased from MilliPore (Livingston, Fleming Road, UK). The autophagy inhibitor 3-Methyladenine (3MA; #sc-205596) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The primary antibody [LC3A/B (D3U4C) rabbit monoclonal antibody—#12741] and secondary antibody (anti-rabbit IgG – HRP-linked antibody—#7074) were purchased from Cell Signaling Technology (Danvers, 3 Trash Lane, MA). Frozen semen was purchased from CRV Lagoa (Sertãozinho, São Paulo, Brazil).
Experimental design
Experiment 1: Autophagy induction on bovine oocytes and cumulus cells exposed to heat shock during IVM
The objective of this study was to determine autophagy activation and efficiency of the autophagy inhibitor 3-methyladenine (3MA) in cumulus-oocyte complexes (COCs) exposed to heat shock during IVM. 3MA inhibits the positive regulator of autophagy known as class III phosphatidylinositol-3-kinase (PI3K). This experiment was arranged in a 2 × 2 factorial design to determine the effect of temperature (38.5 or 41 °C) and autophagy inhibitor (0 or 10 mM 3MA) during IVM on autophagy activity by measuring LC3 protein level (autophagy marker). COCs were distributed on the following experimental groups: Control (38.5 °C for 22 h) and Heat Shock (41 °C for 16 h followed by 38.5 °C for 6 h36,37), in the presence of 0 or 10 mM 3MA during IVM38. After IVM, COCs were denuded so that denuded oocytes and respective cumulus cells were processed for western blotting analysis to determine autophagy activity by LC3-I and LC3-II level. This experiment was replicated 3 times using 60 oocytes/treatment/replicate and cumulus cells of 60–70 COCs/treatment/replicate (Fig. 1).

adapted from Master’s Thesis83.
Schematic representation of the experimental design. COCs underwent IVM at Control (38.5 °C for 22 h) and Heat Shock (41 °C for 16 h followed by 38.5 °C for 6 h) temperatures in the presence of 0 or 10 mM 3MA. After 22 h IVM, COCs were either denuded and processed for western blotting and RT-qPCR analysis or submitted to IVF for 18 h at 38.5°C, followed by IVC for 8 days. Embryonic development was recorded on Days 3 (cleavage rate, %) and 8 (blastocyst rate, %) after IVF. IVM, in vitro maturation; IVF, in vitro fertilization; IVC, in vitro culture; 3MA, 3-Methyladenine. The figure was
Experiment 2: The role of autophagy on developmental competence of heat-shocked bovine oocytes
The objective was to determine the role of autophagy on developmental potential of bovine oocytes exposed to heat shock during IVM. This experiment was arranged in a 2 × 2 factorial design as described for experiment 1. COCs were distributed on the following experimental groups: Control (38.5 °C for 22 h) and Heat Shock (41 °C for 16 h followed by 38.5 °C for 6 h) in the presence of 0 or 10 mM 3MA during IVM. After 22 h IVM, COCs were subjected to in vitro fertilization (IVF) and in vitro culture (IVC) as described below. Cleavage rate was determined at day 3 and the percentage of oocytes and cleaved embryos that reached the blastocyst stage was determined on day 8 post-IVF, respectively. This experiment was replicated 5 times using 30 COCs/treatment/replicate (Fig. 1).
Experiment 3: The effect of autophagy inhibition on gene expression of bovine oocytes exposed to heat shock
The objective was to determine the effect of autophagy on mRNA abundance of bovine oocytes submitted to heat shock during IVM. This experiment was arranged in a 2 × 2 factorial design and COCs were randomly distributed on the same experimental groups as described before. After IVM, pools of 30 oocytes were denuded, processed and stored at – 80 °C until RT-qPCR analysis using Biomark HD System. This experiment was replicated 5 times using 30 oocytes/treatment/replicate (Fig. 1).
Methods
In vitro maturation
Bovine ovaries (crossbred Bos taurus indicus) were collected at slaughterhouse and transported within 2 h to the laboratory in sterile saline [0.9% (w/v) NaCl containing 100 U/mL penicillin-G and 100 µg/mL streptomycin] at 37 °C. COCs were collected by slicing 2–8 mm follicles into Oocyte Collection Medium [Tissue Culture Medium-199 (TCM-199) containing L-glutamine and phenol red supplemented with 2.2 mg/mL sodium bicarbonate, 1% (v/v) fetal bovine serum (FBS) containing 2 U/mL heparin, 0.01 µg/mL streptomycin, 0.01 U/mL penicillin-G]. Grade I-III COCs39 were selected and washed once on pre-IVM medium [TCM-199 HEPES supplemented with 10% (v/v) FBS, 50 μg/mL gentamicin and 0.2 mM sodium pyruvate]. Groups of 10 COCs were placed on 50 µL drops of IVM medium [TCM-199-Bicarbonate containing 10% (v/v) FBS, 50 μg/mL gentamicin, 0.2 mM sodium pyruvate, 10 μg/mL FSH, 10 μg/mL hCG, and 1 μg/mL 17-β estradiol] in the presence of 0 or 10 mM 3-MA. IVM drops were covered with mineral oil at control (38.5 °C for 22 h under 5% CO2) or heat shock (41 °C for 16 h under 7% CO2 followed by 38.5 °C for 6 h under 5% CO2)40,41 temperatures in humidified air.
Western blotting analysis
After IVM COCs were transferred to microcentrifuge tubes, vortexed in pre-warmed phosphate buffered saline (10 mM PBS) containing 0.1% (w/v) hyaluronidase for 5 min. Denuded oocytes were washed three times in 10 mM PBS containing 1 mg/mL PVP (polyvinylpyrrolidone) and suspended in 15 μL of buffer solution [1.5 M Tris–HCl pH 6.8 containing 50% (v/v) glycerol, 25% (v/v) β-mercaptoethanol, 10% (v/v) SDS, and 0.05% (v/v) bromophenol blue], and 15 μL of PBS-PVP, boiled at 90 °C for 5 min and stored at -80 °C. After vortexing, the remaining cumulus cells were washed in 1 mL PBS at 5,000 × g for 5 min, followed by a second wash in 500 μL PBS at 5,000 × g for 5 min. The pellet of cumulus cells was subjected to 5 cycles of thermal shock (1 min in liquid nitrogen followed by 1 min at 37 °C), resuspended in 15 μL of PBS-PVP and 15 μL of buffer solution, boiled at 90 °C for 5 min and stored at – 80 °C. Proteins were separated by 15% (w/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel at 60 mA. Gels were transferred to a polyvinylidenedifluoride membrane at 15 V for 1 h at room temperature (RT). Membranes were then incubated in blocking buffer [5% (w/v) skim powdered milk diluted in Tris (TBS) saline with 0.1% (v/v) Tween-20—(TBS-T)] for 1 h at RT. After blocking, membranes were washed 3 times in washing buffer [TBS-T] at RT and incubated with primary rabbit monoclonal antibody anti-LC3—I and II [1:1000]26,42 diluted in TBS-T containing 5% bovine serum albumin (BSA) at 4 °C overnight. Membranes were washed in TBS-T, incubated with secondary antibody [1:2000] anti-rabbit IgG diluted in TBS-T containing 5% BSA for 1 h at RT, washed 3 times, and incubated with chemiluminescent reagent (SuperSignal West Pico PLUS Chemiluminescent Substrate, 1:1) for 1 min at RT. Chemiluminescence was captured by Alliance LD4 Gel Documentation System (UVITEC Cambridge). The proteins LC3-I (16 kDa) and II (14 kDa) were identified according to molecular weight. Protein abundance was analyzed by UVIBANDMAX software, version 15.06a, through band size and intensity.
In vitro fertilization and culture
After 22 h IVM, COCs were washed on pre-IVF medium (TCM-199 HEPES supplemented with 3 mg/mL BSA fraction V, 50 µg/mL gentamicin, and 0.2 mM sodium pyruvate) and on IVF medium [modified Tyrode's albumin-lactate-pyruvate (TALP) medium43 containing 6 mg/mL essentially fatty acid free (EFAF)-BSA, 50 µg/mL gentamicin, 0.2 mM sodium pyruvate, 100 µg/mL heparin, and 41.66 µL/mL PHE (penicillamine 2.7 µg/mL, hypotaurine 1 µg/mL, epinephrine 0.33 µg/mL in 0.9% (w/v) NaCl]. Groups of 10 COCs were placed in 90 µL drop of IVF medium covered with mineral oil at 38.5°C and 5% CO2 in humidified air. Frozen-thawed straws from two different bulls (Bos taurus indicus) were used for each replicate. Viable spermatozoa were purified by centrifugation (9,000 × g for 5 min) on Percoll gradient (45% and 90%) followed by centrifugation (9,000 × g for 2.5 min) on SP-TALP medium (TALP medium containing 6 mg/mL EFAF-BSA, 50 µg/mL gentamicin, and 0.2 mM sodium pyruvate). COCs were inseminated with 1 × 106 sperm/mL at 38.5 °C and 5% CO2 in humidified air. After 18 h IVF, presumptive zygotes were denuded by 5 min vortexing in pre-IVF medium and washed three times in modified potassium simplex optimization medium (KSOM medium – Embryomax) supplemented with 10% (v/v) FBS, 1.25 µg/mL gentamicin, and 4 µl/mL non-essential amino acids (KSOMaaFBS). Groups of 25–30 presumptive zygotes were placed in 50 µL drops of KSOMaaFBS covered with mineral oil at 38.5°C and 5% CO2 in humidified air for 8 days. The percentage of oocytes that cleaved was determined at day 3 after IVF and the percentage of oocytes and cleaved embryos that reached the blastocyst stage was determined at day 8 after IVF.
cDNA synthesis and gene expression analysis
Total RNA was extracted from pools of 30 denuded oocytes (n = 5 pools/treatment/replicate) using Qiagen RNeasy Micro Kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer’s instructions. DNAse treatment was performed in all samples during RNA isolation according to the manufacturer’s instructions. The cDNA synthesis was performed using all the RNA with the High Capacity Reverse Transcription kits (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions.
Gene expression analysis of bovine oocytes was performed using Applied BiosystemsTaqMan Assays, specific for Bos taurus species as previously described by Razza et al.44,45. A total of 96 candidate genes were analyzed (Table 1). Prior to qPCR thermal cycling, each cDNA sample was submitted to sequence-specific preamplification process as follows: 1.25 µL assay mix (Taqman Assay was pooled to a final concentration of 0.2 × for each of the 96 assays), 2.5 µL TaqManPreAmp Master Mix (Applied Biosystems, #4391128) and 1.25 µL cDNA. The reactions were activated at 95 °C for 10 min followed by denaturing at 95 °C for 15 s, annealing and amplification at 60 °C for 4 min for 14 cycles. Preamplified products were diluted fivefold prior to RT-qPCR analysis. The preamplification process is a required step for analysis in the Biomark HD system due to the nanoliter scale of qPCR reactions. A recent study using the same microfluidic platform demonstrated that preamplification step performed on 96.96 Biomark HD Array for bovine oocytes was uniform and reliable45.
For gene expression analysis, the sample solution consisted of 2.25 µL cDNA (preamplified products), 2.5 µL of TaqMan Universal PCR Master Mix (2 ×, Applied Biosystems) and 0.25 µL of 20 × GE Sample Loading Reagent (Fluidigm); and the assay solution: 2.5 µL of 20 × TaqMan Gene Expression Assay (Applied Biosystems) and 2.5 µL of 2 × Assay Loading Reagent (Fluidigm). The 96.96 Dynamic Array Integrated Fluidic Circuits (Fluidigm) chip was used for data collection. After priming, the chip was loaded with 5 µL of each assay solution and 5 µL of each sample solution. The qPCR thermal cycling was performed in the United Biomark HD System (Fluidigm, South San Francisco, CA, USA) using TaqMan GE 96 × 96 Standard protocol. Briefly, the protocol consisted of one stage of Thermal Mix (50 °C for 2 min, 70 °C for 20 min and 25°C for 10 min) followed by a Hot Start stage (50 °C for 2 min and 95 °C for 10 min), followed by 40 cycles of denaturation (95 °C for 15 s), primer annealing and extension (60 °C for 60 s).
The RefFinder software (GeNorm and Normfinder) was used to determine the most stable genes among samples. The retrieved reference genes were peptidyl-prolylisomerase-A (PPIA) and ribosomal protein L15 (RPL15). Therefore, the data were normalized by the formula: ΔCq = (target gene Cq)—(Geometric Mean of PPIA and RPL15 genes). The data were then transformed (twofold change-ΔCq) and the result was used for statistical analyses46.
Statistical analysis
Assumptions for analysis of variance (ANOVA) (normally distributed data and homogeneity of variance) were determined by JMP, Version 11 (SAS Institute Inc., Cary, NC, 1989–2007). Logarithmic and square root transformation were applied to obtain normal distribution whenever required. Parametric data were analyzed by least-squares ANOVA using General Linear Models (GLM) procedure of SAS (SAS, 1989). Dependent variables were LC3-II/LC3-I ratio, percentage of cleaved embryos, percentage of oocytes and cleaved embryos that developed to the blastocyst stage and fold change of analyzed genes. Independent variables were temperature, autophagy inhibitor, and replicate. The statistical model considered all the main effects and all possible interactions. Differences between individual means were further analyzed by completing pair-wise comparisons (probability of difference analysis; SAS Institute, Inc.). Non-parametric data (HSPA5, PFKP, and XBP1 mRNA) were analyzed by the Kruskal–Wallis test. Differences of P < 0.05 were considered significant.
Results
Experiment 1: Autophagy induction on bovine oocytes and cumulus cells exposed to heat shock during IVM
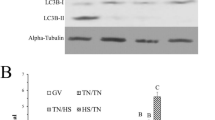
The abundance of LC3-I and -II were differently detected in cumulus cells and oocytes, demonstrating cellular specificity to stress response during exposure to heat shock (Fig. 2 and Supplementary Data). The LC3-II/LC3-I ratio was used to evaluate autophagy induction, as LC3-I is conjugated with phosphatidylethanolamine (PE) to become LC3-II, which is part of the autophagosome membrane28. On oocytes, exposure to heat shock during IVM increased (P < 0.05) LC3-II/LC3-I ratio compared to oocytes at 38.5 °C, indicating the induction of autophagy by heat shock. Autophagy inhibition with 3MA did not change oocyte LC3-II/LC3-I ratio regardless of temperature (Fig. 2B). On cumulus cells, there was no effect of temperature on LC3-II/LC3-I ratio. However, addition of 3MA at 38.5 and 41 °C reduced (P < 0.05) LC3-II/LC3-I ratio, demonstrating that the drug was able to decrease autophagy activity in cumulus cells (Fig. 2C).
Induction of autophagy on COCs submitted to heat shock during IVM. Representative western blotting images of single cropped blots for oocytes and cumulus cells showing both types of LC3 protein (Full-length blots are presented in Supplementary Fig. S1 online) (A). Quantification of LC3-II/LC3-I ratio on oocytes (B) and cumulus cells (C). Results are least-squares means ± SEM. Different letters in each bar represent significant difference (P < 0.05).
Experiment 2: The role of autophagy on developmental competence of heat-shocked bovine oocytes
In the absence of 3MA, heat shock (IVM-41 °C) did not affect cleavage rate (Fig. 3A). However, inhibition of autophagy reduced cleavage rate (P < 0.01) in heat-shocked oocytes (3MA-41 °C) when compared to all other groups (Fig. 3A). Moreover, the proportion of cleaved embryos was greater (P < 0.01) for oocytes matured in 0 mM 3MA than 10 mM 3MA at 38.5 °C (Fig. 3A). Bovine oocytes exposed to heat shock in the absence (IVM-41 °C; P < 0.05) or presence of 10 mM 3MA (3MA-41 °C; P < 0.01) reduced the proportion of oocytes that developed to the blastocyst stage at day 8 post-insemination compared with oocytes matured without 3MA at 38.5 °C (Fig. 3B). Addition of 10 mM 3MA at 38.5 and 41 °C reduced (P < 0.05) blastocyst rate compared to oocytes matured at the same temperature without 3MA, respectively. There was no effect of heat shock on the proportion of cleaved embryos that reached the blastocyst stage, regardless of 3MA (Fig. 3C). However, inhibition of autophagy reduced the proportion of cleaved embryos that developed to the blastocyst stage at 38.5 °C (P < 0.05) and 41 °C (P < 0.01) compared to oocytes matured in 0 mM 3MA at 38.5 °C (Fig. 3C).

adapted from Master’s Thesis83.
The impact of autophagy inhibition during IVM of heat-shocked bovine oocytes on the proportion of oocytes that cleaved (A) and developed to the blastocyst stage (B), and the proportion of cleaved embryos that developed to blastocyst (C). Results are least-squares means ± SEM. Different letters in each bar represent significant difference (P < 0.05). The figure was
Taken together, these results showed that autophagy activity during bovine oocyte maturation has a physiological role in early cleavage and development. Moreover, it has an important pro-survival effect in oocytes subjected to heat shock.
Experiment 3: Inhibition of autophagy affects gene expression of bovine oocytes exposed to heat shock
Exposure of bovine oocytes to heat shock in the presence of 10 mM 3MA (3MA-41 °C) reduced transcript abundance of oocyte HAS2 (P < 0.05; Fig. 4A), MTIF3 (P < 0.05; Fig. 4C), and HSF1 (P = 0.05; Fig. 4E) compared to heat-shocked oocytes in 0 mM 3MA (IVM-41 °C). There was a similar tendency for SREBF2 (P = 0.06, Fig. 4D) and HSPA1A (P = 0.06, Fig. 4E). GREM1 (P < 0.05; Fig. 4A) mRNA abundance were lower in heat-shocked oocytes matured in 10 mM 3MA (3MA-41 °C) than oocytes matured at 38.5 °C (3MA-38.5 °C). There was a tendency for similar pattern of expression for HSF1 (P = 0.06). Inhibition of autophagy during oocyte maturation at 38.5 °C (3MA-38 °C) reduced BMP15 (P < 0.05) and IGF2 (P < 0.05) mRNA abundance in relation to heat shocked oocytes without 3MA (IVM-41 °C; Fig. 4A, B). String analysis of the significant genes revealed that BMP15, HAS2, and GREM1 were frequently co-mentioned on publications (see Supplementary Fig. S2 online). There was no effect of temperature, autophagy inhibitor, and temperature x inhibitor interaction for all the other genes evaluated (see Supplementary Table S1 online). This reduced difference in oocyte mRNA abundance between groups was also evidenced in Heatmap analysis, where the majority of genes were similarly expressed in all groups (see Supplementary Fig. S3 online).

adapted from Master’s Thesis83.
Effect of autophagy inhibition during IVM of heat-shocked bovine oocytes on mRNA abundance of differentially expressed genes according to the following functional categories: oocyte maturation (A), oocyte competence to embryonic development (B), energy metabolism (C), lipid metabolism (D), and heat shock response (E). Results are least-squares means ± SEM. Different letters in each bar represent significant difference (P ≤ 0.05). †P = 0.06. The figure was
Discussion
This study demonstrated that the bovine oocyte was able to undergo autophagy after exposure to adverse conditions. Autophagy acted as a pro-survival response during oocyte maturation as the developmental capacity of heat-shocked oocytes was markedly reduced in the presence of the autophagy inhibitor. The negative effect of autophagy inhibition also extended to gene expression. For example, mRNA abundance for genes related to oocyte maturation, energy metabolism and heat shock response were reduced in heat-shocked oocytes.
Activation of autophagy is usually studied through gene expression and protein abundance analysis of molecules involved in the autophagy pathway. The conversion of LC3-I to LC3-II is used as an autophagy marker, since LC3-II is necessary for autophagosome formation28,47. The present study provided evidence that oocyte autophagy was induced by heat shock during IVM as LC3-II/LC3-I ratio was increased on heat-shocked oocytes compared to control. However, heat-induced increase in autophagosome formation was not observed on cumulus cells. These results indicate a divergence between oocytes and cumulus cells response to stress condition48,49. It is well known that the ability of the oocyte to respond to stress is reduced during maturation due to limited transcriptional capacity50. The oocyte relies on translation of stored mRNA to activate pathways to restore homeostasis51. On the other hand, cumulus cells are somatic cells fully able to respond to stress conditions. Therefore, autophagy activation may play a greater role as a protection strategy for oocytes than cumulus cells.
Class III phosphatidylinositol-3-kinase (PI3K) is a positive regulator of autophagy, stimulating the initiation and maturation of autophagosome52,53 and 3MA acts inhibiting class III PI3K. Incubation of bovine COCs with 10 mM 3MA for 22 h did not reduce oocyte LC3-II/LC3-I ratio at the end of maturation period. However, a reduction in LC3-II/LC3-I ratio was clearly observed in cumulus cells that surrounded the oocytes. Therefore, the inhibitory effect of 3MA on COCs autophagy may be occurring indirectly through cumulus cells, impairing cleavage and blastocyst development. It is well known that cumulus cells are essential for oocyte survival and mediate several biological events regulating oocyte growth and maturation54,55. It has been shown that incubation of bovine oocytes with 5 mM 3MA also did not reduce LC3-II/LC3-I ratio at the end of 24 h maturation56. It is possible that the lack of 3MA-induced drop LC3-II/LC3-I ratio is due to the kinetics of LC3-II expression in the oocyte. It has been demonstrated that oocyte LC3-II protein increases during oocyte maturation, reaching a peak at the middle of maturation, and decreasing after that until the end of maturation period26.
Exposure of oocytes to heat shock did not affect cleavage rate, but reduced development to the blastocyst stage. At least part of the deleterious effects of heat shock on oocyte function is mediated by cellular and molecular damage to the oocyte. For example, heat shock reduced mitochondrial activity36, caused cytoskeleton disorganization35,36, protein denaturation, mRNA degradation as well as apoptosis32,57,58,59. In the present study, inhibition of autophagy prone the oocyte more susceptible to heat shock reducing the ability to cleave and reach the blastocyst stage. Therefore, it is likely that autophagy exerted a protective mechanism during oocyte maturation under stress condition. Lack of autophagy through class III PI3K inhibition may have blocked another protective mechanism, such as heat shock response, preventing oocyte recycling of damaged organelles or protein to restore homeostasis.
Moreover, this study demonstrated that autophagy was also required to standard in vitro maturation since cleavage and blastocyst development were reduced upon autophagy inhibition at physiological temperature. Similar results were observed with autophagy manipulation during porcine oocyte maturation27,28, corroborating with the hypothesis that recycling of cellular compounds is a strategy used by the oocyte for proper maturation.
The importance of autophagy on clearance of maternal and paternal mRNA after fertilization is already known19,20,21,22,23. However, the role of autophagy on mRNA abundance of bovine oocytes is not fully understood60. During maturation, the oocyte relies on mRNA stored during oocyte growth phase in order to adapt and support early embryonic development. Therefore, it is likely that autophagy regulates mRNA abundance depending on the environmental condition. Indeed, inhibition of the autophagy pathway in heat-shocked oocytes affected mRNA abundance of HAS2, GREM1, MTIF3, and HSF1. Likewise, such alteration on mRNA abundance exerted downstream effects modulating oocyte developmental competence in the absence of autophagy.
Class III PI3K inhibition in heat-shocked COCs reduced HAS2 and GREM1 mRNA abundance, indicating that the ability of the COCs to produce hyaluronidase and undergo cumulus cells expansion could be compromised. In addition to the report of heat shock reduction on cumulus cells expansion of bovine COCs matured at 41 °C61, there is also evidence that autophagy pathway is involved in the regulation of this event62,63. Inhibition of poly (ADP-ribose) polymerase enzymes (PARPs) during maturation of porcine oocytes lead to HAS2 downregulation62. Therefore, autophagy inhibition might have reduced HAS2 mRNA abundance via PARPs. It has also been shown that GREM1 stimulates cumulus cell expansion and granulosa cell luteinization through bone morphogenic protein (BMP) regulation64,65. Consequently, reduction in HAS2 and GREM1 mRNA abundance in heat-shocked oocytes lacking the autophagic response could lead to poor oocyte maturation, reducing subsequent oocyte developmental ability.
Besides the effect on mRNA abundance of genes related to cumulus cells expansion, class III PI3K inhibition under heat shock also influenced energy balance during oocyte maturation. While MTIF3 expression was not affected by heat shock, autophagy inhibition under high temperature reduced MTIF3 expression. This gene encodes a translation initiation factor involved in mitochondrial protein synthesis66 required for energy production by oxidative phosphorylation67. Thus, a deficiency of oxidative phosphorylation due to alteration on MTIF3 expression may disrupt oocyte energy balance, impairing further embryonic development.
Heat-induced cellular pathways were also modulated in oocytes unable to activate the autophagic response, suggested by the reduced mRNA abundance of HSF1 in heat-shocked oocytes matured with class III PI3K inhibitor. This gene is a major player of the heat shock response (HSR) to maintain cytoplasmic protein homeostasis, as the induction of heat shock proteins (HSPs) following cellular stress is a conserved response preventing aggregation of denatured or misfolded proteins68. Heat shock transcription factor 1 (HSF1) is the major transcriptional regulator responsible for the expression of HSPs and it is highly expressed in oocytes69. Both autophagy and HSR can be activated during heat shock as protein management systems70. In this case, the lack of autophagy activity could induce a selective translation of HSR genes to reduce the damage caused by exposure to heat shock.
Interestingly, class III PI3K inhibition during maturation of bovine oocytes under physiological temperature (38.5 °C) reduced BMP15 and IGF2 mRNA abundance compared with heat-shocked oocytes without the inhibitor. BMP15 regulates differentiation of cumulus cells71,72. It seems like autophagy can regulate BMP15 mRNA abundance since it was increased with autophagy activation73, and decreased with autophagy inhibition, as demonstrated here. On the other hand, IGF2 is a growth factor identified on bovine oocytes74, and it improves bovine embryo production75, in addition to porcine oocyte nuclear maturation76. The alteration on both transcripts can be associated with the reduction of cleavage and blastocyst rate.
Several genes evaluated in this experiment were not affected by heat shock and/or autophagy inhibition. The resistance of heat-shocked bovine oocytes to changes in mRNA abundance during IVM has been demonstrated in previous studies46,77. For example, exposure of oocytes from Holstein and Nelore cows—breeds of contrasting heat tolerance—to 12 h heat shock during IVM had little effect on oocyte mRNA abundance investigated by large scale microarray46. Similar results were demonstrated by Payton et al.78. In contrast, germinal vesicle (GV) oocytes exposed to seasonal summer heat stress did not show resistance to modulate gene expression78,79,80. Oocyte DNA changes configuration during follicular development, demonstrated by different levels of chromatin condensation at GV stages81. The transcriptional and translational capacity of the oocyte is elevated from primordial to antral follicular stages, to store maternal mRNA for further oocyte and early embryonic development51. During either in vivo or in vitro oocyte maturation, the transcription rate is minimum as the chromatin condenses77,82. Therefore, the lack of change in gene expression observed for most of the genes studied is probably due to oocyte chromatin configuration during maturation.
In conclusion, this study provided novel evidence that autophagy is an adaptive response required for survival of heat-shocked oocytes, in addition to its importance during physiological maturation. It also revealed some potentially relevant mechanisms triggered by autophagy inhibition that lead to the deleterious effect of heat shock on oocyte developmental capacity.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mizushima, N. Autophagy: Process and function. Genes Dev.21, 2861–2873 (2007).
Wirawan, E. et al. Autophagy: for better or for worse. Cell Res.22, 43–61. https://doi.org/10.1038/cr.2011.152 (2012).
Yadav, P. K. et al. Germ cell depletion from mammalian ovary: Possible involvement of apoptosis and autophagy. J. Biomed. Sci.25, 36. https://doi.org/10.1186/s12929-018-0438-0 (2018).
Sumpter, R. Jr. & Levine, B. Autophagy and innate immunity: Triggering, targeting and tuning. Semin. Cell Dev. Biol.21, 699–711. https://doi.org/10.1016/j.semcdb.2010.04.003 (2010).
Yoshida, G. J. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: From pathophysiology to treatment. J. Hematol. Oncol.10, 67. https://doi.org/10.1186/s13045-017-0436-9 (2017).
Guillén, C. & Benito, M. mTORC1 veractivation as a key aging factor in the progression to type 2 diabetes mellitus. Front. Endocrinol.9, 621. https://doi.org/10.3389/fendo.2018.00621 (2018).
Bagherniya, M. et al. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. https://doi.org/10.1016/j.arr.2018.08.004 (2018).
Medeiros, T. C. & Graef, M. Autophagy determines mtDNA copy number dynamics during starvation. Autophagy.15, 178–179. https://doi.org/10.1080/15548627.2018.1532263 (2018).
Hale, B. J. et al. Heat stress induces autophagy in pig ovaries during follicular development. Biol. Reprod.97, 426–437. https://doi.org/10.1093/biolre/iox097 (2017).
Ganesan, S. et al. Short-term heat stress results in increased apoptotic signaling and autophagy in oxidative skeletal muscle in Susscrofa. J. Therm. Biol.72, 73–80. https://doi.org/10.1016/j.jtherbio.2018.01.003 (2018).
Li, Z., Li, Y., Zhou, X., Dai, P. & Li, C. Autophagy involved in the activation of the Nrf2-antioxidant system in testes of heat-exposed mice. J. Therm. Biol.71, 142–152. https://doi.org/10.1016/j.jtherbio.2017.11.006 (2018).
Kurz, T., Terman, A. & Brunk, U. T. Autophagy, ageing and apoptosis: The role of oxidative stress and lysosomal iron. Arch. Biochem. Biophys.462, 220–230 (2007).
Lee, J., Giordano, S. & Zhang, J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signaling. Biochem. J.441, 523–540 (2012).
Zuo, W. et al. Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: The role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology86, 103–115. https://doi.org/10.1016/j.neuropharm.2014.07.002 (2014).
Ferrucci, M. et al. Ambiguous effects of autophagy activation following hypoperfusion/ischemia. Int. J. Mol. Sci.19, 2756. https://doi.org/10.3390/ijms19092756 (2018).
Liu, J. et al. Aflatoxin B1 is toxic to porcine oocyte maturation. Mutagenesis30, 527–535. https://doi.org/10.1093/mutage/gev015 (2015).
Han, J. et al. Deoxynivalenol exposure induces autophagy/apoptosis and epigenetic modification changes during porcine oocyte maturation. Toxicol. Appl. Pharmacol.300, 70–76. https://doi.org/10.1016/j.taap.2016.03.006 (2016).
Yang, L.-L. et al. Toxic effects and possible mechanisms of hydrogen sulfide and/or ammonia on porcine oocyte maturation in vitro. Toxicol. Lett.285, 20–26. https://doi.org/10.1016/j.toxlet.2017.12.019 (2018).
Sato, M. & Sato, K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science334, 1141–1144 (2011).
Sato, M. & Sato, K. Maternal inheritance of mitochondrial DNA: Degradation of paternal mitochondria by allogeneic organelle autophagy, allophagy. Autophagy.8, 424–425. https://doi.org/10.4161/auto.19243 (2012).
Tsukamoto, S. & Tatsumi, T. Degradation of maternal factors during preimplantation embryonic development. J. Reprod. Dev.64, 217–222. https://doi.org/10.1262/jrd.2018-039 (2018).
Tsukamoto, S. et al. The role of autophagy during the oocyte-to-embryo transition. Autophagy.4, 1076–1078. https://doi.org/10.4161/auto.7065 (2008).
Xu, Y. N. et al. Autophagy influences maternal mRNA degradation and apoptosis in porcine parthenotes developing in vitro. J. Reprod. Dev.58, 576–584. https://doi.org/10.1262/jrd.2012-005 (2012).
Tsukamoto, S. et al. Autophagy is essential for preimplantation development of mouse embryos. Science4, 1076–1078 (2008).
Escobar, M. L. et al. Analysis of different cell death processes of prepubertal rat oocytes in vitro. Apoptosis15(4), 511–526. https://doi.org/10.1007/s10495-009-0448-1 (2010).
Lee, S. et al. Quantitative analysis in LC3-II protein in vitro maturation of porcine oocyte. Zygote.22, 404–410. https://doi.org/10.1017/S0967199413000269 (2013).
Song, B. S. et al. Induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes. Reprod. Fertil. Dev.26, 974. https://doi.org/10.1071/RD13106 (2014).
Shen, X.-H. et al. Autophagy is required for proper meiosis of porcine oocytes maturing in vitro. Sci. Rep.8, 12581. https://doi.org/10.1038/s41598-018-29872-y (2018).
Sartori, R. et al. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J. Dairy Sci.85, 2803–2812. https://doi.org/10.3168/jds.S0022-0302(02)74367-1 (2002).
Paula-Lopes, F. F. et al. Heat stress induced alteration in bovine oocytes: Functional and cellular aspects. Anim. Reprod.9, 395–403 (2012).
Ju, J. C. & Tseng, J. K. Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol. Reprod. Dev.68, 125–133. https://doi.org/10.1002/mrd.20054 (2004).
Roth, Z. & Hansen, P. J. Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol. Reprod.71, 1898–1906. https://doi.org/10.1095/biolreprod.104.031690 (2004).
Tseng, J. K. et al. Influences of follicular size on parthenogenetic activation and in vitro heat shock on the cytoskeleton in cattle oocytes. Reprod. Domest. Anim.39, 146–153. https://doi.org/10.1111/j.1439-0531.2004.00493.x (2004).
Ju, J. C. et al. Heat shock reduces developmental competence and alters spindle configuration of bovine oocytes. Theriogenology64, 1677–1689. https://doi.org/10.1016/j.theriogenology.2005.03.025 (2005).
Roth, Z. & Hansen, P. J. Disruption of nuclear maturation and rearrangement of cytoskeletal elements in bovine oocytes exposed to heat shock during maturation. Reproduction129, 235–244. https://doi.org/10.1530/rep.1.00394 (2005).
Rodrigues, T. A. et al. Thermoprotective effect of insulin-like growth factor 1 on in vitro matured bovine oocyte exposed to heat shock. Theriogenology86, 2028–2039. https://doi.org/10.1016/j.theriogenology.2016.06.023 (2016).
Rodrigues, T. A. et al. Follicular fluid exosomes act on the bovine oocyte to improve oocyte competence to support development and survival to heat shock. Reprod. Fertil. Dev.31(5), 888–897. https://doi.org/10.1071/RD18450 (2019).
Park, M. R. et al. Possible involvement of Class III phosphatidylinositol-3-kinase in meiotic progression of porcine oocytes beyond germinal vesicle stage. Theriogenology75(5), 940–950. https://doi.org/10.1016/j.theriogenology.2010.11.002 (2011).
Leibfried, L. & First, N. L. Characterization of bovine follicular oocytes and their ability to mature in vitro. J. Anim. Sci.48, 76–86. https://doi.org/10.2527/jas1979.48176x (1979).
Rivera, R. M. & Hansen, P. J. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction121, 107–115 (2001).
Cavallari, F. C. et al. Effects of melatonin on production of reactive oxygen species and developmental competence of bovine oocytes exposed to heat shock and oxidative stress during in vitro maturation. Zygote27, 180–186. https://doi.org/10.1017/S0967199419000236 (2019).
Ferder, I. C. et al. Meiotic gatekeeper STRA8 suppresses autophagy by repressing Nr1d1 expression during spermatogenesis in mice. PLoS Genet. 155, e10080841–e10080929. https://doi.org/10.1371/journal.pgen.1008084 (2019).
Parrish, J. J. et al. Capacitation of bovine sperm by heparin. Biol. Reprod.38, 1171–1180. https://doi.org/10.1095/biolreprod38.5.1171 (1988).
Razza, E. M. et al. Treatment with cyclic adenosine monophosphate modulators prior to in vitro maturation alters the lipid composition and transcript profile of bovine cumulus-oocyte complexes and blastocysts. Reprod. Fertil. Dev.30(10), 1314–1328. https://doi.org/10.1071/RD17335 (2018).
Fontes, P. K. et al. Bona fide gene expression analysis of samples from the bovine reproductive system by microfluidic platform. Anal. Biochem.596, 113641. https://doi.org/10.1016/j.ab.2020.113641 (2020).
Ticianelli, J. S. et al. Gene expression profile in heat-shocked Holstein and Nelore oocytes and cumulus cells. Reprod. Fertil. Dev.29, 1787. https://doi.org/10.1071/RD16154 (2016).
Lin, F. H. et al. Role of autophagy in modulating post-maturation aging of mouse oocytes. Cell Death Dis.9(3), 308. https://doi.org/10.1038/s41419-018-0368-5 (2018).
Saadeldin, I. M. et al. Differences between the tolerance of camel oocytes and cumulus cells to acute and chronic hyperthermia. J. Therm. Biol.74, 47–54. https://doi.org/10.1016/j.jtherbio.2018.03.014 (2018).
Yin, C. et al. Heat stress induces distinct responses in porcine cumulus cells and oocytes associated with disrupted gap junction and trans-zonal projection colocalization. J. Cell. Physiol.234(4), 4787–4798 (2018).
Hyttel, P. et al. Oocyte growth, capacitation and final maturation in cattle. Theriogenology47, 23–32. https://doi.org/10.1016/S0093-691X(96)00336-6 (1997).
Roth, Z. Effect of heat stress on reproduction in dairy cows: Insights into the cellular and molecular responses of the oocyte. Annu. Rev. Anim. Biosci.5, 151–170. https://doi.org/10.1146/annurev-animal-022516-022849 (2016).
Vinod, V. et al. “How can I halt thee?” The puzzles involved in autophagic inhibition. Pharmacol. Res.82, 1–8. https://doi.org/10.1016/j.phrs.2014.03.005 (2014).
Ravanan, P. et al. Autophagy: The spotlight for cellular stress responses. Life Sci.188, 53–67. https://doi.org/10.1016/j.lfs.2017.08.029 (2017).
Coticchio, G. et al. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update.21, 427–454. https://doi.org/10.1093/humupd/dmv011 (2016).
Lonergan, P. & Fair, T. Maturation of oocytes in vitro. Annu. Rev. Anim. Biosci.4, 255–268. https://doi.org/10.1146/annurev-animal-022114-110822 (2016).
Xu, D. et al. SIRT2 functions in aging, autophagy, and apoptosis in post-maturation bovine oocytes. Life Sci.232, 116639. https://doi.org/10.1016/j.lfs.2019.116639 (2019).
Castells-Roca, L. et al. Heat shock response in yeast involves changes in both transcription rates and mRNA stabilities. PLoS ONE6(2), e17272. https://doi.org/10.1371/journal.pone.0017272 (2011).
Fang, N. N., Zhu, M., Rose, A., Wu, K. P. & Mayor, T. Deubiquitinase activity is required for the proteasomal degradation of misfolded cytosolic proteins upon heat-stress. Nat. Commun7, 12907 (2016).
Zander, G. & Krebber, H. Quick or quality? How mRNA escapes nuclear quality control during stress. RNA Biol.14(12), 1642–1648. https://doi.org/10.1080/15476286.2017.1345835 (2017).
Lee, S. E. et al. Rapamycin rescues the poor developmental capacity of aged porcine oocytes. Asian-Austral. J. Anim. Sci.27(5), 635–647. https://doi.org/10.5713/ajas.2013.13816 (2014).
Ahmed, J. A., Nashiruddullah, N., Dutta, D., Biswas, R. K. & Borah, P. Cumulus cell expansion and ultrastructural changes in in vitro matured bovine oocytes under heat stress. Iran. J. Vet. Res.18, 203–207 (2017).
Kim, D. H. et al. The effect of poly(ADP-ribosyl)ation inhibition on the porcine cumulus-oocyte complex during in vitro maturation. Biochem. Biophys. Res. Commun.483, 752–758 (2016).
Chen, Z. et al. Effects of melatonin on maturation, histone acetylation, autophagy of porcine oocytes and subsequent embryonic development. Anim. Sci. J.88(9), 1298–1310. https://doi.org/10.1111/asj.12779 (2017).
Pangas, S. A., Jorgez, C. J. & Matzuk, M. M. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J. Biol. Chem.279, 32281–32286 (2004).
Assidi, M. et al. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol. Reprod.79, 209–222. https://doi.org/10.1095/biolreprod.108.067686 (2008).
Kuzmenko, A. et al. Mitochondrial translation initiation machinery: Conservation and diversification. Biochimie100, 132–140. https://doi.org/10.1016/j.biochi.2013.07.024 (2014).
Christian, B. E. & Spremulli, L. L. Evidence for an active role of IF3mtin the initiation of translation in mammalian mitochondria. Biochemistry48, 3269–3278 (2009).
Garbuz, D. G. Regulation of heat shock gene expression in response to stress. Mol. Biol.51, 352–367. https://doi.org/10.7868/S0026898417020100 (2017).
Metchat, A. et al. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates Hsp90alpha expression. J. Biol. Chem.284, 9521–9528 (2009).
Dokladny, K. et al. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J. Biol. Chem.288, 14959–14972 (2013).
Caixeta, E. S. et al. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction146, 27–35. https://doi.org/10.1530/REP-13-0079 (2013).
Machado, M. F. et al. Fibroblast growth factor 17 and bone morphogenetic protein 15 enhance cumulus expansion and improve quality of in vitro produced embryos in cattle. Theriogenology84, 390–398. https://doi.org/10.1016/j.theriogenology.2015.03.031 (2015).
Chi, D. et al. LC3-dependent autophagy in pig 2-cell cloned embryos could influence the degradation of maternal mRNA and the regulation of epigenetic modification. Cell. Reprogram.19(6), 354–362. https://doi.org/10.1089/cell.2017.0016 (2017).
Yoshida, Y. et al. Expression of growth factor ligand and their receptor mRNAs in bovine ova during in vitro maturation and after fertilization in vitro. J. Vet. Med. Sci.60, 549–554. https://doi.org/10.1292/jvms.60.549 (1988).
Byrne, A. T. et al. Regulation of apoptosis in the bovine blastocyst by insulin and the insulin-like growth factor (IGF) superfamily. Mol. Reprod. Dev.62(4), 489–495. https://doi.org/10.1002/mrd.10153 (2002).
Sirotkin, A. V. et al. Evidence that growth factors IGF-I, IGF-II and EGF can stimulate nuclear maturation of porcine oocytes via intracellular protein kinase A. Reprod. Nutr. Dev.40, 559–569. https://doi.org/10.1051/rnd:2000137 (2000).
Payton, R. R. et al. Impact of heat-stress exposure during meiotic maturation on oocyte, surrounding cumulus cell, and embryo RNA populations. J. Reprod. Dev.57, 481–491. https://doi.org/10.1262/jrd.10-163m (2011).
Gendelman, M. & Roth, Z. In vivo vs in vitro models for studying the effects of elevated temperature on the GV-stage oocyte, subsequent developmental competence and gene expression. Anim. Reprod. Sci.134, 125–134. https://doi.org/10.1016/j.anireprosci.2012.07.009 (2012).
Gendelman, M. & Roth, Z. Incorporation of coenzyme Q10 into bovine oocytes improves mitochondrial features and alleviates the effects of summer thermal stress on developmental competence. Biol. Reprod.87(5), 118. https://doi.org/10.1095/biolreprod.112.101881 (2012).
Ferreira, R. M. et al. The infertility of repeat-breeder cows during summer is associated with decreased mitochondrial DNA and increased expression of mitochondrial and apoptotic genes in oocytes. Biol. Reprod.94, 1–10. https://doi.org/10.1095/biolreprod.115.133017 (2016).
Lodde, V. et al. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol. Reprod. Dev.74, 740–749. https://doi.org/10.1002/mrd.20639 (2007).
Payton, R. R. et al. General features of certain RNA populations from gametes and cumulus cells. J. Reprod. Dev.56, 583–592. https://doi.org/10.1262/jrd.10-007a (2010).
Latorraca, L.B. Oocyte response to heat stress. Master Thesis in Pharmacology and Biotechnology—São Paulo State University, Institute of Biosciences. https://hdl.handle.net/11449/181505 (2019).
Acknowledgements
This research was supported by São Paulo Research Foundation (FAPESP; 2012/50533-2 and 2017/13082-6) and Coordination for the Improvement of Higher Level Education—Personnel (CAPES)—Program Attraction of Young Talents (CSF-PAJT—88887.068701/2014-00). The authors thank Drª Flávia R. O. Barros for her contribution to the gene expression experiment. This manuscript has been adapted from Ms. Latorraca Master’s Thesis83.
Author information
Authors and Affiliations
Contributions
L.B.L.: conducted in vitro embryo production, western blotting analysis, statistics, and prepared the manuscript. W.B.F.: designed the experiments, and collected oocytes for gene expression analysis. C.M.: collected oocytes for gene expression analysis. M.T.M.: conducted in vitro embryo production. P.K.F. and M.F.G.N.: conducted gene expression analysis. F.F.P.-L.: coordinated the studies, designed the experiments, run statistical analysis, and prepared the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Latorraca, L.B., Feitosa, W.B., Mariano, C. et al. Autophagy is a pro-survival adaptive response to heat shock in bovine cumulus-oocyte complexes. Sci Rep 10, 13711 (2020). https://doi.org/10.1038/s41598-020-69939-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69939-3
- Springer Nature Limited