Abstract
Altica deserticola (Coleoptera: Chrysomelidae) is a monophagous insect that feeds on, and is thus a harmful pest of, liquorice. Both adults and larvae feed on leaves, causing serious damage to leaf blades. It will even lead to the extinction of liquorice, resulting in significant economic losses. Leaf-disc tests were used to determine the feeding preference of A. deserticola on leaves of Glycyrrhiza uralensis and G. glabra and explore the underlying mechanism of liquorice feeding resistance to A. deserticola by comparing leaf hardness and thickness, cuticle thickness, and nitrogen and tannin content in the two plants. The results showed that larvae and adults have the same feeding preferences, i.e., both preferably fed on G. uralensis, indicating a higher resistance in this species. The hardness, thickness, and the thickness of the stratum corneum of the leaves of G. glabra were significantly greater than those of G. uralensis. Nitrogen content was higher in G. uralensis, while total tannin, tannic acid, and catechin content were higher in G. glabra. The thick cuticle and hard texture of G. glabra leaves may be an important physical trait for effectively resisting A. deserticola feeding, while high tannin and low nitrogen content may also be important.
Similar content being viewed by others
Introduction
Glycyrrhiza uralensis Fisch. ex DC. and G. glabra Linn. are perennial herbs of the family Leguminosae1. They are medicinal liquorices listed in the Chinese Pharmacopoeia2. Their roots and rhizomes have many functions in Chinese traditional medicine, such as relieving coughs3, reducing phlegm4, antiasthmatic5, protecting the liver6, anti-HIV7, and inhibiting the proliferation of cancerous cells8. Glabridin from the belowground organs of G. glabra has a skin-whitening effect9. It is thus favoured by medical and cosmetic industries. However, overexploitation has increasingly decreased wild resources of liquorice, and both abovementioned species are endangered in China10,11. The contradiction between supply and demand of these plants has been increasingly prominent. In recent years, cultivated liquorice has effectively alleviated this contradiction. However, during cultivation, frequent outbreaks of pests considerably decrease the yield and quality of liquorice12.
Altica deserticola Latreille (Coleoptera: Chrysomelidae) is a monophagous insect and the most harmful pest of liquorice, feeding on its leaves13. It usually breaks dormancy in April, enters dormancy at the end of September, and produces 3 or 4 generations per year14. Both the adults and larvae feed on liquorice leaves, causing serious damage to the leaf blades, and thereby weakening the photosynthetic capacity of the plants and reducing the yield and quality of liquorice roots and rhizomes15. Only chemical control via spraying chemical pesticides is presently adopted by farmers to combat this beetle, which easily leads to the presence of pesticide residues in the medicinal materials of the plants. Therefore, searching for liquorice varieties with higher resistance to A. deserticola will boost cultivation enthusiasm and industrial development of liquorice. According to our field observation, we found that there was much higher population density of the beetle and more severe damage caused by the pest in G. uralensis than G. glabra fields. Whether the difference in pest density and damage was caused by variation in the biological characteristics of the two liquorices or by differences in local climate or cultivation management measures, such as different water or fertilizer management strategies, among different plants remains unclear.
Some physical and chemical characteristics of leaves can usually affect the feeding behaviour or intensity of herbivorous insects16. In the ordinary course of events, the insects tended to feed on tender, soft, nitrogen-rich leaves, and avoided those with poor palatability or phytotoxins17. In the present paper, the feeding preference of A. deserticola for G. uralensis and G. glabra leaves was investigated and the hardness, thickness, cuticle thickness, and nitrogen and tannin content of the two liquorices were compared to reveal the underlying mechanism in the differences in feeding intensity between the two species. Our findings may provide theoretical reference for breeding liquorice varieties with increased resistance to the beetle.
Materials and Methods
Investigation in liquorice field
On 10th July 2018, we selected two adjacent 1-ha plots in G. uralensis and G. glabra fields with the same soil conditions and agricultural regime in Shawan Farm (45°12′N, 85°28′E). A five-point sampling method was used for randomly selecting five quadrats (10 m × 10 m) along the diagonals and at the centre point of each field. Number of liquorice individuals and damaged plants (with at least one hole or notch on its leaves), density of adult and larval beetles, and damage rate of the liquorice individuals in each quadrat were counted, and the average values from five sampling plots were calculated.
Plant and insect samples
A. deserticola adults were collected from a population of Glycyrrhiza aspera Pall in the eastern suburb of Shihezi, Xinjiang, China (44°32′N, 86°10′E). All adults were housed in a light incubator under 12 h of illumination at 25 °C and 12 h of darkness at 20 °C (light intensity, 200 μ mol•m−2•s−1) and were fed with fresh leaves of G. aspera daily. The fertilized eggs were collected from leaves of G. aspera, incubated in a light incubator, and hatched in ~6 days. The larvae were also fed fresh leaves of G. aspera daily; they pupated in ~15 days and emerged into adults after 6–8 days. To avoid the effect of leaf age and cultivation condition, including soil, climate, water, and fertilizer factors on the physical and chemical characteristic of the leaves and feeding preference of the beetle, the fully expanded fresh leaves of G. uralensis and G. glabra at the same age were collected from the position of the fifth leaf from the top of the two liquorices cultivated at the Liquorice Resource Center of Shihezi University, Shihezi, Xinjiang, China (44°18′N, 86°05′E), and these two liquorice species were cultivated under the same conditions. The mean annual precipitation and temperature in the region were 125–207.7 cm and 6.5–7.2 °C, respectively.
Feeding preference of A. deserticola for the two liquorices
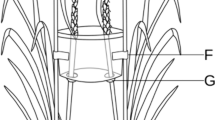
A leaf-disc method was used to determine the feeding preference of the adults or larvae of A. deserticola for the leaves of G. uralensis and G. glabra. The leaves of 30 different plants for each species were randomly selected. They were rinsed with clean water, dried with filter paper, and 1-cm-diameter discs were obtained with a disc cutter punch. Ten leaf discs of each species (total of 20 leaf discs) were placed annularly and alternately in a petri dish (9 cm diameter, Taixing Mingtai Scientific Instruments and Equipment Co., Ltd., Jiangsu, China) over a wet sponge covered with filter paper (Ø9 cm, Hangzhou Special Paper Co., Ltd., Hangzhou, China). Thirty healthy second-instar larvae (hatched for ~6 days) or adults with the same body size were selected and placed at the centre of the filter paper surrounded with the leaf discs, one per dish after starvation for 5 h, and a total of 30 petri dishes were used for the method shown in Fig. 1. Petri dishes with leaf discs but without A. deserticola were used as controls. The larvae or adult beetles were allowed to feed in each experiment for ~24 h. The leaf discs were then pressed and dried, and the leaf area consumed (%) was determined using a HP Scanjet 5300C scanner (Hewlett-Packard, Loveland, CO, USA) and Adobe Photoshop CS6 (Adobe, San Jose, CA, USA). The leaf area consumed was considered to be the percentage of the total damaged area to the total area of the 10 discs.
Mechanical and chemical properties of leaves of the two liquorices
Leaf hardness
Penetrability of the leaves of G. uralensis and G. glabra (maximum penetrability value represented the leaf hardness) were detected using a texture analyser (TA. XT plus, Stable Micro Systems, Godalming, Surrey, UK) with its accompanying software Exponent 32. Measurements were taken under the following settings: HDP/CH detection base, SMS P/2 N sharp probe, 2 mm•s−1 speed before puncture, 1 mm•s−1 speed during puncture, 10 mm•s−1 speed after puncture, and 20 g puncture trigger value. Thirty healthy and fully expanded leaves of each liquorice were randomly selected, and each leaf was tested three times to obtain average values.
Leaf thickness and cuticle thickness
Healthy, fully expanded leaves of G. uralensis and G. glabra from 10 individual plants of each liquorice were cut into small pieces (1 cm × 0.5 cm) and placed in FAA solution (70% alcohol: glacial acetic acid: formaldehyde = 18:1:1) for 48 h. Transverse sections of the leaves (8 μm thick) were prepared using conventional paraffin sectioning18. The sections were stained with safranin and fast green, sealed with optical resin, observed under a light microscope (Olympus BX51, Olympus Optical, Tokyo, Japan), and photographed with an Olympus DP70 system. Leaf and cuticle thickness of the adaxial and abaxial surface were measured by Motic Images Advanced 3.2 (Motic, Hong Kong), calculated their average value.
Leaf nitrogen content
We randomly selected 150 plants and collected one healthy and fully expanded leaf from each plant for 30 leaves per sample. The leaf samples were dried to constant weight, pulverized with a grinder (HAY-201, Hao You Electrical Appliance Factory, Zhongshan, China), and sieved through a 1.98-mm mesh, and then a 0.1-g sample was accurately weighed. Nitrogen content of the leaves was measured using a Kjeldahl apparatus (K9840; Haineng Instrument Co., Ltd., Jinan, China) after digestion with sulfuric acid–hydrogen peroxide (H2SO4–H2O2) as described by Kirk19. Five samples were tested five times and their average values were calculated.
Tannin content
Leaf samples of the two species were dried to constant weight, pulverized with a grinder (HAY-201), and sieved through a 1.98-mm mesh and 0.2 g of leaf powder was accurately weighed. The total tannin content was determined using the Folin–Ciocalteu procedure20 and tannic acid was used as a standard. The content of tannic acid21, ellagic acid22, gallic acid23, and catechin24 were detected by high-performance liquid chromatography (Agilent 1200; Agilent Technologies, CA, USA). Five samples of each plant were tested, and their average value was calculated. Setting conditions were as follows:
Tannic acid: the mobile phase contained solvent A: 0.07% acetic acid 15% and solvent B: methanol 85%, isocratic elution. The flow rate was 0.5 mL•min−1 and the volume injected was 10 µL. The temperature of the column was 25 °C, and UV detector was set at a wavelength of 275 nm.
Ellagic acid: the mobile phase contained solvent A: 0.1% acetic acid and solvent B: acetonitrile. The gradient was 12–20% B for 16 min, 20–25% B for 4 min. The flow rate was 1.0 mL•min−1 and the volume injected was 20 µL. The temperature of the column was 30 °C, and UV detector was set at a wavelength of 265 nm.
Gallic acid: the mobile phase contained solvent A: 0.1% acetic acid and solvent B: acetonitrile. The gradient was 5–7.5% B for 10 min. The flow rate was 1.0 mL•min−1 and the volume injected was 10 µL. The temperature of the column was 25 °C, and UV detector was set at a wavelength of 267 nm.
Catechin: the mobile phase contained solvent A: 0.1% acetic acid 68% and solvent B: methanol 32%, isocratic elution. The flow rate was 1 mL•min−1 and the volume injected was 10 µL. The temperature of the column was 30 °C, and UV detector was set at a wavelength of 254 nm.
Data Analysis
The SPSS 19.0 software (IBM Corp., New York, USA) was used to analyse the data. Differences in leaf area consumed (%), leaf hardness and thickness, cuticle thickness, leaf nitrogen and tannin content between the two liquorices were analysed using a T-test. Multiple comparison analysis was used for comparing the differences in content of the four kinds of tannins for each liquorice species. The charts were produced using Origin 2016 (OriginLab, Hampton, USA).
Results
Population density of Altica deserticola and the damage rate of liquorices
The average density of adult and larval populations in the G. uralensis field reached 13.8 and 3.2/m2, respectively (Table 1). Those in the G. glabra fields were only 1.8 and 0.124/m2, respectively (Table 1). The average damage rates of G. uralensis and G. glabra were 86.7% and 2.36%, respectively (Table 1).
Comparison on the consumed amount of leaves by A. deserticola between the two liquorices
Both adults and larvae of A. deserticola only fed on the leaves of G. uralensis, while all the leaf discs of G. glabra in culture dishes remained intact. The consumption percentage of adults to leaf area reached 12.58% (Fig. 2), and the consumption percentage of larvae to leaf area reached 10.68% (Fig. 3).
Comparison of leaf hardness
The leaves of G. glabra are leathery with a hard texture, while those of G. uralensis are soft textured (Fig. 4). There was a significant difference in the hardness value of the leaf between the two plants.
Comparison of blade and cuticle thickness
The leaves of G. glabra were significantly thicker than those of G. uralensis (Table 2; P = 0.01). The leaf cuticle thickness on the adaxial and abaxial side in G. glabra was also significantly greater than that in G. uralensis (Table 2; P = 0.002).
Comparison of nitrogen content
The leaf nitrogen content in G. uralensis was higher than that in G. glabra (Fig. 5), and there was a significant difference in the nitrogen content between the two liquorices (P = 0.002).
Comparison of tannin contents
The total tannin content in the leaves of G. glabra was significantly higher than that of G. uralensis (Fig. 6; P = 0.003). In both species, the tannic acid content was the highest followed by catechin with both accounting for >92% of the total tannin content in G. glabra and 86% of that in G. uralensis. Hence, we concluded that they were the main constituents of tannins in liquorice leaves. The content of gallic acid and ellagic acid in the leaves of the two liquorices was relatively low, especially in G. glabra leaves (7% of the total tannin content; Fig. 7).
The content of four kinds of tannins in the leaves of two liquorice (Glycyrrhiza) species. Different capital letters denote significant differences between means of the columns (P < 0.01), and different lowercase letters denote significant differences between means of the four kinds of tannins in the same liquorice species (P < 0.01).
Discussion
Consumption of plant leaves by insects causes loss of photosynthetic organs, reduces net photosynthetic rate and biomass accumulation, and thus inhibits plant growth25,26. A. deserticola is a pest, whose various generations overlap and insects with different developmental stages coexist. It will cause extreme damage to G. uralensis leaves, since both the adults and the larvae are dependent on these leaves for food. When devastating outbreaks of the pest occur, there are often 10 ~ 20 adults and larvae of the insects found on a liquorice plant, which eat the leaves reducing the plant’s photosynthetic ability causing the liquorice to wither and die, resulting in a significant decrease in the production of roots and rhizome. E.g. G. uralensis planted in Shawan Farm was investigated on 10th July 2018, and ~85% of its leaves had been eaten by A. deserticola (Table 1). Therefore, it is of great scientific and economic significance to study the feeding preferences and its mechanisms of A. deserticola on liquorice leaves.
In the present study, we found that A. deserticola preferred eating G. uralensis leaves over those of G. glabra when the two types of plants coexisted under the same conditions, which eliminated the influence of environmental differences on the feeding preferences of A. deserticola. This was consistent with the results of our previous field observation. Therefore, the feeding preference of A. deserticola for leaves of G. glabra and G. uralensis is likely to be related to the physical and chemical properties of the leaves themselves.
Physical properties of the leaves, including hardness, thickness, and the presence of trichomes and wax on the surface can significantly affect feeding behaviour of the insects27. Huang28 showed that tea varieties with thick leaves were of better resistance to Myllocerinus aurolineatus Voss than those with thin leaves. Hoffman and Rao29 reported that the hardness degree of the host plant leaves significantly affected the behaviours of Oulema melanopus, which preferred softer leaves. In the present study, some physical characteristics of the leaves of the two liquorices tested were selected for analysis. Combined with the feeding preference of A. deserticola, it can be seen that A. deserticola preferred to eat thin and soft leaves with thin cuticles.
Nitrogen was recognized as the most important limiting nutrient for herbivorous insects. The C:N ratios of the herbivores were considerably lower than those of their potential foods, but the insect required nutrient-rich resources to rapidly build nutrient-rich bodies30. To meet such high nitrogen demand, the insect must feed on nitrogen-rich plants. In the present study, we found the nitrogen content in the leaves of G. uralensis was significantly higher than that in G. glabra, which was consistent with the feeding preference of A. deserticola. This indicated that nitrogen content in leaves was an important factor affecting the feeding behaviour of A. deserticola, i.e. the higher the nitrogen content in leaves the higher the feeding preference of the pest.
Tannins are secondary metabolites of plants. They are natural polyphenolic compounds and widely exist in leguminous plants. Previous studies reported that leaves of liquorice plants contained tannins, and tannic acid, catechin, ellagic acid, and gallic acid were four major components31,32,33,34. We determined the content of total tannins and their four components in the leaves of the two liquorices. Our results showed that the content of total tannins in the leaves of G. glabra was significantly higher than that in those of G. uralensis. Tannin is an important defensive substance in plants against their pests, which lengthens insect developmental times35. Sun36 found a significant negative correlation between the tannin content of leaves in different poplar varieties and the feeding intensity of Saperda populnea (Coleoptera: Cerambycidae). Therefore, tannins affect the palatability of insects and thus the feeding preferences of phytophagous insects37. G. glabra leaves have high tannin content, which resulted in poor palatability. This may be another reason why A. deserticola only feeds on the leaves of G. uralensis.
In summary, the feeding preference of A. deserticola for the leaves of the two liquorices was the result of a combination of various factors. The physical and chemical characteristics, such as leaf hardness, leaf thickness, cuticle thickness, and nitrogen and tannin content of leaves, may be important factors affecting the feeding preference of A. deserticola. Tannic acid was the tannin component with the highest content in the leaves of G. uralensis followed by catechin. The content of these two substances in the leaves of G. glabra was significantly higher than those in the leaves of G. uralensis. Therefore, we speculate that the differences in content of the two tannin components may be one of the reasons for the feeding preference of the beetles.
To gain more accurate results, we should add these two substances to the leaves of G. uralensis and investigate whether there is a difference in leaf consumption between the added group and the non-added groups under the same conditions. Colour and volatile compounds of plant leaves could obviously affect the feeding behaviours of some other insects38,39. Thus, whether the differences in colour and volatile compounds of the two plant leaves significantly affect the feeding behaviour of the beetle should be further studied.
References
Cui, H. B. Flora of China. 167–175 (Science Press, Beijing, China 1998).
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. 80 (China Medical Science Press, Beijing, China 2015).
Gong, J. Clinical analysis on laryngeal cough in children treated by Glycyrrhiza and Balloonflower decoction with additional integrants. J. Practical Traditional Chin. Med. 26(6), 384–385 (2010).
Frieri, M., Narayan, A. R., Huang, Y. C., Zhang, Y. H. & Li, X. Interleukin-8 and nitrite production from A549 human type II alveolar epithelial cells with Glycyrrhiza uralensis and budesonide. Ann. Allergy Asthma & Immunol. 98(1), A9–A9 (2007).
Patel, S. et al. Evaluation of anti-asthmatic activity of Glycyrrhiza glabra. Biosci. Biotechnol. Research Asia. 6(2), 761–766 (2009).
Ma, T., Cao, Y., Bai, H. & Chen, Y. J. Protective effect of the extracts of Radix Glycyrrhiza on the liver injury induced by pentachloronitrobenzene. J. Shenyang Pharmaceutical University. 19(4), 275–277 (2002).
Manfredi, K. P., Vallurupalli, V., Demidova, M., Kindscher, K. & Pannell, L. K. Isolation of an anti-HIV diprenylated bibenzyl from Glycyrrhiza lepidota. Phytochemistry. 58(1), 153–157 (2001).
Zhang, B. et al. Antineoplastic activity of isoliquiritigenin, a chalcone compound, in androgen-independent human prostate cancer cells linked to G2/M cell cycle arrest and cell apoptosis. Eur. J. Pharmacol. 821, 57–67 (2018).
Cui, F. L., Zhang, T. J., Li, T., Lin, T. X. & Huang, J. S. Study on the liquorice ingredients in cosmetics. Detergent & Cosmetics 40(3), 19–22 (2017).
Zhang, J. T., Xu, B. & Li, M. Genetic diversity of populations of an endangered medicinal plant species (Glycyrrhiza uralensis) in different environments of North China. J. Med. Plants Research. 4(9), 830–836 (2010).
Zhou, Y. H. Discussion on Revision of the National Key Protected Wild Medicinal Species List. Modern Chin. Med. 14(9), 1–12 (2012).
Wei, S. L., Wang, W. Q. & Wang, H. Study on liquorice resources and its sustainable utilization in central and western China. Chin. J. Chinese Materia Med. 28(3), 202–206 (2003).
Xu, Y. Integrated pest control technology for licorice in Altay region. Rural Technol. 5, 36–37 (2017).
Li, S. F. & Xing, H. T. Preliminary observation on Altica glycyrrhizae. Xinjiang Farm Research Sci. & Technol. 6, 25–28 (1989).
Xiao, X. P., Su, Y. T., Wang, X. & Tang, L. P. Control of diseases and pests in licorice. Special Economic Animal and Plant. 18(9), 52–53 (2015).
Liu, P. et al. Research progress of insect adaptability to their host plants. Biological Disaster Sci. 39(4), 250–254 (2016).
Sagers, C. L. Manipulation of host plant quality, herbivores keep leaves in the dark. Functional Ecol. 6, 741–743 (1992).
Gan, Y. & Xu, F. The coexistence of binucleate and trinucleate pollen in Mitrephora macclurei Weerasooriya & R. M. K. (Annonaceae). Grana. 58(2), 129–132 (2019).
Kirk, P. L. Kjeldahl method for total nitrogen. Analytical Chem. 22(2), 354–358 (1950).
Price, L. Rapid visual estimation and spectrophotometric determination of tannin content of Sorghum grain. J. Agric. Food Chem. 25(6), 1268–1273 (1977).
Murdiati, T. B., Mcsweeney, C. S. & Lowry, J. B. Metabolism in sheep of gallic acid, tannic acid and hydrolysable tannin from Terminalia oblongata. Crop & Pasture Sci. 43(6), 1307–1319 (1992).
Gasperotti, M., Masuero, D., Vrhovsek, U., Guella, G. & Mattivi, F. Profiling and accurate quantification of rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J. Agric. & Food Chem. 58(8), 4602–4616 (2010).
Ovando-Martínez, M., Gámez-Meza, N., Molina-Domínguez, C. C., Hayano-Kanashiro, C. & Medina-Juarez, L. A. Simulated gastrointestinal digestion, bioaccessibility and antioxidant capacity of polyphenols from Red Chiltepin (Capsicum annuum L. Var. glabriusculum) Grown in Northwest Mexico. Plant Foods Hum. Nutr. 73(2), 116–121 (2018).
Persic, M., Mikulic-Petkovsek, M., Slatnar, A., Solar, A. & Veberic, R. Changes in phenolic profiles of red-colored pellicle walnut and hazelnut kernel during ripening. Food Chem. 252, 349–355 (2018).
Yin, J. H. et al. Effects of cutting on morphology, growth and biomass of Stipa grandis. Chin. J. Grassland. 36(5), 89–94 (2014).
Hou, Y. R. & An, S. Z. Temporal variation of water soluble carbo-hydrate in Seriphidium transiliense under different mowingintensities and it how to transfer during seasonal change. Acta Prataculturae Sinca. 24(4), 48–56 (2015).
Qin, J. D. The Relationship Between Insects and Plants, Discussion of the Interaction and Evolution Between Insects and Plants. 1–227 (Science Press, Beijing, China 1987).
Huang, Y. H., Zhang, J. Y., Zhang, Y. L., Yang, Y. & Wang, Y. J. Investigation on the resources of tea tree insect-resistant varieties and research on resistance mechanism-IV. Tea Communication. 4, 5–6 (1994).
Hoffman, G. D. & Rao, S. Oviposition site selection on oats: the effect of plant architecture, plant and leaf age, tissue toughness, and hardness on cereal leaf beetle, Oulema melanopus. Entomol. Exp. Appl. 141(3), 232–244 (2011).
Elser, J. J. et al. Nutritional constraints in terrestrial and freshwater food webs. Nature. 408(6812), 578–580 (2000).
Cheel, J. et al. Variations in the chemical profile and biological activities of licorice (Glycyrrhiza glabra L.), as influenced by harvest times. Acta Physiol. Plant. 35(4), 1337–1349 (2013).
Hamad, G. M., Taha, T. H., El-Deeb, N. M. & Alshehri, A. M. A. Advanced trends in controlling Helicobacter pylori infections using functional and therapeutically supplements in baby milk. J. of Food Sci & Technol. 52(12), 8156 (2015).
Rahman, H. et al. Glycyrrhiza glabra HPLC fractions, identification of Aldehydo Isoophiopogonone and Liquirtigenin having activity against multidrug resistant bacteria. BMC Complementary Altern. Med. 18(1), 140 (2018).
Komes, D. et al. Consumer acceptability of liquorice root (Glycyrrhiza glabra L.) as an alternative sweetener and correlation with its bioactive content and biological activity. Int. J. Food Sci. Nutr. 67(1), 14 (2016).
Coley, P. D. & Barone, J. A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 27, 305335 (1996).
Sun, P., Guo, S. P. & Li, H. X. Relationship between tannin content of poplar and damage of Saperda populnea. J. Northeast Forestry University. 36(5), 51–52 (2008).
Liu, Z. D., Li, D. M., Gong, P. Y. & Wu, K. J. Life table studies of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), on different host plants. Envi-ronmenta Entomol. 33, 1570–1576 (2004).
Cooney, L. J. et al. Red leaf margins indicate increased polygodial content and function as visual signals to reduce herbivory in Pseudowintera colorata. New Phytologist. 194(2), 488–497 (2012).
Li, T., Blande, J. D. & Holopainen, J. K. Atmospheric transformation of plant volatiles disrupts host plant finding. Sci. Rep. 6, 33851 (2016).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31360047).
Author information
Authors and Affiliations
Contributions
C.H.L. and C.P.Y. performed the experiments, analysed the data and wrote the paper. M.M. designed the research work and was responsible for revision of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, H., Chen, P. & Ma, M. Feeding preference of Altica deserticola for leaves of Glycyrrhiza glabra and G. uralensis and its mechanism. Sci Rep 10, 1534 (2020). https://doi.org/10.1038/s41598-020-58537-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58537-y
- Springer Nature Limited