Abstract
Repeated cycles of post-remission high-dose cytarabine (Ara-C) have been suggested to improve survival in core binding factor (CBF) acute myeloid leukaemia (AML). High-dose Ara-C used for induction regimens has also been reported to be associated with increased treatment-related mortality (TRM). Few data are available about intermediate-dose Ara-C serving as induction therapy. The aim of our study was to compare the tolerance and outcomes of standard- and intermediate-dose levels of Ara-C as induction in CBF AML and to analyse the clinical heterogeneity of the two AML entities under these induction settings. We retrospectively investigated the outcomes in adults with CBF AML induced with regimens based on standard-dose Ara-C at 100 to 200 mg/m2 or intermediate-dose Ara-C at 1,000 mg/m2. In total, 152 patients with t(8; 21) and 54 patients with inv(16) AML were administered an induction regimen containing anthracyclines plus either standard- or intermediate-dose Ara-C. After a single course of induction, the complete remission (CR) rate in the inv(16) cohort was 52/52 (100%), higher than the 127/147 (86.4%) in the t(8; 21) cohort (P = 0.005). Intermediate-dose Ara-C (HR = 9.931 [2.135–46.188], P = 0.003) and negative KITmut (HR = 0.304 [0.106–0.874], P = 0.027) independently produced an increased CR rate in the t(8; 21) cohort. Positive CD19 expression (HR = 0.133 [0.045–0.387], P = 0.000) and sex (male) (HR = 0.238 [0.085–0.667], P = 0.006) were associated with superior leukaemia-free survival (LFS) in the t(8; 21) cohort independently of KITmut status or the induction regimen. We conclude that intermediate-dose Ara-C is superior to standard-dose Ara-C for induction of remission in t(8; 21) AML, and CD19 status and sex independently confer prognostic significance for LFS. The KITmut status alone does not have an independent effect on survival in t(8; 21) AML. More intensive induction therapy is unnecessary in inv(16) AML.
Similar content being viewed by others
Introduction
Acute myeloid leukaemia (AML) with t(8; 21)(q22; q22) and AML with inv(16)(p13.1q22)/t(16; 16)(p13.1; q22) are together referred to as core binding factor (CBF) AML1,2,3,4, which constitutes approximately 5–8% of all de novo AML cases5,6,7. Both have been recognized as distinct diseases by the World Health Organization classification of myeloid neoplasms and acute leukaemia8.
Overall, CBF AML has a relatively favourable clinical outcome compared to other cytogenetic subtypes. Repeated cycles of high-dose cytarabine (Ara-C) for intensification during post-remission treatment can improve survival outcomes9,10,11,12. However, there is considerable clinicopathological heterogeneity within this AML subset, as demonstrated by the relapse incidence reaching up to 40% and the overall survival (OS) rate of 40–60%13,14,15,16,17.
Ara-C at a daily dose of 100 to 200 mg/m2 for 7 days as induction therapy is the most widely applied strategy in most centres. Several clinical trials evaluating high-dose Ara-C as induction therapy in AML have been conducted, with results differing and the majority reporting increased treatment-related toxicities18,19,20,21,22,23. Consensus has not been reached on the benefit of higher doses of Ara-C in the induction stage. A randomized trial showed no response and survival benefits but excessive toxic effects for high-dose Ara-C induction compared to intermediate-dose Ara-C induction in newly diagnosed AML patients aged 18 to 60 years, suggesting a plateau in the dose-response relationship above intermediate-dose Ara-C20. In view of this, the use of high-dose Ara-C for induction remains controversial.
However, few data are available regarding the impact of intermediate-dose Ara-C induction on clinical outcomes specifically in CBF AML. Additionally, in recent years, next-generation sequencing (NGS) technology has been widely used in AML. In this scenario, we conducted a comparison between standard- and intermediate-dose levels of Ara-C during induction in adults with CBF AML. Meanwhile, we analysed the impacts of clinicopathological characteristics (i.e., immunophenotyping, cytogenetics, and molecular biology data) and NGS-identified genetic lesions on clinical outcome under these induction settings.
Results
Patient baseline clinicopathological and genetic characteristics
In total, 206 patients with CBF AML consisting of 152 t(8; 21) and 54 inv(16) cases who fulfilled the enrolment criteria were included in the final analysis. The median age was 34 years (range 16–65 years), with male and female sex accounting for 107 (51.9%) and 99 (48.1%) patients, respectively. Supplementary Table S1 gives details on the data regarding patient clinicopathological and genetic characteristics at diagnosis according to CBF subtype.
Induction therapy safety and response in CBF AML
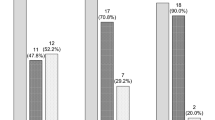
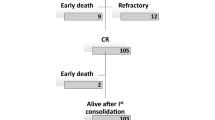
The distribution of analysed patients was even in terms of all clinicopathological and genetic parameters between the two induction arms for both CBF subtypes (data not shown). The induction strategies were also balanced between both CBF cohorts, with the standard dose being given in 83 patients (54.6%) and the intermediate dose being given in 69 patients (45.4%) in the t(8; 21) cohort and the standard dose being given in 31 patients (57.4%) and the intermediate dose being given in 23 patients (42.6%) in the inv(16) cohort (P = 0.722). In the 152 t(8; 21) patients, five patients died early of septic shock or cerebral haemorrhage during or after induction, with two patients (2.4%) being in the standard-dose arm and three patients (4.3%) being in the intermediate-dose arm. In the 54 inv(16) patients, there were 2 early deaths, with one in each induction arm. There was no significant difference in early mortalities between the two arms (P = 0.772). The remaining 147 patients with t(8; 21) and 52 patients with inv(16) were evaluable for response assessment. The overall CR rate in the t(8; 21) cohort was 127/147 patients (86.4%), with 63/81 patients (77.8%) responding in the standard-dose arm and 64/66 patients (97.0%) responding in the intermediate-dose arm (P = 0.001). All 52 patients in the inv(16) cohort achieved an overall CR after a single course of induction. (Supplementary Table S2).
Compared with the standard-dose arm, the intermediate-dose arm showed similar incidences of grade 3 and 4 toxicities and no delayed neutrophil and platelet recoveries. Overall, non-haematological toxicities were mild and controllable, without any neurotoxicity documented.
Analysis of factors impacting CR in t(8; 21) AML
After analysis with a univariate chi-square test, in the t(8; 21) cohort, patients with KIT mutations (KITmuts) had a lower overall CR rate than those without KITmuts (P = 0.025), and the codon change D816 was more associated with the significant difference than N822 (P = 0.024 for D816; P = 0.256 for N822). In the standard-dose arm, a trend existed but did not reach a statistical significance (P = 0.107). An elevated fusion transcript level ( > 150 copies/ABL copies) was found to have an inferior impact on the CR rate only in the standard-dose arm (P = 0.046), while this effect was not obvious in the intermediate-dose arm (P = 1.000). The intermediate-dose Ara-C induction regimen raised the overall CR rate by nearly 20% versus the standard-dose Ara-C regimen (97.0 vs. 77.8%, P = 0.001). (Supplementary Table S2).
Logistic regression analysis revealed that in the entire t(8; 21) cohort, the factors independently associated with a higher CR rate were intermediate-dose induction (HR = 9.931 [2.135–46.188], P = 0.003) and negative KITmut status (HR = 0.304 [0.106–0.874], P = 0.027). The stratified analysis identified fusion transcript level and KITmut status as factors independently impacting the CR rate in the standard-dose arm, while no factor was identified in the intermediate-dose arm. Moreover, both univariate chi-square and multivariate logistic regression analyses yielded a consistent and robust statistical significance for the impact of induction regimen on the CR rate (P < 0.01 for both analyses), suggesting its powerful predictive capability, as demonstrated by the considerably high hazard ratio (HR) value (HR = 9.931). (Table 1).
No statistical difference was observed regarding CR rate between t(8; 21) patients with 2 or more concomitant additional chromosomal abnormalities and those without, as well as patients between patients with and without FLT3-ITD mutations, which has been well documented in cytogenetically normal AML.
Analysis of LFS and OS in t(8; 21) AML
With a median follow-up of 12.7 (range 1–44) months, the inv(16) cohort showed a trend towards superior LFS versus the t(8; 21) cohort, but it did not reach statistical significance (P = 0.066). OS between the two cohorts showed no statistical difference (P = 0.306). (Fig. 1).
In the t(8; 21) cohort, survival estimates were evaluable in 127 patients who attained CR1 and had received at least one consolidation cycle based on high-dose Ara-C. The median follow-up time was 11.4 months (range 1.1–43.9 months). In univariate Kaplan-Meier analysis, patients with positive KITmut status and negative CD19 expression exhibited poorer LFS than those with negative KITmut status and positive CD19 expression either in the entire t(8; 21) cohort, the standard-dose Ara-C arm, or the intermediate-dose Ara-C arm (P = 0.002, 0.021 and 0.037 for KIT, respectively; P = 0.000, 0.002 and 0.002 for CD19, respectively; Table 2 and Fig. 2). In the entire t(8; 21) cohort, male sex was shown to be associated with significantly superior LFS compared with female sex in the univariate Kaplan-Meier analysis (P = 0.006). Subgroup analysis indicated that this differences between male sex female sex was also present in the standard-dose arm (P = 0.019), while the LFS between males and females was not significantly different in the intermediate-dose arm (P = 0.132). When the screened factors were subjected to Cox regression models, the difference in LFS affected by CD19 and patient sex remained significant independently of KITmut status in the entire t(8; 21) cohort and the standard-dose arm. Although KITmut status was associated with inferior LFS in the entire t(8; 21) cohort and both induction arms according to the univariate Kaplan-Meier analysis, as was the 2 or more concomitant additional chromosomal abnormalities in standard-dose arm, these covariates were not confirmed in the multivariate Cox models. (Table 2).
Kaplan-Meier plots of LFS according to CD19 expression or patient sex in the entire t(8; 21) cohort and in both treatment arms. (a–c) According to cohort- and induction-stratified outcome analysis, patients with negative CD19 expression exhibited poorer LFS than those with positive CD19 expression in the entire t(8; 21) cohort (a), the standard-dose Ara-C arm (b), and the intermediate-dose Ara-C arm (c) (P = 0.000, 0.002 and 0.002, respectively). (d,e) Female patients exhibited poorer LFS than male patients in both the entire t(8; 21) cohort (d) and the standard-dose arm (e) (P = 0.004 and 0.019, respectively). (f) Additionally, female patients showed slightly inferior LFS compared with male patients in the intermediate-dose arm, but the difference was not significant (P = 0.132).
Univariate analysis demonstrated mutated NRAS to be associated with a superior LFS in the entire t(8; 21) cohort, with marginal significance (P = 0.069). Additionally, FLT3-TKD and NOTCH1 mutations showed an association with poor LFS (P = 0.050 and 0.089, respectively), with NOTCH1 showing independent significance in the multivariate model (HR = 9.993 [1.888–52.900], P = 0.009). (Table 2).
Because of the small number of patients who reached the study end-points, we were unable to perform multivariate Cox analysis for LFS in the inv(16) cohort.
Combination of CD19 and sex can further refine risk stratification in t(8; 21) AML
Given that CD19 and patient sex each showed an independent impact on LFS, we subsequently analysed their combined effect on clinical outcome in t(8; 21) AML. Given that there was no significant difference in LFS between the two induction arms, a Kaplan-Meier comparison was conducted collectively in the entire t(8; 21) cohort. When CD19 was combined with patient sex, the risk stratification was more obvious, with male patients with positive CD19 expression exhibiting the most superior LFS and female patients without CD19 expression showing the worst LFS (3-year LFS rate: 96% vs. 25%; median LFS duration: not reached vs. 8.6 months). (Fig. 3).
Kaplan-Meier plots of LFS according to CD19 expression combined with patient sex in the entire t(8; 21) cohort. CD19 expression in combination with patient sex led to a more obvious survival stratification for t(8; 21) AML, with male patients positive for CD19 expression having the most superior LFS and female patients negative for CD19 expression having the worst LFS (3-year LFS rate: 96% vs. 25%; median LFS duration: not reached vs. 8.6 months).
Discussion
Although CBF AML has a relatively favourable prognosis compared with other non-M3 AML subtypes, there are yet heterogeneities across different CBF subtypes, making it possible to further improve the therapeutic effects in t(8; 21) AML patients. Dose-reduced induction chemotherapy has been suggested to predict a poor clinical outcome, as previously reported24,25, so our cohort of CBF patients were induced with adequate dose intensity and assessed for response after a single induction cycle. We identified the induction regimen and KITmut status as the independent factors impacting the overall CR rate in the entire t(8; 21) cohort. The fusion transcript level and KITmut status were independent factors in the standard-dose arm by subgroup analysis but not in the intermediate-dose arm. Patients who underwent intermediate-dose Ara-C induction had a nearly 20% increase in the overall CR rate with acceptable TRM similar to that of the standard-dose arm and could overcome the inferior influence of factors such as KITmut status, which predict an impaired induction response. In addition, our data showed that there was no need to give more intensive Ara-C regimens as induction therapy for inv(16) patients, as indicated by the high CR rate as well as the low amount of patients who experienced relapse, irrespective of the dosage level.
Of the KITmuts, the codon change D816 was more associated with the significant difference in response than was N822 in t(8; 21) AML, a finding that is supported by in vitro studies demonstrating a positive association of the D816 mutation with stronger KIT phosphorylation and subsequent signalling activation26,27. In clinical practice, sequencing results are often not available fast enough, making the development of approaches that can quickly screen for the existence of KIT-D816 necessary. For instance, the ddPCR methodology can accomplish relatively quick evaluations, facilitating the timely administration of more intensive induction regimens in the distinctive KIT-D816-mutated t(8; 21) AML subset28.
No statistical difference was observed concerning the overall CR rate between t(8; 21) patients with 2 or more additional chromosomal abnormalities and those without, as well between patients with and without FLT3-ITD mutation. In the inv(16) cohort, the overall CR rate reached 100% regardless of the induction regimen, which is similar to the improved CR rates reported by others29,30.
Controversial prognostic values of KITmuts have been reported in CBF AML. Most studies confirmed KITmuts as predicting a higher risk of relapse and shorter OS7,11,31,32,33,34,35,36. In our t(8; 21) cohort, KITmuts showed a significant predictive value for LFS, with KIT-D816 mainly accountable for the difference compared to KIT-D822, consistent with previous reports36. However, the independent prognostic impact of KITmuts on LFS was lost in the multivariate model. We demonstrated that KITmuts alone, as a prognostic factor, did not confer an independently unfavourable impact on survival in t(8; 21) AML, which was similar to other results37,38. The possible explanations for the lack of findings of the effect of KITmuts on survival may be attributable to several explanations. (i) A low variant allele frequency (VAF) of KITmuts might be detected in a considerable percentage of patients owing to the relatively sensitive high-throughput NGS techniques. It has been shown that only KITmuts with a VAF of 25% or higher are independently correlated with an elevated relapse rate in t(8; 21) patients39. (ii) There is diversification of the clonal architecture as the leukaemia develops and progresses, as exemplified by the appearance of late evolutionary signalling clones. Mutations of KIT, FLT3 and RAS have been commonly reported in CBF AML32,40,41,42. A recently published study described multiple signalling clones of KIT, FLT3 and RAS variants during clonal progression43, which negatively affected event-free survival (EFS), while the presence of a single signalling clone showed no such relevance. iii) Post-remission high-dose Ara-C might still exert its role on residual KITmut clones. iv) Mutations in KIT have also been reported to be frequently lost at disease relapse 27,40.
Congruent with data from multicentre studies of children in Germany44 and studies of French adults39, our findings showed mutated NRAS as being associated with favourable LFS, which is worth further attention and clarification. NOTCH1 lesions commonly occur in more than 50% of T cell acute lymphoblastic leukaemia patients45. Activated Notch signalling contributes to the crosstalk between leukaemia cells and surrounding mesenchymal stromal cells, leading to malignant properties and chemoresistance46,47. We reported for the first time that mutated NOTCH1 was an adverse prognostic factor in t(8; 21) AML. Due to the low incidence of mutated NOTCH1 (10/152, 6.6%) in our t(8; 21) cohort, further confirmation of its clinical impact will be required.
The 3 + 7 combination scheme as induction therapy has still exerted its cornerstone role in modern therapies of AML. Because the anthracycline dosage in AML has reached its plateau (60–90 mg/m2), improved outcomes from induction with these 3 + 7 regimens depend on the dose adjustment of Ara-C. In an attempt to provide sufficient dose intensity, after failure on the first course of AML induction treatment, we prefer to change the drug combination scheme rather than to use the same combination for re-induction. Since a high dose (2,000–3,000 mg/m2 every 12 hours for 3 days or more) of Ara-C may increase the risk of induced toxicity or even early death, we did not include a high dose Ara-C in our AML induction strategy. In the current context, and also considering the tolerance of Chinese patients with AML, we designed an improved 3 + 7 regimen including intermediate-dose Ara-C at the late stage (days 5 to 7) of the induction course. Comparatively, the CR rates were compared after sequential double induction (i.e., two consecutive courses) from studies by Lowenberg et al.20, in which there was no significant difference in the remission rate between the two schemes. In addition, mutational data were not included in the outcome analysis of their relatively early investigations.
In our t(8; 21) cohort, although intermediate-dose Ara-C provided a remission advantage, the regimen as induction did not translate into an LFS benefit, which may be partly explained by repetitive cycles of post-remission high-dose Ara-C continuously preventing leukaemia recurrence. In line with our result, dose-intensified induction had no association with improved survival in the setting of post-remission intensification therapy in adult patients with CBF AML11.
Previous studies on the efficacy and outcome of CBF AML have rarely involved immunophenotypic data. A Japanese finding reported that CD19 status was significantly associated with CR rate (expression group 95.7% vs. non-expression group 83.8%, P = 0.049)48. However, we did not replicate this result either in the entire t(8; 21) cohort or in both induction arms. Intriguingly, in our study, negative CD19 expression and female sex presented a significantly higher relapse rate and a shorter LFS than positive CD19 expression and male sex, similar to a previous result49. CD19 expression remained a robust predictor of relapse in the multivariate Cox model, underscoring its independent prognostic implications. Furthermore, CD19 expression combined with sex can be used to further risk-stratify t(8; 21) patients, providing an unambiguous prognostication refinement for t(8; 21) AML.
There were some limitations in our study, including its non-prospective design and missing values owing to the unavailability of laboratory records, as well as patient loss to follow-up after referral to regional institutions. All of these factors can impair the power of the statistical comparisons. Prospectively randomized studies with larger sample sizes are warranted to redefine a high-risk subset from the relatively favourable but heterogeneous t(8; 21) AML populations.
Taken together, although sharing a common CBF lesion, these two AML subtypes display distinct clinical outcomes, with t(8; 21) AML showing relatively obvious heterogeneity. More intensive induction is unnecessary in inv(16) AML, where a trend towards slightly superior LFS is shown over t(8; 21) AML. Intermediate-dose Ara-C-containing induction may provide an optimized treatment option for t(8;21) patients, especially for those carrying elevated levels of fusion transcript and/or KITmuts, with acceptable tolerability and no increased early mortality. General KITmuts alone are not an independent factor for survival in t(8; 21) AML. CD19 expression and sex significantly affect LFS, and their combination can be used to risk-stratify t(8; 21) AML patients, thereby guiding individually risk-adapted post-remission therapy.
Patients and Methods
Patient selection
In the present study, we observed a cohort of 206 newly diagnosed CBF AML patients (aged 16 to 65 years) including 152 patients with t(8; 21)(q22; q22) [t(8; 21) patients] and 54 patients with inv(16)(p13q22)/t(16; 16)(p13; q22) [inv(16) patients] from August 2014 to March 2018. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Changzhou First People’s Hospital and Shengjing Hospital of China Medical University. All patients provided signed informed consent for receiving therapies and using their records. For patients under 18 years of age, informed consent was also obtained from a parent and/or legal guardian. Patients who were eligible met the following requirements: t(8; 21) or inv(16) AML diagnoses established according to the WHO criteria50; age over 16 but less than 65 years; availability of records on chromosomal karyotype and NGS data; remission induction using a standard-dose or an intermediate-dose Ara-C-containing regimen; and at least one course of consolidation had been received for those achieving a complete remission (CR).
Immunophenotyping by flow cytometry
Untreated bone marrow (BM) samples were freshly collected at first diagnosis. Four-colour immunophenotyping was performed by flow cytometry on a FACSCalibur instrument (Becton-Dickinson, CA, USA) using mouse antihuman monoclonal antibodies against the following myeloid and lymphoid associated markers labelled by combinatorial immunofluorescence FITC/PE/PerCP/APC sets: 1) CD34/CD10/CD45/CD19; 2) CD7/CD117/CD45/CD33; 3) CD9/CD2/CD45/CD56; 4) CD15/CD38/CD45/HLA-DR; 5) CD16/CD13/CD45/CD11b; 6) CD4/CD64/CD45/CD14; 7) cMPO/cCD79a/CD45/cCD3; and 8) TdT/CD123/CD45/HLA-DR. Cells were stained with different fluorescently labelled monoclonal antibodies according to the manufacturer’s protocol. For intracellular markers, a BD cytofix/cytoperm kit was used. Blasts were obtained and characterized based on combination gating of CD45/side scatter along with other antigen markers. The expression of lineage-specific markers was determined by analysis on a FACSCanto II or FACSAria (Becton-Dickinson). Results were expressed as the mean fluorescence intensity (MFI; arbitrary relative linear units, scaled from 0 to 104), with negative expression defined at a fluorescence intensity of <102, dim expression of 102–103 and strong expression of 103–104. Samples were considered positive if at least 20% of blasts had surface antigen expression.
Conventional karyotyping and FISH analysis
Conventional metaphase karyotyping of untreated BM samples was performed using the G/R-banding method. Twenty metaphases were routinely counted in each patient, with karyotypes described according to the International System for Human Cytogenetic Nomenclature (ISCN)51,52. Interphase FISH was performed using a dual-colour, dual-fusion FISH (D-FISH) fluorescent probe specific for the chimeric genes RUNX1-RUNX1T1 and CBFβ-MYH11. At least 500 cells per sample were analysed, and the number of cells showing abnormal fluorescence signals was calculated. The abnormal cut-off for the D-FISH probe sets was defined as > 0.6% of the 500 interphase cells analysed with the D-FISH probe set.
Total RNA extraction and fusion transcript level measurement
Total RNA was extracted from mononuclear cells in fresh BM samples using TRIzol Reagent (Life Technologies, Grand Island, NY, USA). Fusion transcript levels were measured by real-time quantitative PCR (RQ-PCR) on a ViiA7 Dx PCR System (Applied Biosystems, Life Technologies, CA, USA) with a Leukemia-Related Fusion Gene Detection Kit.
Next-generation sequencing (NGS)
Genomic DNA was extracted from fresh BM samples with the QIAamp DNA Mini Kit (Qiagen GmbH, Germany) following the manufacturer’s instructions. All cases with a sufficient amount of genomic DNA were subjected to comprehensive analysis of gene mutations with the Ion PGM™ or Illumina next-generation sequencer. The detection panel was comprised of 112 potentially mutated genes related to haematological malignancies involved in the following functional categories: epigenetic regulators, signalling pathways, transcription factors, spliceosomes, cohesin complex, tumour suppressors and NPM1. PCR followed by direct Sanger sequencing was used to detect FLT3-ITD, NPM1, CEBPA and other potential complex insertions and/or deletions as previously described53,54,55.
Induction and consolidation treatment
Enrolled patients were administered either of the two regimens as induction chemotherapy: i) the standard-dose Ara-C arm consisted of Ara-C 100–200 mg/m2 i.v. over 24 hours from day 1 to 7 plus daunorubicin 60 mg/m2 or idarubicin 12 mg/m2 i.v. from day 1 to 3; ii) the intermediate-dose Ara-C arm consisted of Ara-C 1,000 mg/m2 i.v. over 2 hours every 12 hours from day 5 to 7, and other agents were the same as those in the standard-dose arm. BM response assessment was performed between days 21 and 28 after induction when peripheral blood (PB) counts recovered. When no PB recovery was noticed or leukaemic blasts persisted or reappeared in the PB, the response assessment was postponed to no later than day 35 after induction. Patients obtaining a first CR received consolidation chemotherapies based on intermediate- or high-dose Ara-C.
Clinical end-points and definitions
The overall CR rate achieved after a single induction course, early mortality, leukaemia-free survival (LFS) and overall survival (OS) were evaluated. In this study, CR and complete remission with incomplete platelet or neutrophil recovery (CRi), which were established according to criteria50, were collectively termed the overall CR. Early mortality was defined as death within 30 days after induction before response assessment. Relapse was defined as the reappearance of 5% or more leukaemic blasts on BM aspirates, the presence of blasts at any percentage in the PB, or recurrence at an extramedullary site of disease for patients who had previously achieved overall CR. LFS was calculated as the interval from the date of overall CR documentation until either first relapse or death. OS was defined as the interval between diagnosis and death from any cause. Patients who underwent haematopoietic stem cell transplantation were censored from survival analysis on the date of transplantation.
Statistical analysis
Median values (and ranges) were calculated for non-normally distributed data. The Mann-Whitney U test was used for analysis of nonparametric continuous variables. Frequencies were compared by the chi-square test for categorical variables after crosstabulation. Continuous variables were dichotomously transformed after subdivision near their median values. The probabilities of survival were estimated using the Kaplan-Meier method and were compared by the log-rank test between subgroups according to predictive factors. Comparisons were stratified by CBF subtype, clinicopathological parameters, and induction regimen. Factors that fulfilled the pre-specified assumption with P-values < 0.15 in univariate analyses were further examined in the multivariate model. Multivariate logistic regression analysis was used for factors impacting the achievement of CR and a multivariate Cox model was used for factors associated with survival end-points. All statistical tests were 2-sided, and P-values < 0.05 were considered statistically significant. Statistical analyses were conducted applying IBM SPSS Statistics 22 for Windows.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Mrozek, K., Marcucci, G., Paschka, P. & Bloomfield, C. D. Advances in molecular genetics and treatment of core-binding factor acute myeloid leukemia. Curr. Opin. Oncol. 20, 711–718, https://doi.org/10.1097/CCO.0b013e32831369df (2008).
Sinha, C., Cunningham, L. C. & Liu, P. P. Core Binding Factor Acute Myeloid Leukemia: New Prognostic Categories and Therapeutic Opportunities. Semin. Hematol. 52, 215–222, https://doi.org/10.1053/j.seminhematol.2015.04.002 (2015).
Speck, N. A. & Gilliland, D. G. Core-binding factors in haematopoiesis and leukaemia. Nature Reviews Cancer 2, 502–513, https://doi.org/10.1038/nrc840 (2002).
Ustun, C. & Marcucci, G. Emerging diagnostic and therapeutic approaches in core binding factor acute myeloid leukaemia. Curr. Opin. Hematol. 22, 85–91, https://doi.org/10.1097/MOH.0000000000000124 (2015).
Motyckova, G. & Stone, R. M. The role of molecular tests in acute myelogenous leukemia treatment decisions. Curr. Hematol. Malig. Rep. 5, 109–117, https://doi.org/10.1007/s11899-010-0049-7 (2010).
Riera, L. et al. Core binding factor acute myeloid leukaemia and c-KIT mutations. Oncol. Rep. 29, 1867–1872, https://doi.org/10.3892/or.2013.2328 (2013).
Cairoli, R. et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood 107, 3463–3468, https://doi.org/10.1182/blood-2005-09-3640 (2006).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405, https://doi.org/10.1182/blood-2016-03-643544 (2016).
Bloomfield, C. D. et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 58, 4173–4179 (1998).
Miyawaki, S. et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood 117, 2366–2372, https://doi.org/10.1182/blood-2010-07-295279 (2011).
Jourdan, E. et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 121, 2213–2223, https://doi.org/10.1182/blood-2012-10-462879 (2013).
Byrd, J. C. et al. Patients with t(8; 21)(q22; q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J. Clin. Oncol. 17(12), 3767–75 (1999).
Appelbaum, F. R. et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. British Journal of Haematology 135, 165–173, https://doi.org/10.1111/j.1365-2141.2006.06276.x (2006).
Schlenk, R. F. et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J. Clin. Oncol. 22, 3741–3750, https://doi.org/10.1200/JCO.2004.03.012 (2004).
Marcucci, G. et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8; 21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J. Clin. Oncol. 23, 5705–5717, https://doi.org/10.1200/JCO.2005.15.610 (2005).
Grimwade, D. et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116, 354–365, https://doi.org/10.1182/blood-2009-11-254441 (2010).
Solh, M., Yohe, S., Weisdorf, D. & Ustun, C. Core-binding factor acute myeloid leukemia: Heterogeneity, monitoring, and therapy. Am. J. Hematol. 89, 1121–1131, https://doi.org/10.1002/ajh.23821 (2014).
Bishop, J. F. et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood 87, 1710–1717 (1996).
Weick, J. K. et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood 88, 2841–2851 (1996).
Lowenberg, B. et al. Cytarabine dose for acute myeloid leukemia. NEJM 364, 1027–1036, https://doi.org/10.1056/NEJMoa1010222 (2011).
Burnett, A. K. et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J. Clin. Oncol. 31, 3360–3368, https://doi.org/10.1200/JCO.2012.47.4874 (2013).
Willemze, R. et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J. Clin. Oncol. 32, 219–228, https://doi.org/10.1200/JCO.2013.51.8571 (2014).
Kern, W. & Estey, E. H. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer 107, 116–124, https://doi.org/10.1002/cncr.21543 (2006).
Mosna, F. et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up. Am. J. Hematol. 90, 515–523, https://doi.org/10.1002/ajh.24000 (2015).
Jourdan, E. et al. Outcome of adult patients with acute myeloid leukemia who failed to achieve complete remission after one course of induction chemotherapy: a report from the BGMT Study Group. Leuk. Lymphoma 42, 57–65, https://doi.org/10.3109/10428190109097676 (2001).
Omori, I. et al. D816V mutation in the KIT gene activation loop has greater cell-proliferative and anti-apoptotic ability than N822K mutation in core-binding factor acute myeloid leukemia. Experimental Hematology 52, 56–64 e54, https://doi.org/10.1016/j.exphem.2017.05.003 (2017).
Tarlock, K. et al. Functional Properties of KIT Mutations Are Associated with Differential Clinical Outcomes and Response to Targeted Therapeutics in CBF Acute Myeloid Leukemia. Clin. Cancer Res. 25, 5038–5048, https://doi.org/10.1158/1078-0432.CCR-18-1897 (2019).
Tan, Y. et al. Monitoring of clonal evolution of double C-KIT exon 17 mutations by Droplet Digital PCR in patients with core-binding factor acute myeloid leukemia. Leuk. Res. 69, 89–93, https://doi.org/10.1016/j.leukres.2018.04.013 (2018).
Delaunay, J. et al. Prognosis of inv(16)/t(16; 16) acute myeloid leukemia (AML): a survey of 110 cases from the French AML Intergroup. Blood 102, 462–469, https://doi.org/10.1182/blood-2002-11-3527 (2003).
Clozel, T. et al. Slow relapse in acute myeloid leukemia with inv(16) or t(16; 16). Haematologica 94, 1466–1468, https://doi.org/10.3324/haematol.2009.010702 (2009).
Nguyen, S. et al. A white blood cell index as the main prognostic factor in t(8; 21) acute myeloid leukemia (AML): a survey of 161 cases from the French AML Intergroup. Blood 99, 3517–3523 (2002).
Boissel, N. et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia 20, 965–970, https://doi.org/10.1038/sj.leu.2404188 (2006).
Kim, H. J. et al. KIT D816 mutation associates with adverse outcomes in core binding factor acute myeloid leukemia, especially in the subgroup with RUNX1/RUNX1T1 rearrangement. Annals of Hematology 92, 163–171, https://doi.org/10.1007/s00277-012-1580-5 (2013).
Park, S. H. et al. Prognostic impact of c-KIT mutations in core binding factor acute myeloid leukemia. Leuk. Res. 35, 1376–1383, https://doi.org/10.1016/j.leukres.2011.06.003 (2011).
Schnittger, S. et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood 107, 1791–1799, https://doi.org/10.1182/blood-2005-04-1466 (2006).
Yui, S. et al. D816 mutation of the KIT gene in core binding factor acute myeloid leukemia is associated with poorer prognosis than other KIT gene mutations. Annals of Hematology 96, 1641–1652, https://doi.org/10.1007/s00277-017-3074-y (2017).
Krauth, M. T. et al. High number of additional genetic lesions in acute myeloid leukemia with t(8; 21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome. Leukemia 28, 1449–1458, https://doi.org/10.1038/leu.2014.4 (2014).
Jung, H. A. et al. Prognostic factor analysis in core-binding factor-positive acute myeloid leukemia. Anticancer Research 34, 1037–1045 (2014).
Duployez, N. et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia. Blood 127, 2451–2459, https://doi.org/10.1182/blood-2015-12-688705 (2016).
Allen, C. et al. The importance of relative mutant level for evaluating impact on outcome of KIT, FLT3 and CBL mutations in core-binding factor acute myeloid leukemia. Leukemia 27, 1891–1901, https://doi.org/10.1038/leu.2013.186 (2013).
Paschka, P. et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16; 16): a study of the German-Austrian AML Study Group (AMLSG). Blood 121, 170–177, https://doi.org/10.1182/blood-2012-05-431486 (2013).
Opatz, S. et al. Exome sequencing identifies recurring FLT3 N676K mutations in core-binding factor leukemia. Blood 122, 1761–1769, https://doi.org/10.1182/blood-2013-01-476473 (2013).
Itzykson, R. et al. Clonal interference of signaling mutations worsens prognosis in core-binding factor acute myeloid leukemia. Blood 132, 187–196, https://doi.org/10.1182/blood-2018-03-837781 (2018).
Klein, K. et al. Clinical Impact of Additional Cytogenetic Aberrations, cKIT and RAS Mutations, and Treatment Elements in Pediatric t(8; 21)-AML: Results From an International Retrospective Study by the International Berlin-Frankfurt-Munster Study Group. J. Clin. Oncol. 33, 4247–4258, https://doi.org/10.1200/JCO.2015.61.1947 (2015).
Girardi, T., Vicente, C., Cools, J. & De Keersmaecker, K. The genetics and molecular biology of T-ALL. Blood 129, 1113–1123, https://doi.org/10.1182/blood-2016-10-706465 (2017).
Takam Kamga, P. et al. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget 7, 21713–21727, https://doi.org/10.18632/oncotarget.7964 (2016).
Marschalek, R. Systematic Classification of Mixed-Lineage Leukemia Fusion Partners Predicts Additional Cancer Pathways. Ann. Lab. Med. 36, 85–100, https://doi.org/10.3343/alm.2016.36.2.85 (2016).
Iriyama, N. et al. CD56 expression is an independent prognostic factor for relapse in acute myeloid leukemia with t(8; 21). Leuk. Res. 37, 1021–1026, https://doi.org/10.1016/j.leukres.2013.05.002 (2013).
Rege, K. et al. Disease features in acute myeloid leukemia with t(8; 21)(q22; q22). Influence of age, secondary karyotype abnormalities, CD19 status, and extramedullary leukemia on survival. Leuk. Lymphoma 40, 67–77, https://doi.org/10.3109/10428190009054882 (2000).
Vardiman, J. W. et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114, 937–951, https://doi.org/10.1182/blood-2009-03-209262 (2009).
Brothman, A. R., Persons, D. L. & Shaffer, L. G. Nomenclature evolution: Changes in the ISCN from the 2005 to the 2009 edition. Cytogenet. Genome Res. 127, 1–4, https://doi.org/10.1159/000279442 (2009).
Simons, A., Shaffer, L. G. & Hastings, R. J. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenet. Genome Res. 141, 1–6, https://doi.org/10.1159/000353118 (2013).
Lin, L. I. et al. Characterization of CEBPA mutations in acute myeloid leukemia: most patients with CEBPA mutations have biallelic mutations and show a distinct immunophenotype of the leukemic cells. Clin. Cancer Res. 11, 1372–1379, https://doi.org/10.1158/1078-0432.CCR-04-1816 (2005).
Rau, R. & Brown, P. Nucleophosmin (NPM1) mutations in adult and childhood acute myeloid leukaemia: towards definition of a new leukaemia entity. Hematol. Oncol. 27, 171–181, https://doi.org/10.1002/hon.904 (2009).
Kiyoi, H. et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood 93, 3074–3080 (1999).
Acknowledgements
The authors are thankful to 1) DeLiang Liu for helping with data collection and management and 2) Yan Lin for handling, conditioning and storing patient samples. We would like to acknowledge all clinical research assistants here.
Author information
Authors and Affiliations
Contributions
B.W. was responsible for clinical data management and manuscript writing, and the manuscript was approved by all the authors; Jh.Z. was responsible for mutation analysis and interpretation; Xy.H. and Hq.L. enrolled patients in the study; Zl.W. performed the statistical analysis; B.Y. was responsible for literature collection and clinical data management. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Zhang, J., Hua, X. et al. Clinical heterogeneity under induction with different dosages of cytarabine in core binding factor acute myeloid leukaemia. Sci Rep 10, 685 (2020). https://doi.org/10.1038/s41598-020-57414-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57414-y
- Springer Nature Limited