Abstract
Obstructive sleep apnea (OSA) is associated with increasing risk of recurrent stroke and mortality. Nasogastric tubes used by dysphagic stroke patients may interfere with nasal continuous positive airway pressure (CPAP) due to air leakage. This study was evaluated the effects and short-term tolerability of high-flow nasal cannula (HFNC) therapy for OSA in stroke patients with nasogastric intubation. The HFNC titration study was performed in post-acute ischemic stroke patients with nasogastric intubation and OSA. Then, participants were treated with HFNC therapy in the ward for one week. Eleven participants (eight males) who were all elderly with a median age of 72 (IQR 67–82) years and a body mass index of 23.5 (IQR 22.0–26.6) completed the titration study. The HFNC therapy at a flow rate up to 50~60 L/min significantly decreased the apnea-hypopnea index from 52.0 events/h (IQR 29.9–61.9) to 26.5 events/h (IQR 3.3–34.6) and the total arousal index from 34.6 (IQR 18.6–42.3) to 15.0 (IQR 10.3–25.4). The oxygen desaturation index was also significantly decreased from 53.0 events/h (IQR 37.0–72.8) to 16.2 events/h (IQR 0.8–20.1), accompanied by a significant improvement in the minimum SpO2 level. Finally, only three participants tolerated flow rates of 50~60 L/minute in one-week treatment period. Conclusively, HFNC therapy at therapeutic flow rate is effective at reducing the OSA severity in post-acute ischemic stroke patients with nasogastric intubation. Owing to the suboptimal acceptance, HFNC might be a temporary treatment option, and CPAP therapy is suggested after the nasogastric tube is removed.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive collapse of the pharyngeal airway during sleep, resulting in intermittent hypoxemia that is usually terminated by arousal from sleep, causing fragmentation of normal sleep1. Moderate-to-severe OSA was found to significantly increase cardiovascular risk, especially the risk of fatal or nonfatal stroke (relative risk, 2.02; 95% confidence interval 1.4–2.9) in a meta-analysis study2. Additionally, OSA has a negative effect on stroke outcome by worsening functional recovery and increasing the risk of recurrent stroke and mortality3 which is improved by proper treatment of OSA4. The most effective treatment for OSA is continuous positive airway pressure (CPAP). However, the compliance to CPAP in stroke patients was poor5, and the effects of CPAP on cerebral hemodynamics are uncertain6,7. CPAP has been reported to decrease cerebral blood flow velocity in acute stroke patients8. In addiction, the use of nasal CPAP has been reported to inhibit swallowing function9 and increase the risk of aspiration pneumonia10. Therefore, the benefits of CPAP in dysphagic stroke patients with OSA are questionable. Furthermore, nearly 30% of stroke patients were initially found to have dysphagia11, and most of them using nasogastric tube for nutrition support. The nasogastric intubation may cause air leakage during noninvasive ventilation therapy by nasal or oronasal masks12. Although CPAP by oral mask for OSA has been reported in some small-scale studies to be as effective as CPAP by nasal mask13,14, oral masks are not popular in clinical practice15 and have not been studied in stroke patients. Therefore, looking for alternative treatments for OSA in dysphagic stroke patients requiring nasogastric tube feeding is warranted.
High-flow nasal cannula (HFNC) oxygen therapy delivers adequately heated and humidified medical gas up to a flow rate of 60 L/minute and has the following physiological effects: anatomical dead space reduction, positive end-expiratory pressure (PEEP) effect, constant fraction of inspired oxygen, and adequate humidification16. By adequately conditioning the gas, HFNC ventilation therapy may enhance mucociliary function and accelerate the clearance of secretions16, which is an important concern for dysphagic stroke patients. The positive effect of HFNC therapy for OSA has been found in some small-scale studies17,18,19,20,21,22,23. McGinley et al. found that HFNC therapy decreased OSA severity in both adults21 and children20. In addition, the 2-week compliance of HFNC therapy for OSA was similar to that of CPAP therapy23. In a preliminary study, HFNC therapy was well tolerated and moderately decreased the apnea-hypopnea index (AHI) and the oxygen desaturation index (ODI) in 10 acute stroke patients22. The patients recruited in their study were all men with a relatively young age (56.8 ± 10.7 y/o) and mild stroke severity (HIHSS, 4 ± 4.3). In addition, central sleep apnea (CSA), which is usually noted in patients with acute stroke with unclear clinical significance and improve over time3, was not excluded in their study. Therefore, their results cannot be generalized to more severe stroke patients with OSA. Furthermore, a low flow rate of 18 L/min was selected to guarantee comfort, and a higher flow rate was suggested to yield a better reduction in sleep apnea severity22.
The main objective of this study was to evaluate the effects and short-term tolerability of HFNC therapy at the therapeutic flow rate for OSA in dysphagic stroke participants with nasogastric intubation.
Materials and Methods
Participants
This study recruited post-acute (one week after the stroke onset) dysphagic ischemic stroke patients, with nasogastric intubation admitted consecutively to the Rehabilitation ward of a teaching hospital. The age of enrolled patient was above 20 years old. After a full clinical examination and neuroimaging studies, they were diagnosed with ischemic stroke and the diagnosis of dysphagia was made by a speech pathologist. Participants with congestive cardiac failure, severely compromised consciousness, chronic obstructive pulmonary disease, history of intracranial hemorrhage or malignance, and unstable medical and neurological conditions such as severe infection or uncontrolled diabetes mellitus were excluded from this study. Participants with CSA or other sleep disordered breathing were also excluded.
The study protocol (Fig. 1) was approved by the Institutional Review Board of Chang Gung Medical Foundation. The committee that approved the research confirmed that all methods were performed in accordance with relevant guidelines and regulations, and that informed consent was obtained from all participants and/or their legal guardians. This study was registered in the WHO International Clinical Trial Registry Platforms with ID number of NCT04173767 on 22/11/2019.
Clinical evaluation
Upon admission, a comprehensive history of stroke risk factors and demographic data was taken. Body mass index and the Barthel Index (BI)24 were measured on the same day as polysomnography (PSG) examination. The BI was used to evaluate the functional outcomes in stroke patients and might represent stroke severity.
Polysomnography
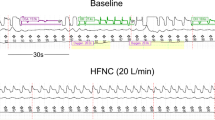
The PSG was tested with a Compumedics Profusion system (Australia). It was conducted at the sleep center from 10:00 pm to 7:00 am. The American Academy of Sleep Medicine (AASM) scoring manual version 2.0.3 was used to diagnose sleep apnea25. The PSG comprised six electroencephalography channels, thoracic and abdominal movement sensors (inductance plethysmography), an oxyhemoglobin saturation detector (finger pulse oximetry), a chin and bilateral anterior tibial surface electromyogram, an electro-oculogram, an electrocardiogram and nasal and oral airflow sensors (nasal pressure cannula and oronasal thermistor). Because the nasal and oral airflow sensors are unreliable while on HFNC, the respiratory inductance plethysmography sum (RIPsum) without calibration was used for scoring apnea and hypopnea events in accordance with AASM’s alternative criteria. Apnea was defined as a peak signal excursion drop ≥ 90% for at least 10 seconds; hypopnea was defined as a reduction in the peak signal excursion ≥ 30% for ≥ 10 seconds with either an arousal or oxygen desaturation ≥ 3%; and the number of times per hour of sleep that the blood’s oxygen saturation level drops by ≥ 3% from baseline was defined as the oxyhemoglobin desaturation index (ODI). As the RIPsum method tend to underestimate apnea events, especially the obstructive apnea events26, the AASM mentioned that previous studies27,28 using this method usually analyzed the combination of hypopneas and apneas29. Therefore, this study did not analyze apneas and hypopneas separately. The diagnosis of OSA was made when >50% of respiratory events were obstructive or mixed type in PSG. The diagnosis of CSA was made when ≥50% of respiratory events were the central type. One author, a board-certified somnologist, visually scored the PSGs.
High-flow nasal cannula ventilation titration
Once OSA was diagnosed, a HFNC ventilation titration study was performed within two weeks in the sleep center with a simultaneously PSG study. An air compressor (AIRVOTM 2, Fisher & Paykel Healthcare, Auckland, New Zealand) delivered room air at a high flow rate through the nasal cannula (OptiflowTM system). A delivery heating tube (900PT500) and humidifier included in the AIRVOTM2 device maintained a temperature of 37 °C and absolute humidity of 44 mg/L. The rule of flow rate adjustment is similar to CPAP titration guideline30 (Fig. 2). The initial flow rate was 20 L/minute and FiO2 was maintained at room air level of 21% and was then titrated gradually in 10 L/min increments when more than two apnea, three hypopnea, or three minutes of loud or unambiguous snoring were found. The time interval between each increase in the flow rate was at least five min. AIRVOTM 2 has a maximum flow rate of 60 L/minute. The titration continued until reach therapeutic flow rate, when the maximum flow was reached or the participant could not tolerate it or no respiratory events (≥2 obstructive apneas, or ≥ 3 hypopneas, or ≥ 3 min of loud or unambiguous snoring) were noted. After the therapeutic flow rate was reached, OSA severity and sleep parameters were measured.
High-flow nasal cannula ventilation therapy
The next day after the titration study, participants began the HFNC treatment for one week in the ward. The HFNC flow rate was kept constant without ramping every night and the beginning flow rate on the first night was set at 20 L/minute. On the following nights, the flow rate was increased in 10 L/minute increments per night if participants could sleep with HFNC more than four hours until the therapeutic flow rate determined by the titration study was reached. The reason why participants could not tolerate HFNC for more than four hours would be recorded and corrected if possible and the flow rate will be kept in the last tolerate flow rate.
Statistical analysis
Data are shown as the median and interquartile range (IQR), if not otherwise specified. The Wilcoxon Signed Ranks test was performed to compare differences in polysomnographic indices between the diagnostic and HFNC titration studies. Statistical power analyses with an effect size of 1, 2-tailed α of 0.05, and power of 0.8 were conducted, using G-Power version 3.1.9.5 software (Heinrich-Heine-University, Düsseldorf, Germany), which resulted in 11 subjects. As participants need nasogastric tube feeding, the expected flow rate of HFNC therapy to achieve therapeutic effect would be higher than that in subjects without nasogastric intubation. Considering a possible 33% drop-out rate because of intolerance to HFNC therapy22, we decided to recruit at least 17 patients. Good response to HFNC therapy was defined as an AHI decrease of more than 50% of the baseline AHI. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (IBM SPSS Statistics 25, SPSS Inc., Chicago, IL).
Results
There were 22 participants enrolled in this study. However, seven of them refused the HFNC titration study due to discomfort during the initial PSG study and two of them were excluded due to CSA or sleep-related hypoventilation due to a medical disorder diagnosed by the initial PSG study. One participant was excluded because of the normal PSG report. Twelve participants received a HFNC titration study, but one of them showed very poor sleep quality in the titration test, which was terminated prematurely without useful results.
The remaining 11 participants (8 males and 3 females) who completed the titration study were all elderly with a median age of 72 (IQR: 67 to 82) years and a BMI of 23.5 (IQR: 22.0 to 26.6). They were severely disabled31 with a Barthel index of 20 (IQR: 10 to 35) and were in the post-acute stroke phase at an average of 1.3 months (IQR: 1.1 to 2.8) after stroke onset. Brainstem stroke was found in only one of them. Severe OSA with an AHI of 52.0 events/h (IQR 29.9 to 61.9)) and sleep efficiency of 68.6% (IQR 39.7 to 76.2) was found in the initial PSG study. The final flow rate of the titration study was 60 L/min in eight participants and 50 L/min in three participants.
The results of initial PSG and HFNC therapy at the therapeutic flow rate are shown in Table 1. After using HFNC therapy at the therapeutic flow rate, the AHI significant decreased to 26.5 events/h (IQR 3.3 to 34.6), with a reduction rate of 49%. The ODI significantly decreased from 53.0 events/h (IQR 37.0 to 72.8) to 16.2 events/h (IQR 0.8 to 20.1), with a reduction rate of 69.4%, which was accompanied by a significant improvement in the minimum SpO2 level. Despite the significant reduction in the respiratory and total arousal index, the sleep architecture parameters including sleep efficiency did not change significantly after HFNC therapy.
However, the effect of HFNC therapy on OSA severity was heterogeneous among participants, as shown in Fig. 3. Six (55%) out of 11 participants were good responders with more than a 50% reduction in the AHI after HFNC therapy. The polysomnographic, demographic, and anthropometric differences between good and poor responders are shown in Table 2. The good responders tended to have a higher AHI (58.8 events/h (IQR 45.4 to 69.9) vs 37.3 events/h (IQR 24.8 to 53.9)) compared to the poor responders. Otherwise, they were similar with respect to the Barthel index, BMI, SpO2 level, and sleep efficiency.
Three of the 11 participants did not complete the 7-day therapy course due to intolerance. The final flow rate at the 7th therapy night was 30 L/min (3 participants), 40 L/min (2 participants), 50 L/min (2 participants) and 60 L/min (1 participant).
Discussion
To the best of our knowledge, this is the first study to evaluate the effect of HFNC therapy for OSA on post-acute ischemic stroke patients with nasogastric intubation and HFNC therapy at the therapeutic flow rate significantly improved OSA severity. One major strength of this study is that it tested the acceptance of HFNC therapy in the ward setting for one week. Although HFNC therapy by ramping the flow rate gradually up to 50~60 L/minute decreases arousals and does not deteriorate sleep efficiency in a well-controlled sleep center, its tolerability in the ward setting is suboptimal, and further study to improve patient acceptance is indicated.
HFNC therapy at the therapeutic flow rate effectively reduces the OSA severity. Previous studies suggested that the possible mechanism of HFNC therapy for OSA is the alleviation of upper airway obstruction by increasing nasopharyngeal pressure16,21. There is no absolute contraindication of HFNC therapy16. However, the influence of nasogastric intubation on the application of HFNC therapy has not yet been reported. The nasal cavity was found to account for two-thirds of the total airway resistance32. Nasogastric intubation was found to significantly increase nasal resistance and total airway resistance in infants33. The relatively large volume of the nasogastric tube to the volume of the nasal cavity might be the main reason that a higher flow rate up to 50~60 L/min is required to significantly improve OSA severity. The 49% reduction in the overall AHI at an therapeutic flow rate is nearly threefold the night-to-night variability in OSA severity of 13%34 and is clearly better than previous studies on normal subjects18 at 20 L/min flow rate (23.9% reduction, from 22.6 ± 15.6 to 17.2 ± 13.2 events/h) and male acute stroke patients without nasogastric intubation22 at 18 L/min flow rate (23.8% reduction, from 40.4 ± 25.7 to 30.8 ± 25.7 events/h). Despite the general therapeutic effect of HFNC therapy observed in this study, the individual response is heterogeneous, and stroke patients with more severe OSA tend to respond better to HFNC therapy. Previous studies in children19 and middle-aged adults18 suggested that hypopnea-predominant OSA responded better to HFNC therapy. Nilius et al. recruited 54 middle-aged, male-predominant adults with a mean BMI of 28 and moderate hypopnea-predominant OSA and found that the response rate was associated with a greater hypopnea rate in a dose-dependent fashion18. Given the employment of the RIPsum method, results of this study did not include separate analysis of hypopnea and apnea events and cannot be compared with their findings18.
The degree of intermittent hypoxia due to OSA is significantly improved after HFNC therapy. In our study, the improvement in the ODI was more obvious than the improvement in the AHI (the reduction rates of the ODI and AHI were 69.4% and 49%, respectively). Our results are in agreement with previous studies in acute stroke patients, which found that HFNC therapy at an 18 L/minute flow rate decreased the ODI moderately from 40.7 ± 28.4 to 31 ± 22.5 events/h, thus a 23.8% reduction in the ODI, while the mean SpO2 level remained unchanged22. In addition, the minimum SpO2 level was significantly improved from 78.0% (IQR 74.0 to 80.0) to 88.0% (IQR 82.0 to 92.0) after HFNC therapy. Therefore, HFNC therapy at a high flow rate above 50 L/min not only improved the frequency of oxygen desaturation but also the severity of oxygen desaturation. The minimum SpO2 level was found to be independently associated with interleukin-6 and C-reactive protein levels in OSA patients35. Nocturnal hypoxia is associated with atrial fibrillation (Af)36, neurological deterioration37 and cerebral white matter hyperdensities38 in stroke patients. Reducing the intermittent hypoxia in dysphagic stroke patients with OSA using HFNC therapy may improve comprehensive stroke treatment.
HFNC therapy with a gradually increasing flow rate up to 50~60 L/min performed at the sleep center decreases arousal events and does not deteriorate sleep architecture and efficiency in post-stroke dysphagic patients with OSA. Previous studies of middle-aged adults23 with flow rates up to 35 L/min, acute stroke patients22 with flow rates of 18 L/min and children19 with flow rates up to 50 L/min also found that HFNC therapy did not deteriorate sleep efficiency. The effect of HFNC on arousal is inconclusive as two studies using a relatively low flow rate (1822 and 2021 L/min) reported a decrease in the arousal index and one study using a relatively high flow rate (3523 L/min) reported the same arousal index. Although the discomfort due to the high airflow rate slightly increased the spontaneous arousal index in this study, the respiratory and total arousal index was significantly decreased by HFNC therapy which is in agreement with studies recruiting acute stroke patients22.
Although HFNC therapy by ramping the flow rate gradually up to 50~60 L/minute does not deteriorate sleep efficiency in a well-controlled sleep center, its acceptance in the ward setting is suboptimal. Therefore, the significant therapeutic effect of HFNC in the sleep center cannot be generalized to the ward setting. Most studies focusing on HFNC therapy for OSA evaluated only one-night therapeutic effects and tolerability18,19,21,22 and very few studies reported short-term adherence. Sowho et al. compared the 2-wk adherence of home-based CPAP and HFNC therapy at flow rates ranging from 10–35 L/min for mild OSA adults and found that the average HFNC therapy usage time was 3.6 ± 1.6 h/night and the percentage of nights with HFNC usage more than 4 h was approximately 50%23. Amaddeo et al. studies 8 children with OSA and found poor one-month compliance in three (60%) out of five children who received HFNC at 20 L/min17. In this study, three (27.2%) out of 11 participants could not complete a 7-day treatment course because of nasal congestion, noise and difficulty initiating sleep. The nasal congestion might be related to the humidity and temperature setting of the HFNC therapy. Only two participants were treated at 50 L/min and one participant was treated at 60 L/min on the 7th night of HFNC therapy. Difficulty in initiating sleep was noted by others because the flow rate at the beginning of therapy was too high to tolerate. However, it requires many resources and is impractical to manually ramp up the flow rate of HFNC gradually and adjust the corresponding temperature and humidity of the gas in the ward according to participants’ response every night. The development of an overnight HFNC ramping protocol that automatically controls the flow rate, humidity and temperature is suggested to improve the patient’s tolerance to a high flow rate in the ward setting or even in the home setting.
There are several limitations of this study. First, the small sample size in this preliminary study makes further analysis of predictors for treatment response such as stroke topography or etiology difficult. Most studies regarding HFNC therapy for OSA were small-scale. Further studies with larger sample sizes are suggested to elucidate this issue. Second, the apnea and hypopnea events were not analyzed separately because of the employment of RIPsum method in this study. Caution should be taken in direct comparison between studies using different sensors for airflow detection. Third, participants in this study were highly selected, which made its generalizability to ischemic stroke patients with different stroke topography, etiology and severity limited. Lastly, the potential bias for underscoring PSG in HFNC titration study might happened since titration was performed and scored by one of our authors.
Conclusion
HFNC therapy at therapeutic flow rate is effective at reducing OSA severity in post-acute ischemic stroke patients fed with a nasogastric tube, especially when the severity of OSA is high. Owing to the suboptimal acceptance, HFNC therapy might be a temporary treatment option for OSA, and nasal CPAP therapy is suggested after dysphagia improves and nasogastric intubation is removed. In addition, the development of an automatic ramping protocol for HFNC therapy is recommended to improve therapeutic effect.
References
Eckert, D. J. & Malhotra, A. Pathophysiology of Adult Obstructive Sleep Apnea. Proc. A Thorac. Soc. 5, 144–153 (2008).
Dong, J.-Y., Zhang, Y.-H. & Qin, L.-Q. Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Atherosclerosis 229, 489–495, https://doi.org/10.1016/j.atherosclerosis.2013.04.026 (2013).
Khot, S. P. & Morgenstern, L. B. Sleep and Stroke. Stroke 50, 1612–1617, https://doi.org/10.1161/STROKEAHA.118.023553 (2019).
Lipford, M. C., Park, J. G. & Ramar, K. Sleep-disordered breathing and stroke: therapeutic approaches. Curr. Neurol. Neurosci. Rep. 14, 431, https://doi.org/10.1007/s11910-013-0431-7 (2014).
Hsu, C. Y. et al. Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure. J. Neurol. Neurosurg. Psychiatry 77, 1143–1149 (2006).
Droste, D. W. et al. Middle cerebral artery blood flow velocity, end-tidal pCO2 and blood pressure in patients with obstructive sleep apnea and in healthy subjects during continuous positive airway pressure breathing. Neurol. Res. 21, 737–741 (1999).
Scala, R., Turkington, P. M., Wanklyn, P., Bamford, J. & Elliott, M. W. Effects of incremental levels of continuous positive airway pressure on cerebral blood flow velocity in healthy adult humans. Clin. Sci. 104, 633–639, https://doi.org/10.1042/CS20020305 (2003).
Scala, R., Turkington, P. M., Wanklyn, P., Bamford, J. & Elliott, M. W. Acceptance, effectiveness and safety of continuous positive airway pressure in acute stroke: A pilot study. Respir. Med. 103, 59–66, https://doi.org/10.1016/j.rmed.2008.08.002 (2009).
Nishino, T., Sugimori, K., Kohchi, A. & Hiraga, K. Nasal Constant Positive Airway Pressure Inhibits the Swallowing Reflex. Am. Rev. Respir. Dis. 140, 1290–1293 (1989).
Su, V. Y.-F. et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. Can. Med. Assoc. J. 186, 415–421, https://doi.org/10.1503/cmaj.131547 (2014).
Barer, D. H. The natural history and functional consequences of dysphagia after hemispheric stroke. J. Neurol. Neurosurg. Psychiatry 52, 236–241, https://doi.org/10.1136/jnnp.52.2.236 (1989).
Gregoretti, C. et al. Evaluation of patient skin breakdown and comfort with a new face mask for non-invasive ventilation: a multi-center study. Intensive Care Med. 28, 278–284, https://doi.org/10.1007/s00134-002-1208-7 (2002).
Anderson, F. E. et al. A Randomized Crossover Efficacy Trial of Oral CPAP (Oracle) Compared with Nasal CPAP in the Management of Obstructive Sleep Apnea. Sleep 26, 721–726, https://doi.org/10.1093/sleep/26.6.721 (2003).
Katz, N., Adir, Y., Etzioni, T., Kurtz, E. & Pillar, G. The SomnuSeal Oral Mask Is Reasonably Tolerated by Otherwise CPAP Noncompliant Patients with OSA. Sleep. Disord. 2013, 6, https://doi.org/10.1155/2013/840723 (2013).
Andrade, R. G. S., Madeiro, F., Genta, P. R. & Lorenzi-Filho, G. Oronasal mask may compromise the efficacy of continuous positive airway pressure on OSA treatment: is there evidence for avoiding the oronasal route? Curr. Opin. Pulm. Med. 22, 555–562 (2016).
Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Iintensive care 3, 15 (2015).
Amaddeo, A. et al. High-flow nasal cannula for children not compliant with continuous positive airway pressure. Sleep Med https://doi.org/10.1016/j.sleep.2019.05.012 (2019).
Nilius, G. et al. Predictors for treating obstructive sleep apnea with an open nasal cannula system (transnasal insufflation). Chest 137, 521–528, https://doi.org/10.1378/chest.09-0357 (2010).
Hawkins, S., Huston, S., Campbell, K. & Halbower, A. High-Flow, Heated, Humidified Air Via Nasal Cannula Treats CPAP-Intolerant Children With Obstructive Sleep Apnea. J. Clin. Sleep. Med. 13, 981–989, https://doi.org/10.5664/jcsm.6700 (2017).
McGinley, B. et al. Effect of a High-Flow Open Nasal Cannula System on Obstructive Sleep Apnea in Children. Pediatrics 124, 179–188, https://doi.org/10.1542/peds.2008-2824 (2009).
McGinley, B. M. et al. A Nasal Cannula Can Be Used to Treat Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 176, 194–200, https://doi.org/10.1164/rccm.200609-1336OC (2007).
Haba-Rubio, J. et al. Effect of transnasal insufflation on sleep disordered breathing in acute stroke: a preliminary study. Sleep. Breath. 16, 759–764, https://doi.org/10.1007/s11325-011-0572-3 (2012).
Sowho, M. et al. Nasal insufflation treatment adherence in obstructive sleep apnea. Sleep. Breath. 19, 351–357, https://doi.org/10.1007/s11325-014-1027-4 (2015).
Shah, S., Vanclay, F. & Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 42, 703–709, https://doi.org/10.1016/0895-4356(89)90065-6 (1989).
Berry, R., Brooks, R. & Gamaldo, C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. 3. (2014).
Sabil, A. et al. Comparison of Apnea Detection Using Oronasal Thermal Airflow Sensor, Nasal Pressure Transducer, Respiratory Inductance Plethysmography and Tracheal Sound Sensor. J. Clin. Sleep. Med. 15, 285–292 (2019).
Heitman, S. J., Atkar, R. S., Hajduk, E. A., Wanner, R. A. & Flemons, W. W. Validation of nasal pressure for the identification of apneas/hypopneas during sleep. Am. J. Respir. Crit. Care Med. 166, 386–391, https://doi.org/10.1164/rccm.2105085 (2002).
Thurnheer, R., Xie, X. & Bloch, K. E. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am. J. Respir. Crit. Care Med. 164, 1914–1919, https://doi.org/10.1164/ajrccm.164.10.2102104 (2001).
Berry, R. B. et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep. Med. 8, 597–619, https://doi.org/10.5664/jcsm.2172 (2012).
Kushida, C. A. et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J. Clin. Sleep. Med. 4, 157–171 (2008).
Carod-Artal, F. J., Trizotto, D. S., Coral, L. F. & Moreira, C. M. Determinants of quality of life in Brazilian stroke survivors. J. Neurol. Sci. 284, 63–68, https://doi.org/10.1016/j.jns.2009.04.008 (2009).
Kowalczyk, D. M., Hardy, E. T. & Lewis, A. F. Airway evaluation in obstructive sleep apnea. Oper. Tech. Otolaryngol. Head. Neck Surg. 26, 59–65, https://doi.org/10.1016/j.otot.2015.03.003 (2015).
Stocks, J. Effect of nasogastric tubes on nasal resistance during infancy. Arch. Dis. Child. 55, 17–21, https://doi.org/10.1136/adc.55.1.17 (1980).
Stepnowsky, C. J., Orr, W. C. & Davidson, T. M. Nightly Variability of Sleep-Disordered Breathing Measured over 3 Nights. Otolaryngol. Head. Neck Surg. 131, 837–843, https://doi.org/10.1016/j.otohns.2004.07.011 (2004).
Arnardottir, E. S. et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep 35, 921–932, https://doi.org/10.5665/sleep.1952 (2012).
Chen, C. Y., Ho, C. H., Chen, C. L. & Yu, C. C. Nocturnal Desaturation is Associated With Atrial Fibrillation in Patients With Ischemic Stroke and Obstructive Sleep Apnea. J. Clin. Sleep. Med. 13, 729–735, https://doi.org/10.5664/jcsm.6594 (2017).
Kim, T. et al. Nocturnal Desaturation is Associated With Neurological Deterioration Following Ischemic Stroke: A Retrospective Observational Study. J Clin Sleep Med (2017).
Patel, S. K., Hanly, P. J., Smith, E. E., Chan, W. & Coutts, S. B. Nocturnal Hypoxemia Is Associated with White Matter Hyperintensities in Patients with a Minor Stroke or Transient Ischemic Attack. J. Clin. Sleep. Med. 11, 1417–1424 (2015).
Acknowledgements
Dr. Chung-Yao, Chen’s work has been funded by the Chang Gung Medical Research Council under Contract No. CMRPG2H0011. Dr. Ho, Dr. Yu, Dr. Yang and Dr. Chia-Ling, Chen declare no potential conflict of interest. Study funded by the Chang Gung Medical Research Council under Contract No. CMRPG2H0011.
Author information
Authors and Affiliations
Contributions
Dr. Ho is contributed to collect the data, perform the analysis, and write the paper. Dr. Chia-Ling, Chen is contributed to design the study, and data and analysis tool. Dr. Yu is contributed to collect the data and perform the analysis. Dr. Yang is contributed to collect the data and data and analysis tool. Dr. Chung-Yao, Chen is contributed to design the study, conceive and collect the data, perform the analysis, and write the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ho, CH., Chen, CL., Yu, CC. et al. High-flow nasal cannula ventilation therapy for obstructive sleep apnea in ischemic stroke patients requiring nasogastric tube feeding: a preliminary study. Sci Rep 10, 8524 (2020). https://doi.org/10.1038/s41598-020-65335-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65335-z
- Springer Nature Limited
This article is cited by
-
Effect of different ventilation modalities on the early prognosis of patients with sleep apnea after acute ischemic stroke–––protocol for a prospective, open-label and randomised controlled trial
BMC Neurology (2023)
-
Nocturnal nasal high-flow oxygen therapy in elderly patients with concomitant chronic obstructive pulmonary disease and obstructive sleep apnea
Sleep and Breathing (2023)
-
Effects of nasal high flow on nocturnal hypercapnia, sleep, and sympathovagal balance in patients with neuromuscular disorders
Sleep and Breathing (2021)